Energy and Heat Conductors vs Insulators Thermal Conductivity





















- Slides: 31

Energy and Heat Conductors vs. Insulators Thermal Conductivity Specific Heat 1

Heat n. Energy that flows from something warm to something cooler n. A hotter substance gives KE to a cooler one n. When heat is transferred (lost or gained), there is a change in the energy within the substance 2

Heat n. Energy that flows from something warm to something cooler n. A hotter substance gives KE to a cooler one n. When heat is transferred (lost or gained), there is a change in the energy within the substance 3

Learning Check H 1 A. When you touch ice, heat is transferred from 1) your hand to the ice 2) the ice to your hand B. When you drink a hot cup of coffee, heat is transferred from 1) your mouth to the coffee 2) the coffee to your mouth Lecture. PLUS Timberlake 99 4

Solution H 1 A. When you touch ice, heat is transferred from 1) your hand to the ice B. When you drink a hot cup of coffee, heat is transferred from 2) the coffee to your mouth 5

Learning Check H 2 When you heat 200 g of water for 1 minute, the water temperature rises from 10°C to 18°C. 200 g 400 g If you heat 400 g of water at 10°C in the same pan with the same amount of heat for 1 minute, what would you expect the final temperature to be? 1) 10 °C 2) 14°C 3) 18°C 6

Solution H 2 2)14°C Heating twice the mass of water using the same amount of heat will raise the temperature only half as much. 200 g 400 g 7

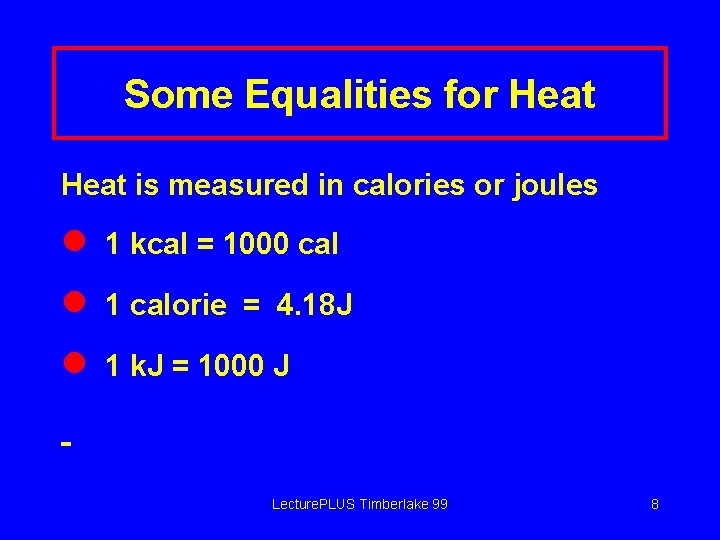
Some Equalities for Heat is measured in calories or joules l 1 kcal = 1000 cal l 1 calorie = 4. 18 J l 1 k. J = 1000 J Lecture. PLUS Timberlake 99 8

Thermal Conductivity & Specific Heat l Why does the metal of your chair feel so much colder than the wood of the desk? l Why do some foods stay hot longer than others? l Why is the beach sand hot, but the water is cool on the same hot day? Lecture. PLUS Timberlake 99 9

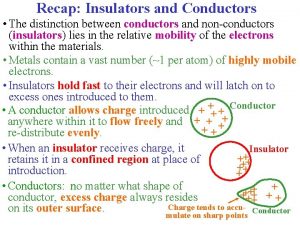
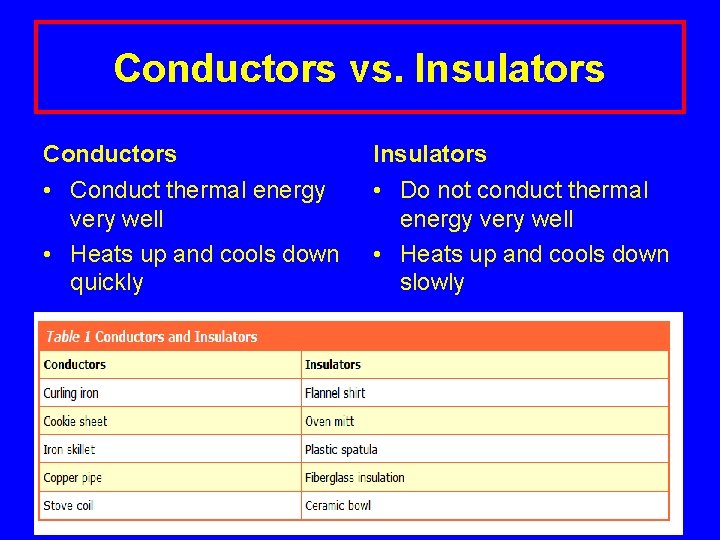
Conductors vs. Insulators Conductors Insulators • Conduct thermal energy very well • Heats up and cools down quickly • Do not conduct thermal energy very well • Heats up and cools down slowly Lecture. PLUS Timberlake 99 10

Thermal Conductivity & Specific Heat l Why does the metal of the seat buckle burn your hand but the cloth does not? (Hint: They are the same temperature!) The metal is a conductor that has a higher thermal conductivity and therefore transfers the heat to your hand very quickly burning your hand! Lecture. PLUS Timberlake 99 11

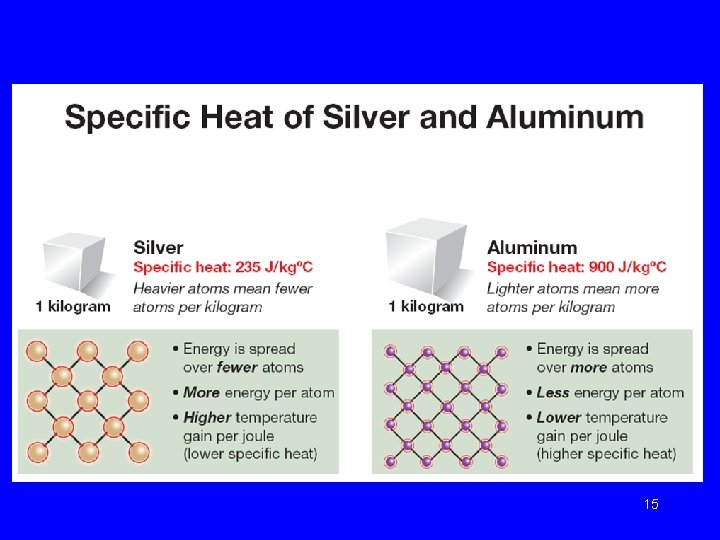
Specific Heat Different substances have different capacities for storing energy It may take 20 minutes to heat water to 75°C. However, the same mass of aluminum might require 5 minutes and the same amount of copper may take only 2 minutes to reach the same temperature. Lecture. PLUS Timberlake 99 12

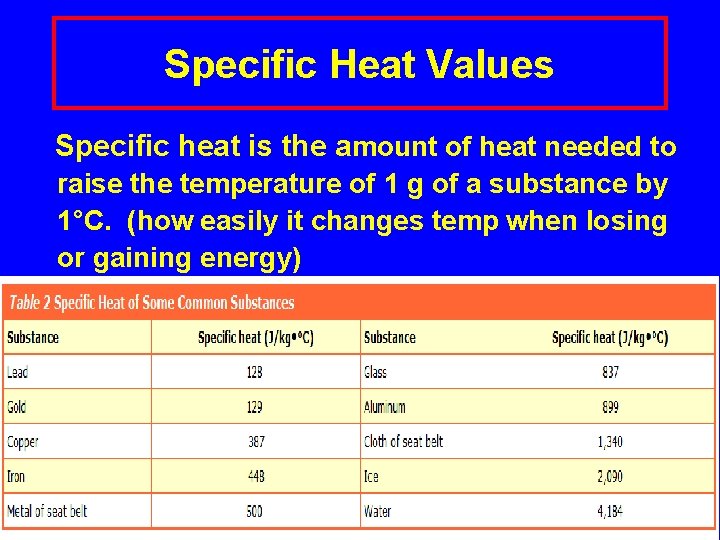
Specific Heat Values Specific heat is the amount of heat needed to raise the temperature of 1 g of a substance by 1°C. (how easily it changes temp when losing or gaining energy) Lecture. PLUS Timberlake 99 13

Learning Check H 3 A. A substance with a large specific heat 1) heats up quickly 2) heats up slowly B. When ocean water cools, the surrounding air 1) cools 2) warms 3) stays the same C. Sand in the desert is hot in the day, and cool at night. Sand must have a 1) high specific heat 2) low specific heat Lecture. PLUS Timberlake 99 14

15

Solution H 3 A. A substance with a large specific heat 2) heats up slowly B. When ocean water cools, the surrounding air 2) warms C. Sand in the desert is hot in the day, and cool at night. Sand must have a 2) low specific heat Lecture. PLUS Timberlake 99 16

Measuring Heat (Q) Requires l Grams of substance (mass) l Temperature change ( T) l Specific heat of the substance (c) Unlike temperature, energy transferred between objects can not be measured directly. Instead, it must be calculated. When calculating energy transferred between objects, you can use the definition of heat as the amount of energy that is transferred between two objects that are at different temperatures. Heat can Lecture. PLUS Timberlake 99 then be expressed in joules (J). 17

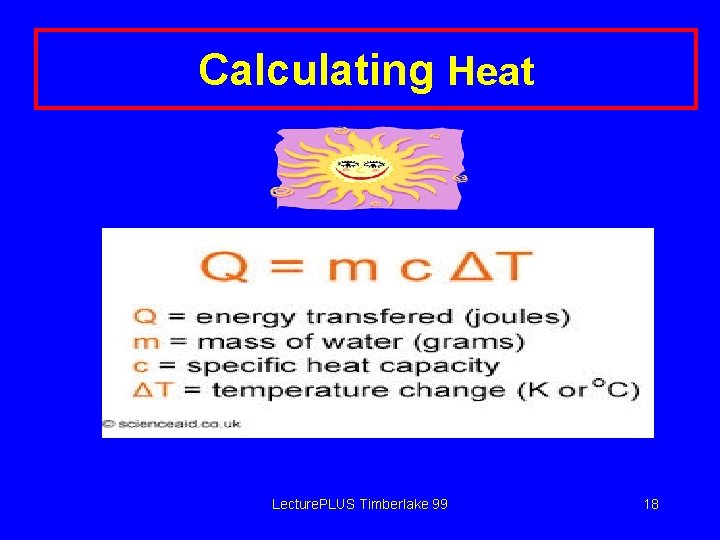
Calculating Heat Lecture. PLUS Timberlake 99 18

Learning Check H 4 A hot-water bottle contains 750 g of water at 65°C. If the water cools to body temperature (37°C), how many joules of heat could be transferred to sore muscles? (SH of water = 4, 184 J/kg*o. C) Lecture. PLUS Timberlake 99 19

Solution H 4 A hot-water bottle contains 750 g of water at 65°C. If the water cools to body temperature (37°C), how many joules of heat could be transferred to sore muscles? (SH of water = 4, 184 J/kg*o. C) Lecture. PLUS Timberlake 99 20

Learning Check H 5 How many joules of heat are needed to raise the temperature of 120 g of water from 15°C to 75°C? (SH of water = 4, 184 J/kg*o. C) Lecture. PLUS Timberlake 99 21

Solution H 5 How many joules of heat are needed to raise the temperature of 120 g of water from 15°C to 75°C? (SH of water = 4, 184 J/kg*o. C) Lecture. PLUS Timberlake 99 22

Learning Check H 6 • What is the specific heat of lead if 57. 0 J are needed to raise the temperature of 35. 6 g of lead by 12. 5 C? Q c = --------m x t Lecture. PLUS Timberlake 99 23

Solution H 6 • What is the specific heat of lead if 57. 0 J are needed to raise the temperature of 35. 6 g of lead by 12. 5 C? Q c = --------m x t Lecture. PLUS Timberlake 99 24

Learning Check H 7 • What is the specific heat of sodium if 123 J are needed to raise the temperature of 4. 00 g of sodium by 25. 0 C? Q c = --------m x t Lecture. PLUS Timberlake 99 25

Solution H 7 • What is the specific heat of sodium if 123 J are needed to raise the temperature of 4. 00 g of sodium by 25. 0 C? Q c = --------m x t Lecture. PLUS Timberlake 99 26

Learning Check H 8 • How many joules of heat are absorbed by 45. 2 g of aluminum if its temperature rises from 12. 5 C to 76. 8 C (See Table 3. 9)? Lecture. PLUS Timberlake 99 27

Solution H 8 • How many joules of heat are absorbed by 45. 2 g of aluminum if its temperature rises from 12. 5 C to 76. 8 C (See Table 3. 9)? Lecture. PLUS Timberlake 99 28

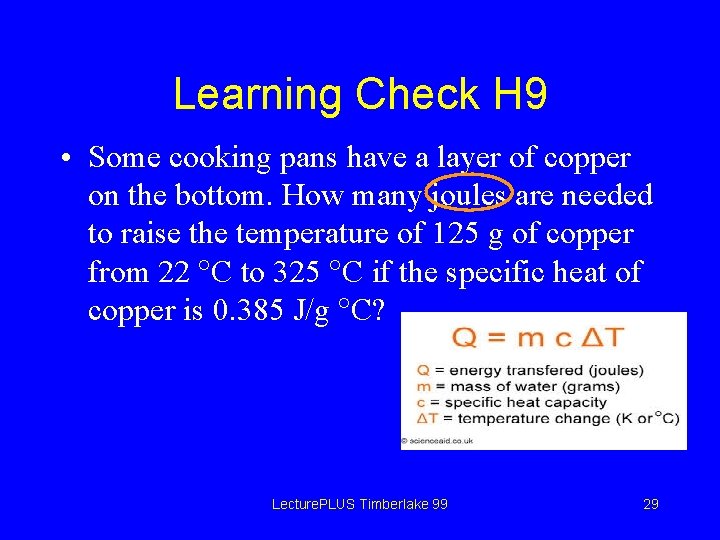
Learning Check H 9 • Some cooking pans have a layer of copper on the bottom. How many joules are needed to raise the temperature of 125 g of copper from 22 C to 325 C if the specific heat of copper is 0. 385 J/g C? Lecture. PLUS Timberlake 99 29

Solution H 9 • Some cooking pans have a layer of copper on the bottom. How many joules are needed to raise the temperature of 125 g of copper from 22 C to 325 C if the specific heat of copper is 0. 385 J/g C? Lecture. PLUS Timberlake 99 30

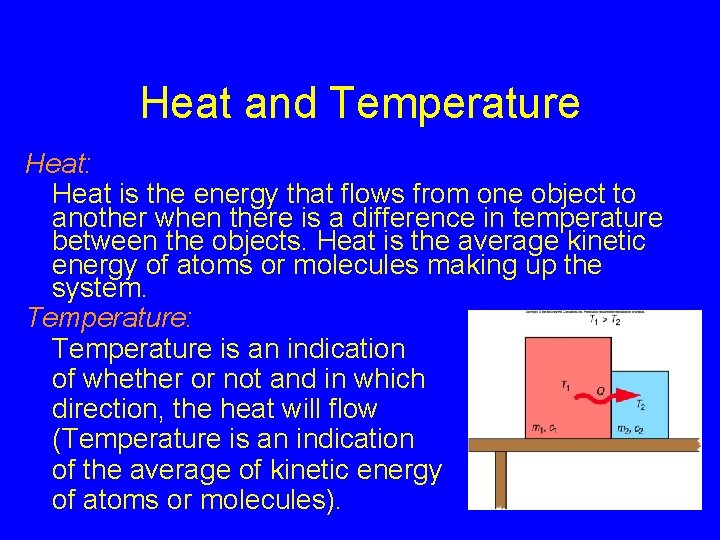
Heat and Temperature Heat: Heat is the energy that flows from one object to another when there is a difference in temperature between the objects. Heat is the average kinetic energy of atoms or molecules making up the system. Temperature: Temperature is an indication of whether or not and in which direction, the heat will flow (Temperature is an indication of the average of kinetic energy of atoms or molecules).
 What is conductor
What is conductor What are conducters
What are conducters Voltage in parallel circuit
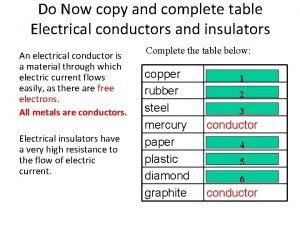
Voltage in parallel circuit Table of conductors and insulators
Table of conductors and insulators Bad conductor of electricity
Bad conductor of electricity Study jams layers of the earth
Study jams layers of the earth Unit of electric field
Unit of electric field Five form of energy
Five form of energy Section 3 using thermal energy worksheet answer key
Section 3 using thermal energy worksheet answer key How are thermal energy and temperature different
How are thermal energy and temperature different Electrical properties of matter
Electrical properties of matter Conductor in science
Conductor in science Thermal conductivity of styrofoam
Thermal conductivity of styrofoam Astm thermal conductivity
Astm thermal conductivity Thermal conductivity detector
Thermal conductivity detector Thermal conductivity of dental materials
Thermal conductivity of dental materials Thermal conductivity detector
Thermal conductivity detector Enhancing thermal conductivity of fluids with nanoparticles
Enhancing thermal conductivity of fluids with nanoparticles Dimensions of thermal conductivity
Dimensions of thermal conductivity T0st
T0st Heat loss symbol
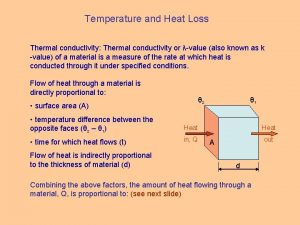
Heat loss symbol Thermal conductivity formula
Thermal conductivity formula Spherical heat equation
Spherical heat equation Thermal conductivity in series
Thermal conductivity in series Difference between heat and thermal energy
Difference between heat and thermal energy Difference between heat and thermal energy
Difference between heat and thermal energy Difference between heat and thermal energy
Difference between heat and thermal energy Heat vs thermal energy vs temperature
Heat vs thermal energy vs temperature What is the difference between thermal energy and heat?
What is the difference between thermal energy and heat? Heat thermal energy and temperature
Heat thermal energy and temperature Temperature and heat
Temperature and heat Chapter 16 thermal energy and heat
Chapter 16 thermal energy and heat