Thermal Energy Transfer Conductors Insulators Essential Standard 6



















- Slides: 31

Thermal Energy Transfer Conductors & Insulators

Essential Standard • 6. P. 3 Understand characteristics of energy transfer and interactions of matter and energy.

Clarifying Objective • 6. P. 3. 3 Explain the suitability of materials for use in technological design based on a response to heat (to include conduction, expansion, and contraction) and electrical energy (conductors and insulators).

Essential Questions • What are examples of INSULATORS? • What are examples of CONDUCTORS? • How does electrical & thermal energy move through conductors? • Why does electrical & thermal energy NOT move through INSULATORS?

Electrical Energy • To understand how conductors & insulators work we have to understand atoms • If you remember atoms are the smallest pieces of matter • They make up everything except photons (which are part of an atom)

What is Electricity • http: //www. youtube. com/watch? v=ZAFW 4 zd Xpb. Y

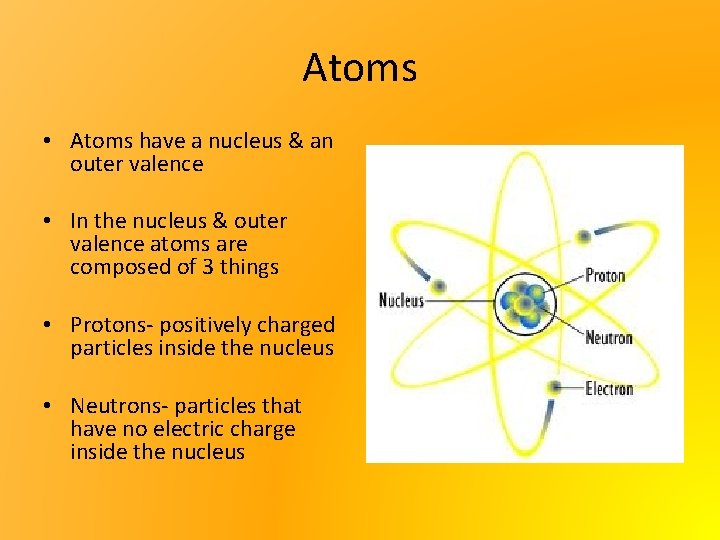
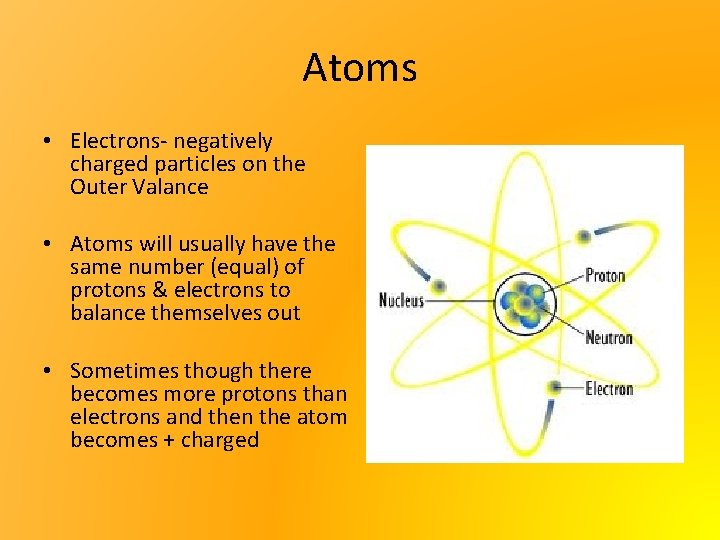
Atoms • Atoms have a nucleus & an outer valence • In the nucleus & outer valence atoms are composed of 3 things • Protons- positively charged particles inside the nucleus • Neutrons- particles that have no electric charge inside the nucleus

Atoms • Electrons- negatively charged particles on the Outer Valance • Atoms will usually have the same number (equal) of protons & electrons to balance themselves out • Sometimes though there becomes more protons than electrons and then the atom becomes + charged

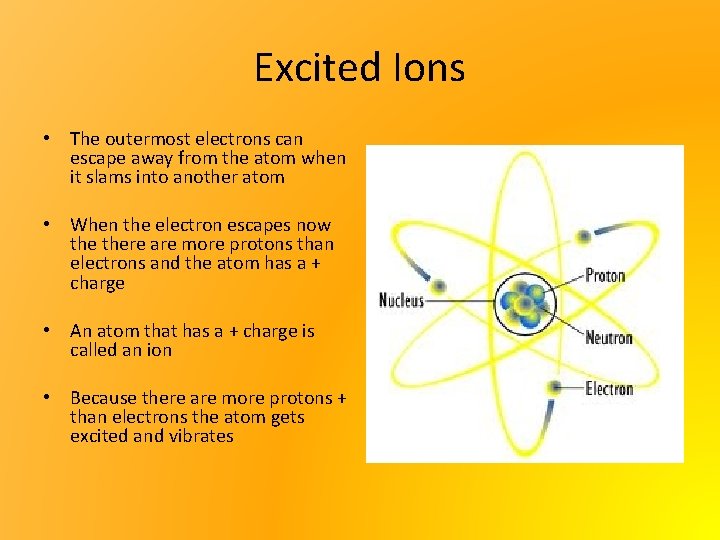
Excited Ions • The outermost electrons can escape away from the atom when it slams into another atom • When the electron escapes now there are more protons than electrons and the atom has a + charge • An atom that has a + charge is called an ion • Because there are more protons + than electrons the atom gets excited and vibrates

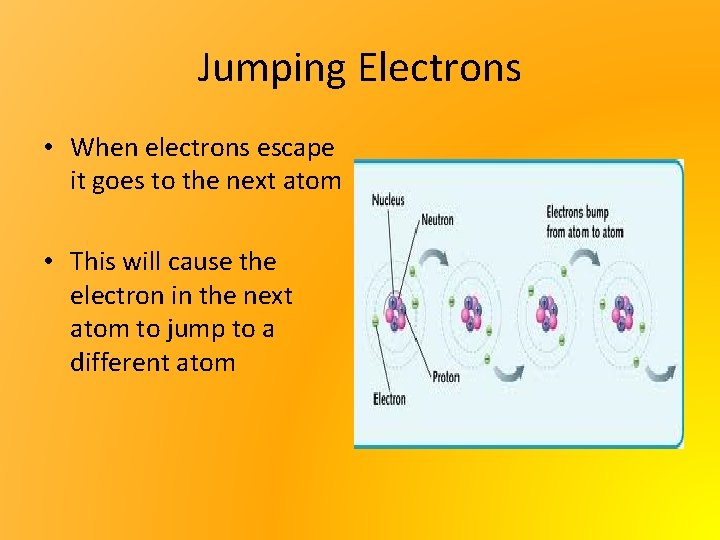
Jumping Electrons • When electrons escape it goes to the next atom • This will cause the electron in the next atom to jump to a different atom

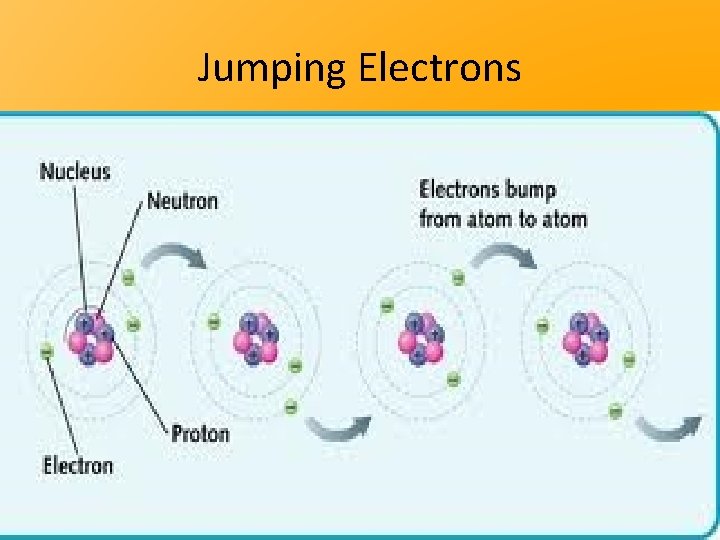
Jumping Electrons

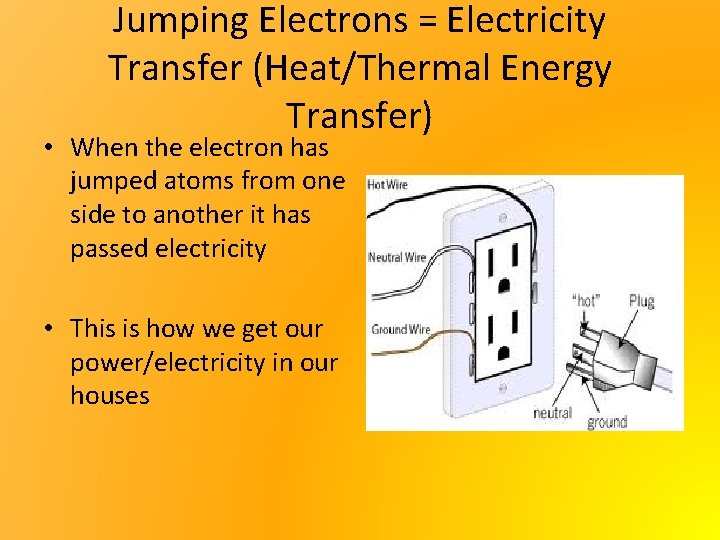
Jumping Electrons = Electricity Transfer (Heat/Thermal Energy Transfer) • When the electron has jumped atoms from one side to another it has passed electricity • This is how we get our power/electricity in our houses

Essential Questions • Get with a partner and answer… • How does electrical & thermal energy move through conductors?

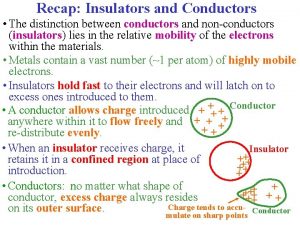
Conductors & Insulators Opposite • Conductors are Insulators OPPOSITES from each other • This means if a material is a good conductor it is a bad insulator • If a material is a good insulator it is a bad conductor

Conductors • http: //www. youtube. com/watch? v=q. Uhxm. XZ w. Pmg • 4: 30 -7: 15

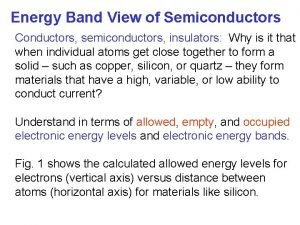
Conductors • Conductors are solids that energy can travel through • This could be thermal energy (heat) electrical energy or other energy • There are good conductors & bad conductors

Good Conductors • Good conductors are solids that have atoms that are very dense • The more dense the atoms the better conductors they will be • This is because the atoms are more tightly compacted together

Good Conductors • Good conductors atoms are close enough to each other that the electrons can bounce from one atom to the next passing the heat or electricity

Bad Conductors • Bad conductors of electricity & thermal energy exist in three different forms • Gasses are bad conductors because the particles are so far apart from each other • Liquids are bad conductors for the same reason • Solids that are not dense are also bad conductors

Why are They Bad Conductors • These are all bad conductors because the atom particles are not dense and they cant jump from atom to atom • The worst kind of conductor is actually called an insulator

Insulators • Insulators are materials used to keep heat & sound contained inside something • Insulators keep heat inside something • Insulators keep electricity inside something

Insulator • http: //www. youtube. com/watch? v=q. Uhxm. XZ w. Pmg • 7: 15 -8: 15

Essential Questions • Get with a partner and answer… • Why does electrical & thermal energy NOT move through INSULATORS?

Good Insulators • Good insulators will NOT allow electrons to move from one end to another • They trap the electrons keeping the heat or electricity inside something

What makes a Good Insulator • Materials that have many different types of atoms make good insulators (Styrofoam cups) • This is because the DIFFERENT types of atoms BOND together • Now not only are the atoms are the same connected together but also those atoms are forming BONDS to other types of atoms

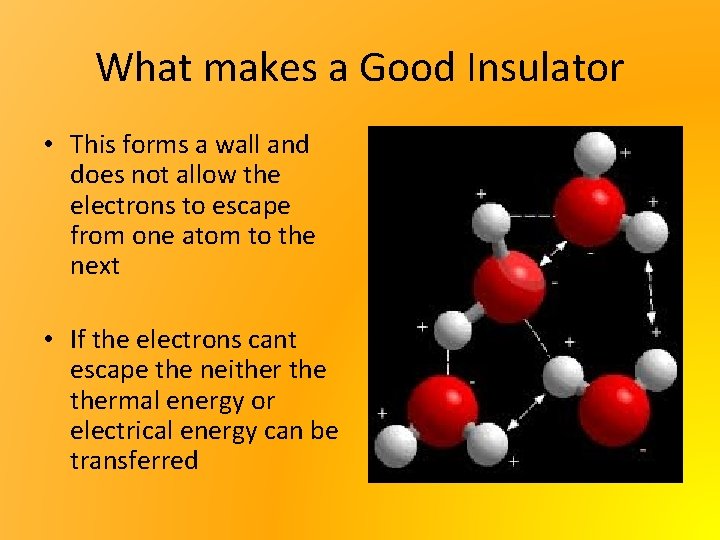
What makes a Good Insulator • This forms a wall and does not allow the electrons to escape from one atom to the next • If the electrons cant escape the neithermal energy or electrical energy can be transferred

Great Conductors • Have to be solids • Metals from the periodic table are the best because they are just one kind of atom & very dense – – – Copper Aluminum Gold Silver People & Animals

Great Insulators • Solid nonmetals that are NOT DENSE – – – – Styrofoam koozies Rubber Plastic Glass Ceramic/Porcelain Clothing

Essential Questions • Get with a partner and answer… • What are examples of INSULATORS? • What are examples of CONDUCTORS?

EOG Questions • Why are some coffee cups composed of ceramic material? • A) Ceramic materials are conductors that limit heat transfer. • B) Ceramic materials are insulators that limit heat transfer. • C) Ceramic materials are conductors that aid heat transfer. • D) Ceramic materials are insulators that aid heat transfer.

EOG Questions • A worker for an electrical company is preparing to fix a power line. Why would he put on rubber gloves before working with any power lines? • A) Rubber is a poor conductor of heat but a good conductor of electricity. • B) Rubber is a good conductor of heat but a poor conductor of electricity. • C) Rubber is a poor conductor of heat and electricity. • D) Rubber is a good conductor of heat and electricity.
 Electricity conductors
Electricity conductors What are conductors
What are conductors Calculate voltage across resistor
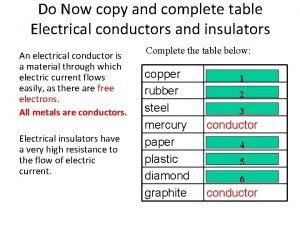
Calculate voltage across resistor Table of conductors and insulators
Table of conductors and insulators Bad conductor of electricity
Bad conductor of electricity Studyjams heat
Studyjams heat Electric field intensity formula
Electric field intensity formula Five types of energy
Five types of energy Section 3 using thermal energy
Section 3 using thermal energy Thermal transfer vs direct thermal printing
Thermal transfer vs direct thermal printing Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis 5 good conductors
5 good conductors Example of heat energy
Example of heat energy Thermal energy transfer
Thermal energy transfer Heat energy
Heat energy How are thermal energy and temperature different
How are thermal energy and temperature different Kinetic energy to thermal energy
Kinetic energy to thermal energy Characteristics of lipids
Characteristics of lipids Gibbs free energy vs standard free energy
Gibbs free energy vs standard free energy Gibbs free energy non standard conditions
Gibbs free energy non standard conditions Gibbs free energy non standard conditions
Gibbs free energy non standard conditions Is scissors a conductor or insulator
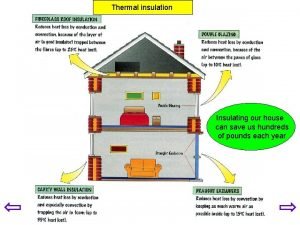
Is scissors a conductor or insulator Insulation in house
Insulation in house Youtube.com
Youtube.com Jeopardy conductor
Jeopardy conductor Victor insulators
Victor insulators How does a rubber rod become negatively
How does a rubber rod become negatively Opacity and translucency in insulators
Opacity and translucency in insulators Testing of insulators
Testing of insulators A disturbance that transfers energy is called
A disturbance that transfers energy is called What is the difference between thermal energy and heat?
What is the difference between thermal energy and heat?