Elements Atoms and Ions The Language of Chemistry




- Slides: 19

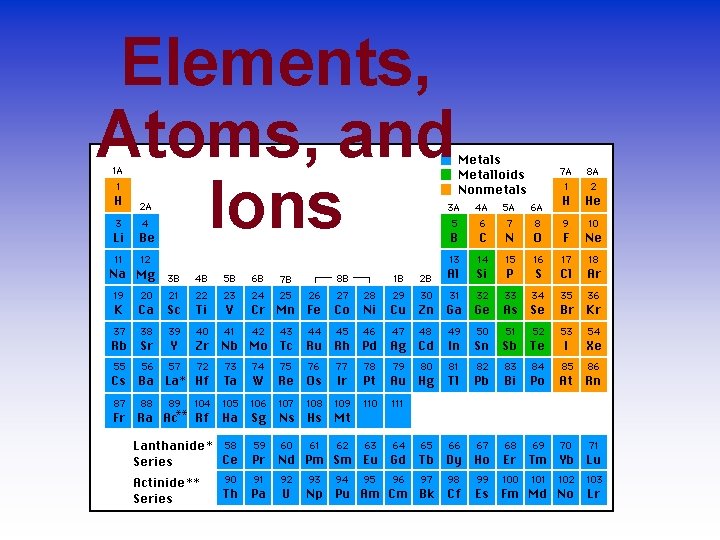
Elements, Atoms, and Ions

The Language of Chemistry • The elements, their names, and symbols are given on the PERIODIC TABLE • How many elements are there?

The Periodic Table Hi, my name’s Dimitri. I like long walks on the beach, meteorology, studying fossil fuels and hot air balloons. Dmitri Mendeleev (1834 - 1907)

Glenn Seaborg (1912 -1999 ) • Discovered 8 new elements. • Only living person for whom an element was named.

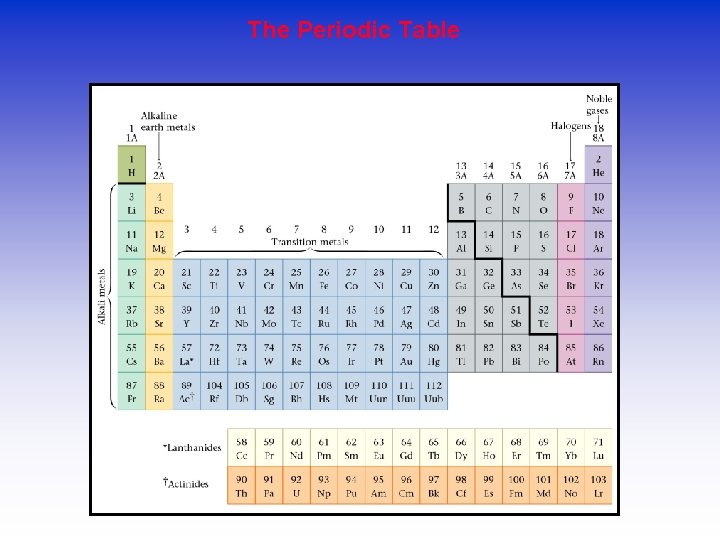
The Periodic Table

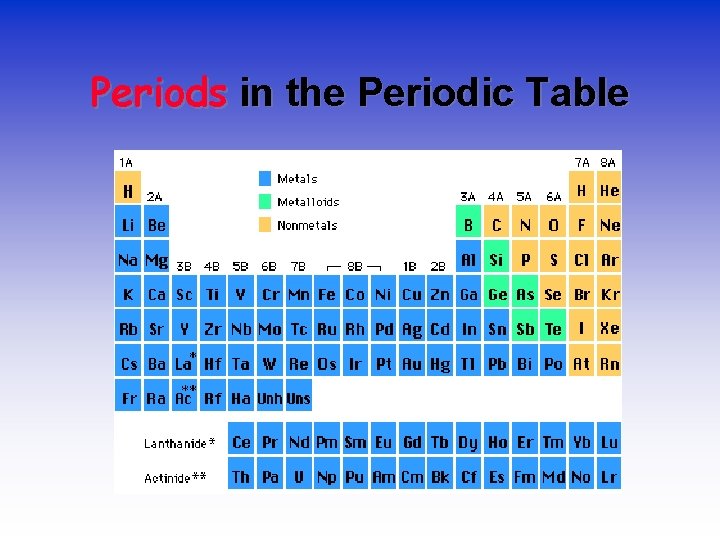
Periods in the Periodic Table

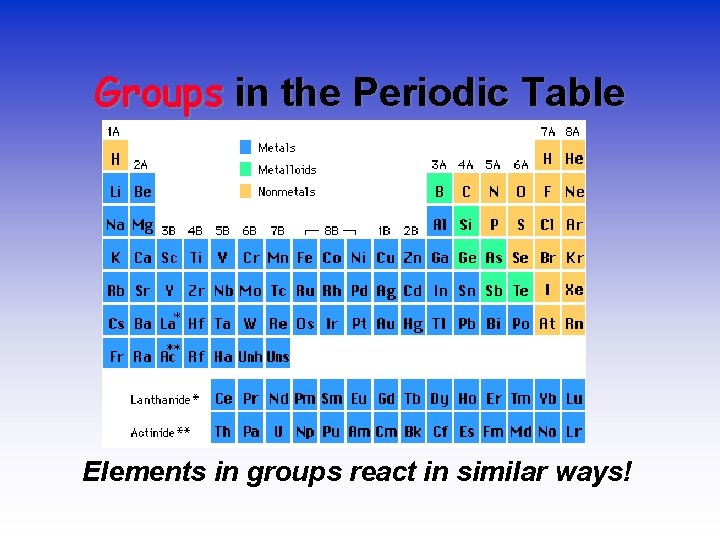
Groups in the Periodic Table Elements in groups react in similar ways!

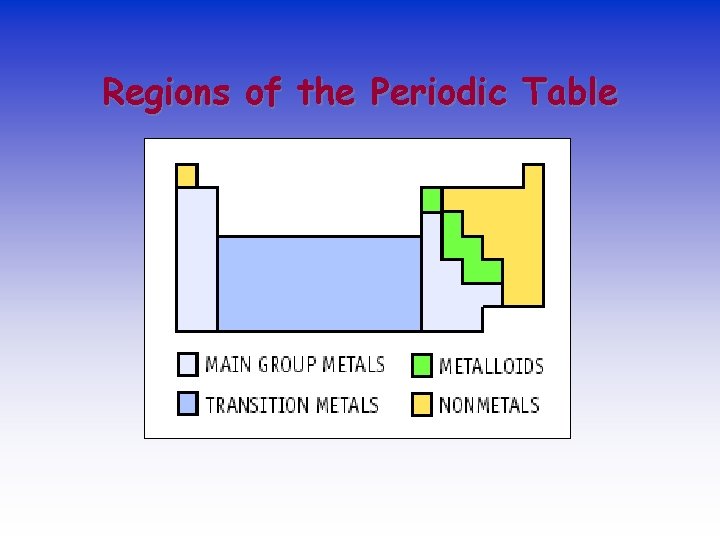
Regions of the Periodic Table

Group 1 A: Alkali Metals Reaction of potassium + H 2 O Cutting sodium metal

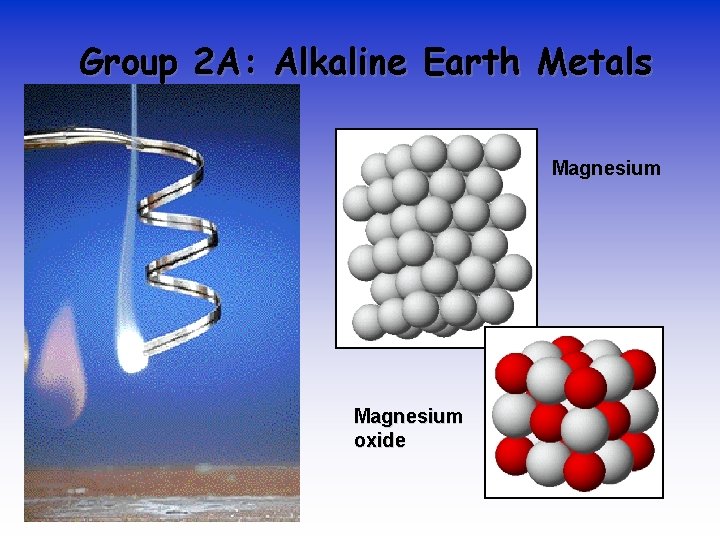
Group 2 A: Alkaline Earth Metals Magnesium oxide

Group 7 A: The Halogens (salt makers) F, Cl, Br, I, At

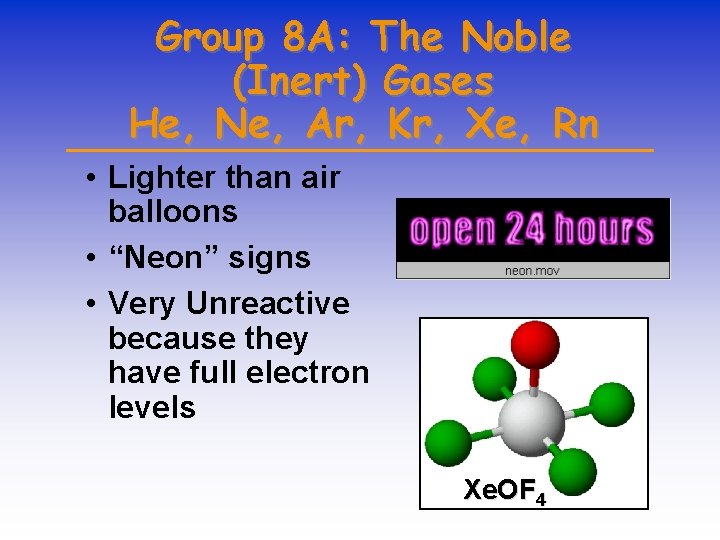
Group 8 A: The Noble (Inert) Gases He, Ne, Ar, Kr, Xe, Rn • Lighter than air balloons • “Neon” signs • Very Unreactive because they have full electron levels Xe. OF 4

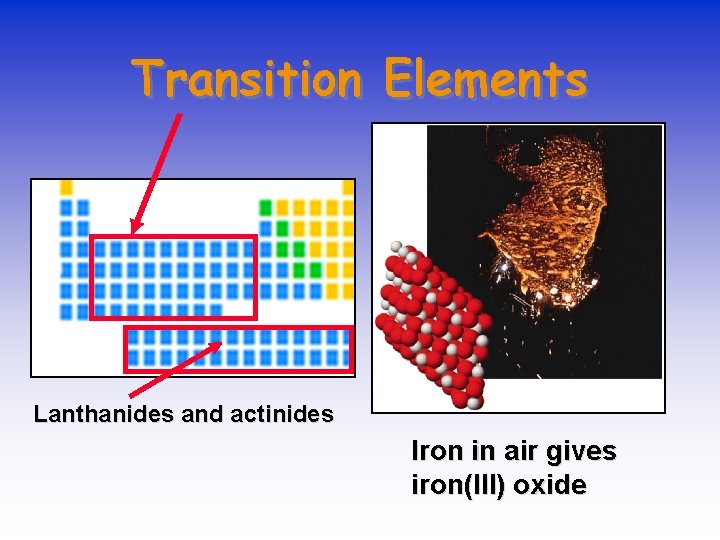
Transition Elements Lanthanides and actinides Iron in air gives iron(III) oxide

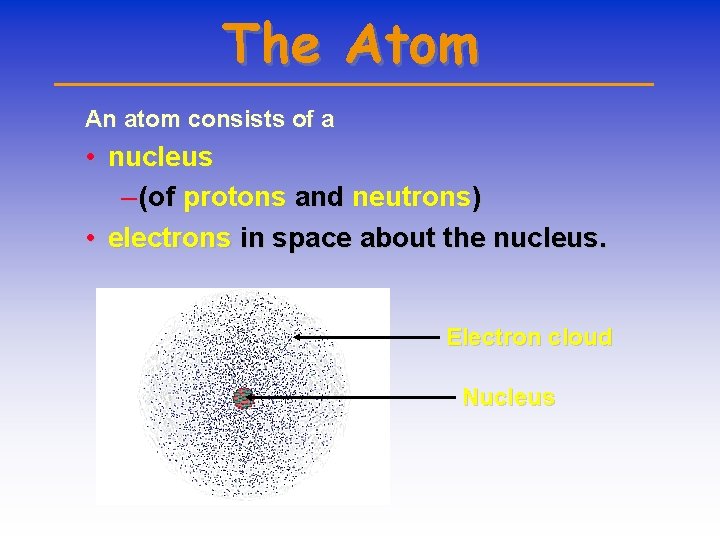
The Atom An atom consists of a • nucleus – (of protons and neutrons) • electrons in space about the nucleus. Electron cloud Nucleus

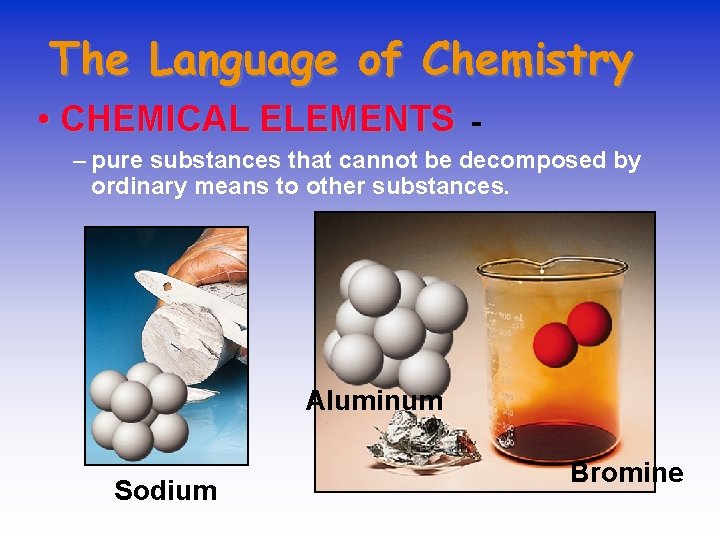
The Language of Chemistry • CHEMICAL ELEMENTS - – pure substances that cannot be decomposed by ordinary means to other substances. Aluminum Sodium Bromine

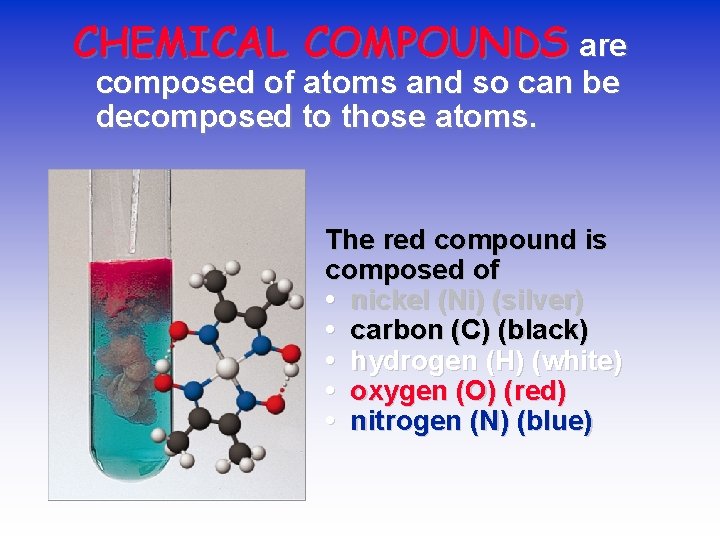
CHEMICAL COMPOUNDS are composed of atoms and so can be decomposed to those atoms. The red compound is composed of • nickel (Ni) (silver) • carbon (C) (black) • hydrogen (H) (white) • oxygen (O) (red) • nitrogen (N) (blue)

Compounds – composed of 2 or more elements in a fixed ratio – properties differ from those of individual elements – EX: table salt (Na. Cl)

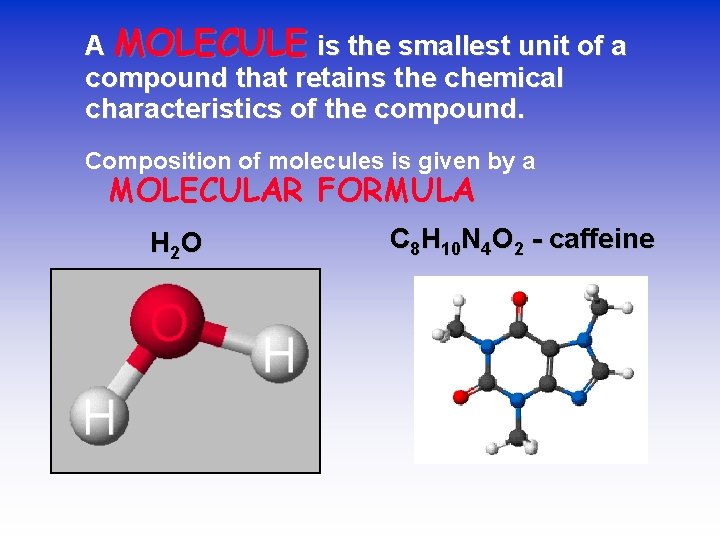
A MOLECULE is the smallest unit of a compound that retains the chemical characteristics of the compound. Composition of molecules is given by a MOLECULAR FORMULA H 2 O C 8 H 10 N 4 O 2 - caffeine

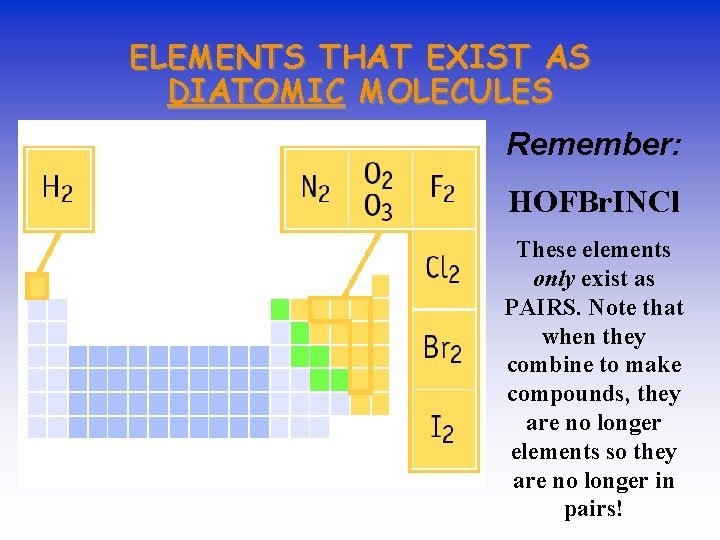
ELEMENTS THAT EXIST AS DIATOMIC MOLECULES Remember: HOFBr. INCl These elements only exist as PAIRS. Note that when they combine to make compounds, they are no longer elements so they are no longer in pairs!
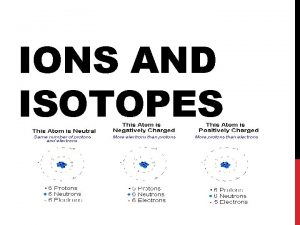
 Positive ions and negative ions table
Positive ions and negative ions table Ion chapter 11
Ion chapter 11 Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms ions and molecules
Atoms ions and molecules Atoms ions and molecules
Atoms ions and molecules Collision theory states that
Collision theory states that Chapter 2 atoms molecules and ions
Chapter 2 atoms molecules and ions How does a positive ion form
How does a positive ion form Atoms or ions are considered isoelectronic if
Atoms or ions are considered isoelectronic if Regents periodic table
Regents periodic table Ions meaning chemistry
Ions meaning chemistry Spectator ions equation example
Spectator ions equation example Ap chemistry electronic structure of atoms
Ap chemistry electronic structure of atoms Substance
Substance Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Chapter 6 section 1 atoms elements and compounds
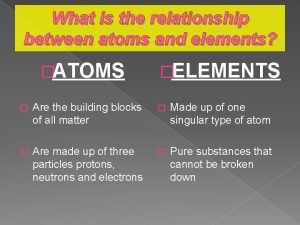
Chapter 6 section 1 atoms elements and compounds What is the relationship between atoms and elements
What is the relationship between atoms and elements