Thermal Energy Transfer in the Hydrosphere Section 2
























- Slides: 36

Thermal Energy Transfer in the Hydrosphere Section 2. 3

Objectives • Define specific heat capacity and use it to calculate thermal energy • Describe the hydrological cycle • Distinguish between different phases of the hydrological cycle • Calculate heat of fusion and heat of vaporization

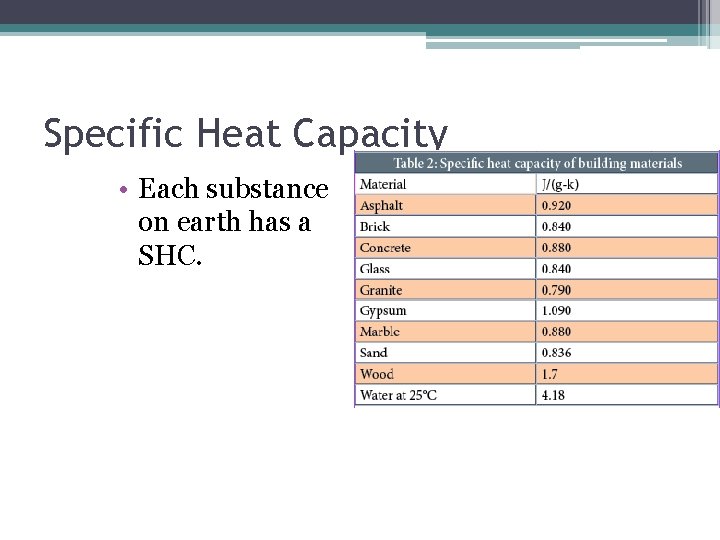
Specific Heat Capacity • Symbolized as “c” • Amount of energy needed to raise temp of 1 g of a substance by 1 degree Celsius • Water c= 4. 19 J/g degree Celsius ▫ This is high • What effect might this have on the climate of Vancouver compared to Calgary?

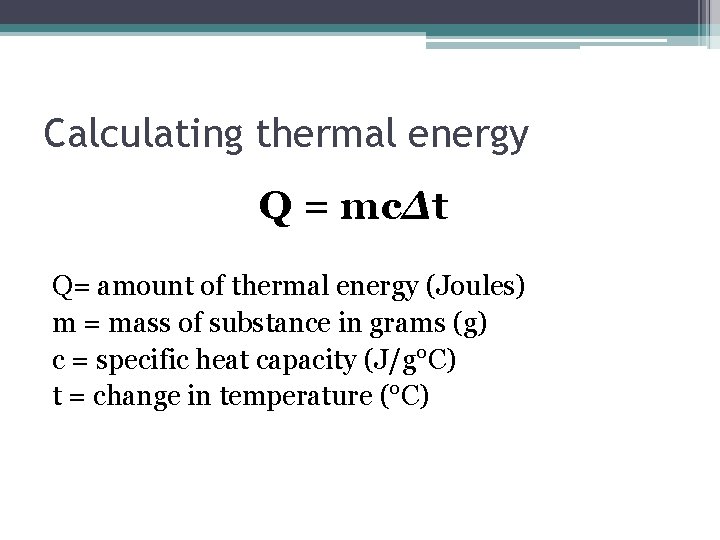
Calculating thermal energy Q = mcΔt Q= amount of thermal energy (Joules) m = mass of substance in grams (g) c = specific heat capacity (J/g°C) t = change in temperature (°C)

Specific Heat Capacity • Each substance on earth has a SHC.

Examples • A house contains 170 g of dry air. The furnace has broken, and the temperature of the air has fallen to 2. 0°C. How much energy is needed to heat the air in the home back to 20°C.

Examples • What is the mass of water that has its temperature raised 35°C and absorbs 100 J of energy?

Water • Water has a very large SHC for its size. • This is due to some of the properties that we discussed in the previous units. ▫ Hydrogen Bonds ▫ Dipoles

Oceans and Mountains Influence Climate • How do these geological features affect weather? ▫ When moist air cools, it cannot hold as much water which may fall in the form of precipitation. ▫ Warm air rises and cool air replaces it this causes the movement of air.

How Oceans Distribute Heat • Oceans are a great storage of heat. ▫ Low albedo ▫ Absorb 90% of incoming solar energy ▫ Large specific heat capacity ▫ Cover lots of the earth’s surface

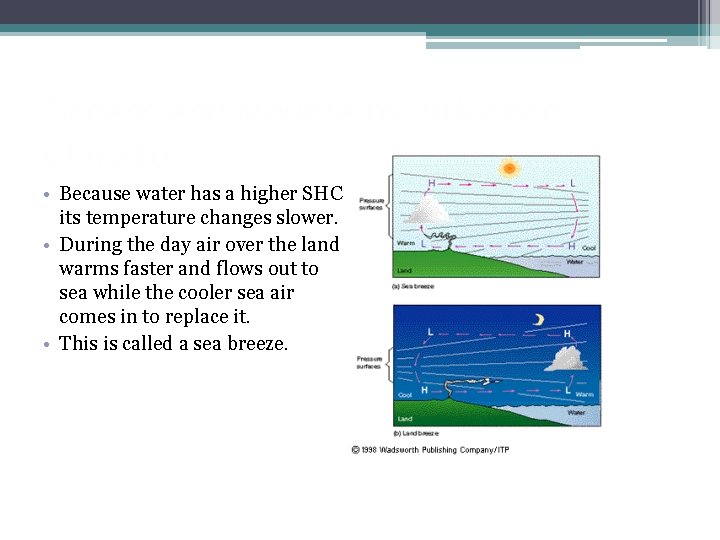
Oceans and Mountains Influence Climate • Because water has a higher SHC its temperature changes slower. • During the day air over the land warms faster and flows out to sea while the cooler sea air comes in to replace it. • This is called a sea breeze.

Oceans and Mountains Influence Climate • During the night the land cools faster than the sea so warm air from the sea rises and is replaced by the cooler air from the land. • This is called a land breeze

Oceans and Mountains Influence Climate • Mountains provide a barrier that forces air to rise over top of them. • As moist air rises it cools and the water held in it condenses. • This leaves very little moisture in the air as it flows quickly down the other side of the mountain. ▫ Areas on the other side of the mountain are in a rain shadow.

Practice • A 50. 0 g mass of water at 25. 0 °C is heated to 50. 0°C on a hot plate. Given that theoretical specific heat capacity of water is 4. 19 J/g°C, determine the value for Q • Answer:

Practice • How much thermal energy must be released to decrease the temperature of 100 g of water by 10. 0 °C, given that theoretical specific heat capacity of water is 4. 19 J/g°C? • Answer:

Practice • Calculate the change in temperature that occurs when 8380 J of thermal energy is added to 100. 0 g of water. c= 4. 19 J/g°C • Answer:

Practice • When 21. 6 J of thermal energy is added to a 2. 0 g mass of iron, the temperature of the iron increases by 24. 0°C. What is the experimental specific heat capacity of iron?

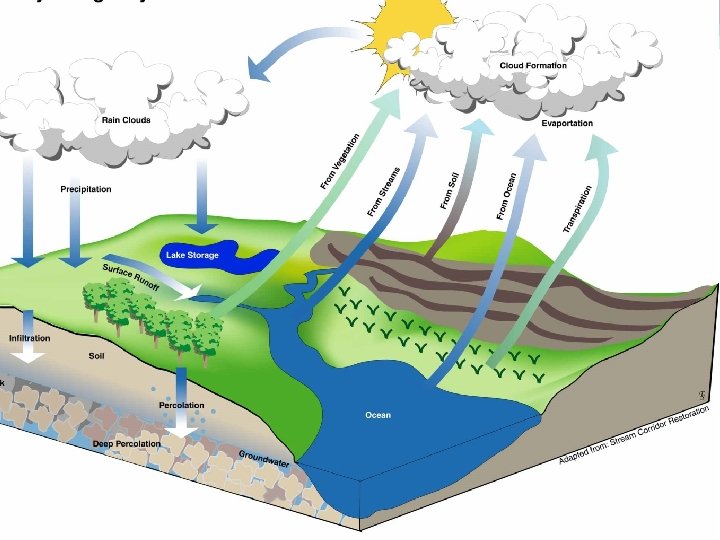
Hydrological Cycle • Water is constantly moving through the biosphere • Changing phases from solid to liquid to gas and back again • Uses evaporation, condensation, transpiration, and precipitation • Add or release thermal energy for phase changes


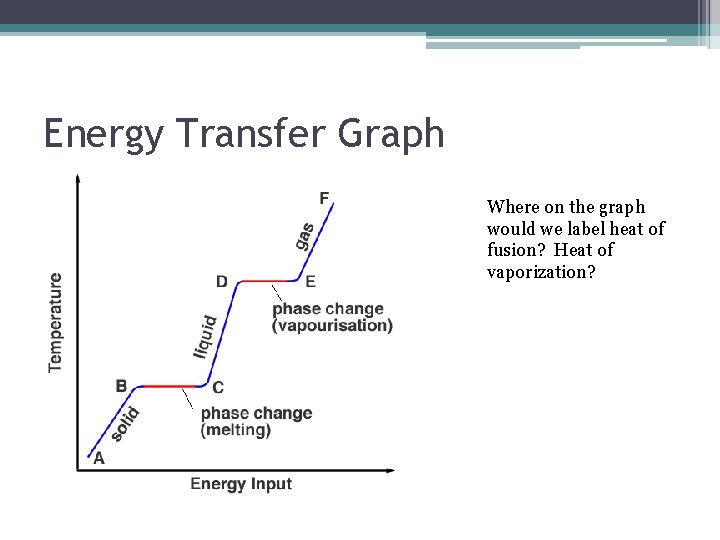
Energy Transfer Types • Heat of fusion ▫ Energy absorbed when 1 mol of a substance changes from solid to liquid • Heat of Solidification ▫ Energy released when 1 mol of a substance changes from liquid to solid • Heat of Vaporization ▫ Energy absorbed when 1 mol of a substance changes from liquid to vapor

Energy Transfer Types cont… • Heat of Condensation ▫ Energy released when 1 mol of a substance changes from vapor to liquid

Energy Transfer Graph Where on the graph would we label heat of fusion? Heat of vaporization?

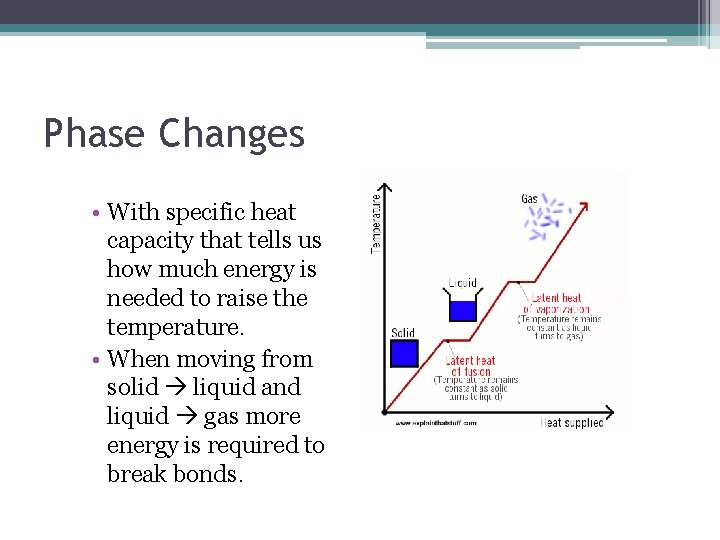
Phase Changes • With specific heat capacity that tells us how much energy is needed to raise the temperature. • When moving from solid liquid and liquid gas more energy is required to break bonds.

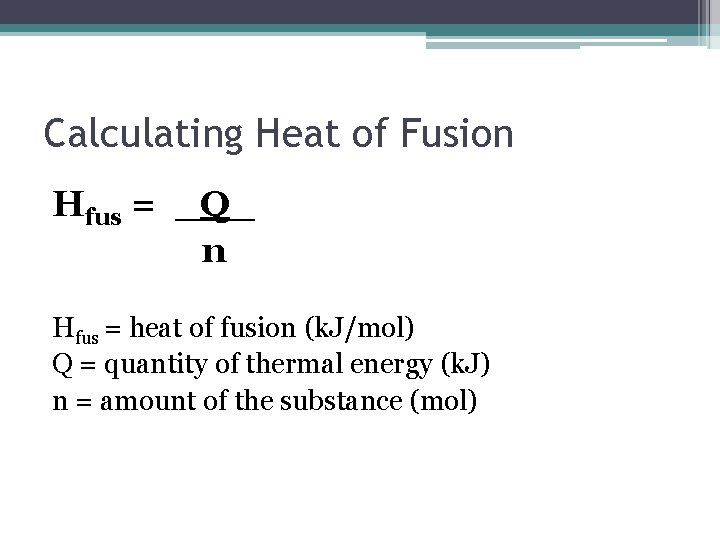
Heat of Fusion • The amount of energy required to melt a substance is referred to as the heat of fusion. • Q = n. Hfus ▫ Q = energy required ▫ n = number of moles ▫ Hfus = J/mol

Example • A beaker contains 360. 4 g of water in the solid state at 0°C. How much energy is required to convert the solid ice to liquid water?

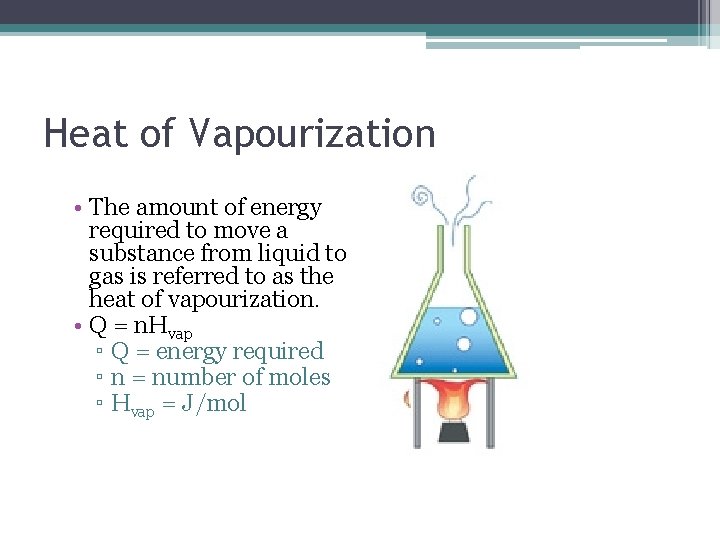
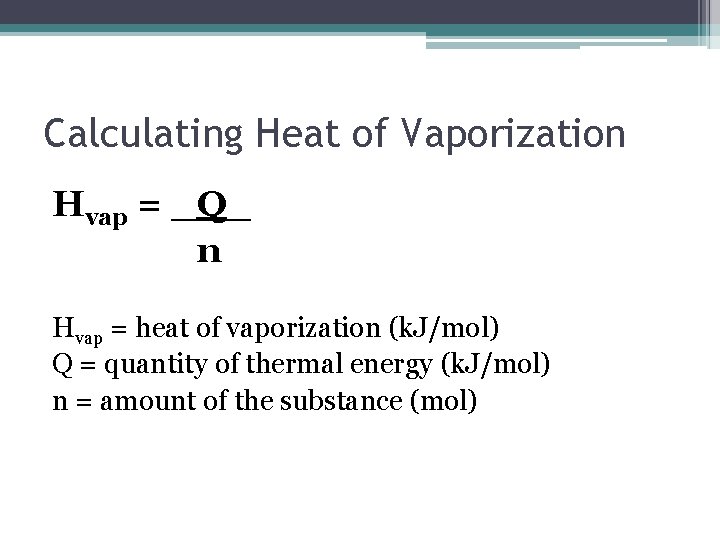
Heat of Vapourization • The amount of energy required to move a substance from liquid to gas is referred to as the heat of vapourization. • Q = n. Hvap ▫ Q = energy required ▫ n = number of moles ▫ Hvap = J/mol

Example • A beaker contains 360. 4 g of water in the liquid state at 100°C. How much energy is required to convert the liquid water to water vapour?

Summary Chart Energy Transfer Type Phase changes Energy absorbed or released?

Calculating Heat of Fusion Hfus = _Q_ n Hfus = heat of fusion (k. J/mol) Q = quantity of thermal energy (k. J) n = amount of the substance (mol)

Practice • When 27. 05 k. J of thermal energy is added to 4. 50 mol of ice at 0. 0°C, the ice melts completely. What is the experimental heat of fusion of water? • Answer:

Practice • When 5. 00 g of ice melts, 1. 67 k. J of thermal energy is absorbed. Calculate the experimental heat of fusion of ice. The molar mass, M, of ice is 18. 02 g/mol. • Answer:

Practice • When 0. 751 k. J of thermal energy is added to 0. 125 mol of ice at 0. 0°C, the ice changes phase. Calculate the experimental heat of fusion of ice.

Calculating Heat of Vaporization Hvap = _Q_ n Hvap = heat of vaporization (k. J/mol) Q = quantity of thermal energy (k. J/mol) n = amount of the substance (mol)

Practice • When 150 g of water changes from liquid to vapor phase, 339 k. J of energy is absorbed. Determine the experimental heat of vaporization of water, given that the molar mass, M, of water is 18. 02 g/mol • Answer:

Practice • When 8. 70 k. J of thermal energy is added to 2. 50 mol of liquid methanol, all the methanol enters the vapor phase. Determine the experimental heat of vaporization of methanol.

Phase Changes and Global Energy Transfer • Phase changes in the hydrologic cycle play a role in global transfer of thermal energy • The transfer of energy warms the air, which rises • This can cause thunderstorms or hurricanes