Dynamic arrest in colloidal systems from glasses to








- Slides: 66

Dynamic arrest in colloidal systems: from glasses to gels Francesco Sciortino Email: francesco. sciortino@phys. uniroma 1. it

Outline Routes to gelation in colloidal systems. Hard-Sphere Glasses § Attractive Glasses Phase-separation driven gels (D. Weitz) §Competing Interactions arrested states §Equilibrium Gels §

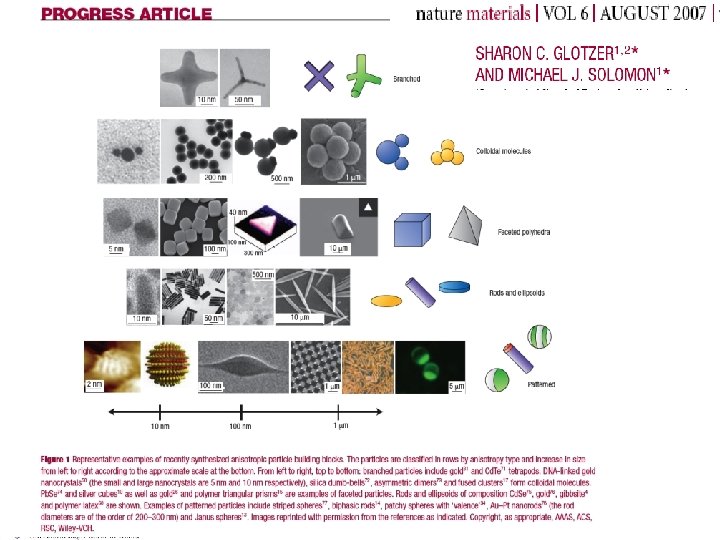
Colloids…. . Greek for Glue…. Nano and micromiter sized particles dispersed in a solvent (proteins…. . ) From a physicist point of view… • Effective interactions …. . • Super-atoms with designed interactions…. • Realization of theoretical models (hard-spheres). Test for integral equations approaches. • Size comparable to light wavelength… (confocal microscopy)

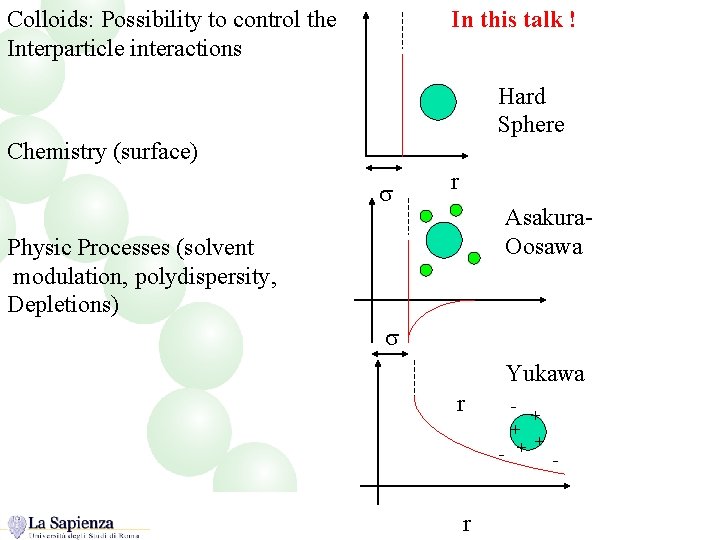
Colloids: Possibility to control the Interparticle interactions In this talk ! Hard Sphere Chemistry (surface) s r Asakura. Oosawa Physic Processes (solvent modulation, polydispersity, Depletions) s Yukawa r + + - + + r

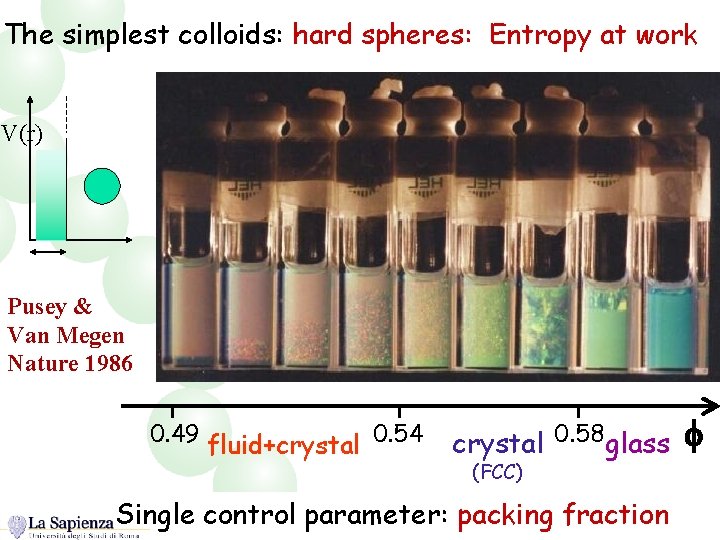
The simplest colloids: hard spheres: Entropy at work V(r) Pusey & Van Megen Nature 1986 0. 49 fluid+crystal 0. 54 crystal 0. 58 glass (FCC) Single control parameter: packing fraction

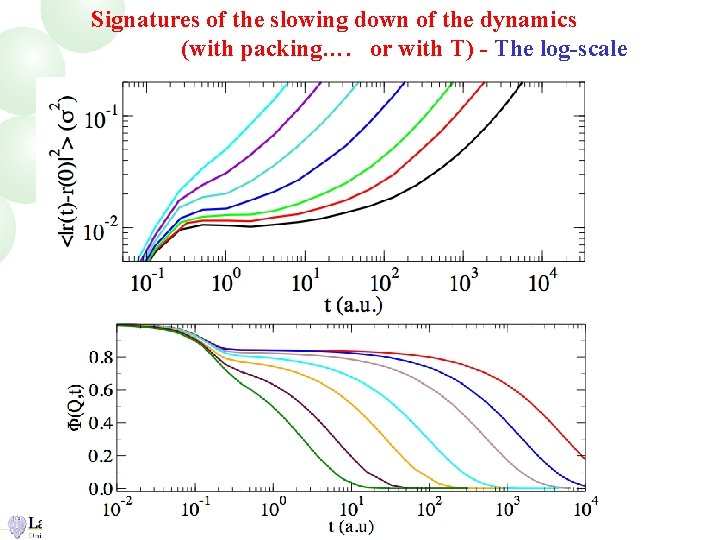
Signatures of the slowing down of the dynamics (with packing…. or with T) - The log-scale

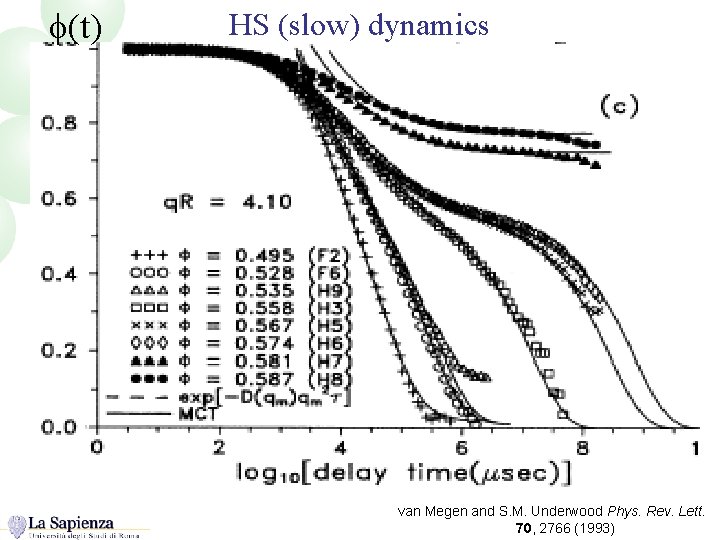
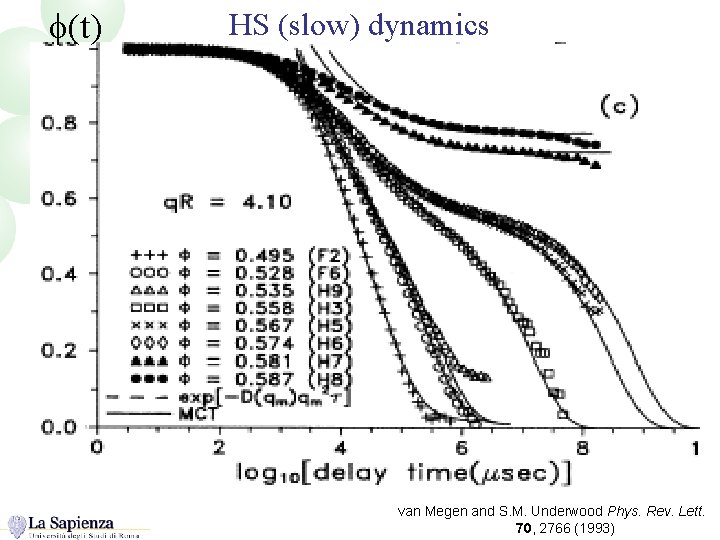
f(t) HS (slow) dynamics van Megen and S. M. Underwood Phys. Rev. Lett. 70, 2766 (1993)

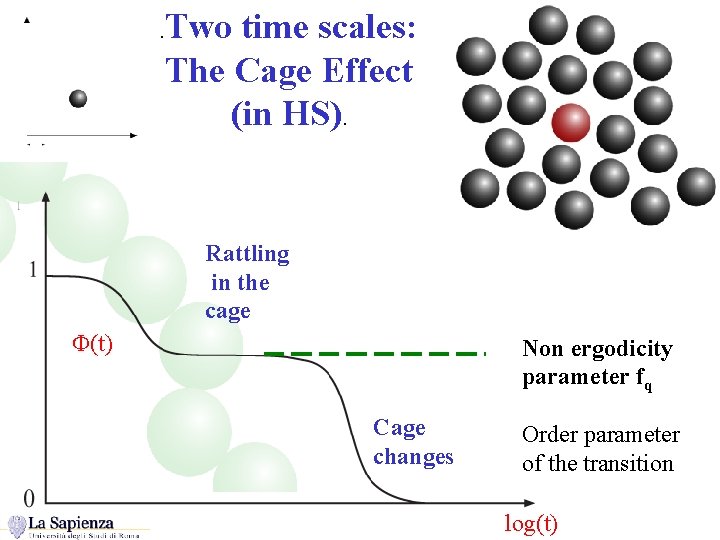
Two time scales: The Cage Effect (in HS). . Rattling in the cage F(t) Non ergodicity parameter fq Cage changes Order parameter of the transition log(t)

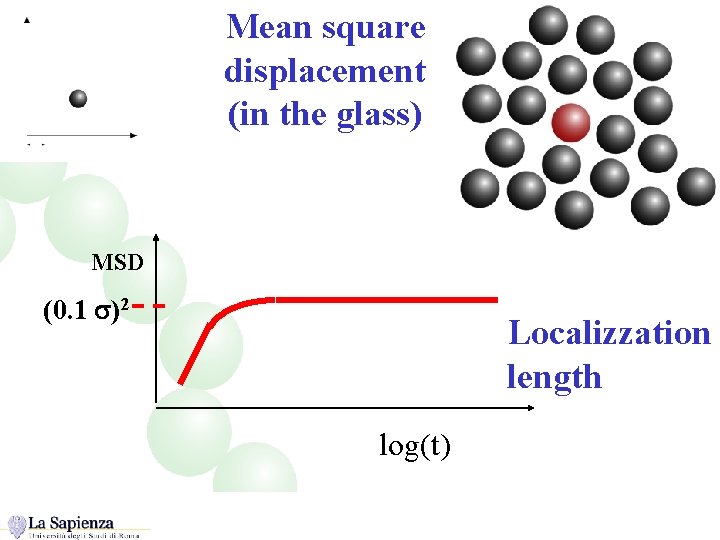
Mean square displacement (in the glass) MSD (0. 1 s)2 Localizzation length log(t)

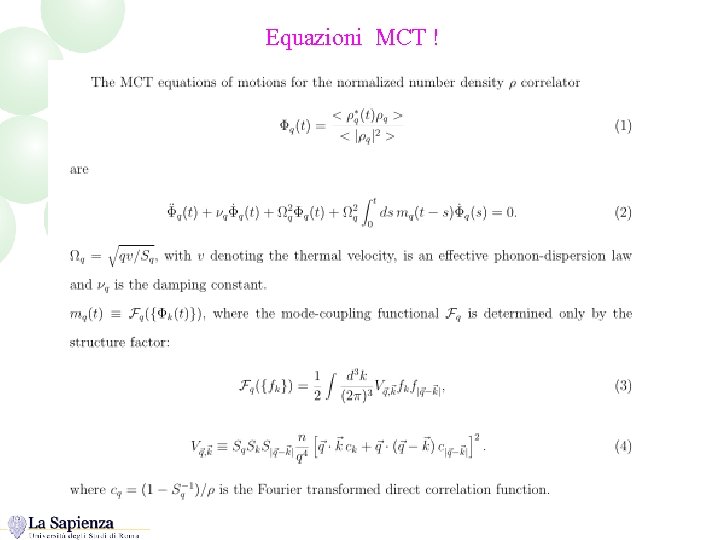
Equazioni MCT !

f(t) HS (slow) dynamics van Megen and S. M. Underwood Phys. Rev. Lett. 70, 2766 (1993)

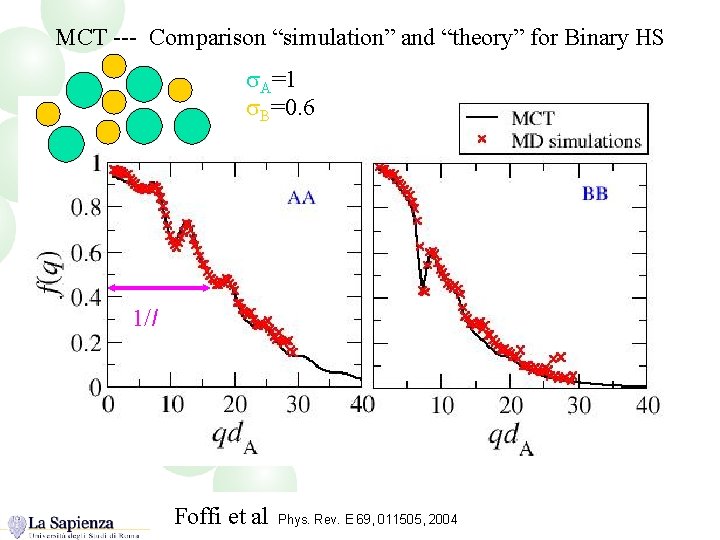
MCT --- Comparison “simulation” and “theory” for Binary HS s. A=1 s. B=0. 6 1/l Foffi et al Phys. Rev. E 69, 011505, 2004

The effect of short-range attraction on the Phase Diagram hard spheres large range short range Anderson and Lekkerkerker, Nature 2001

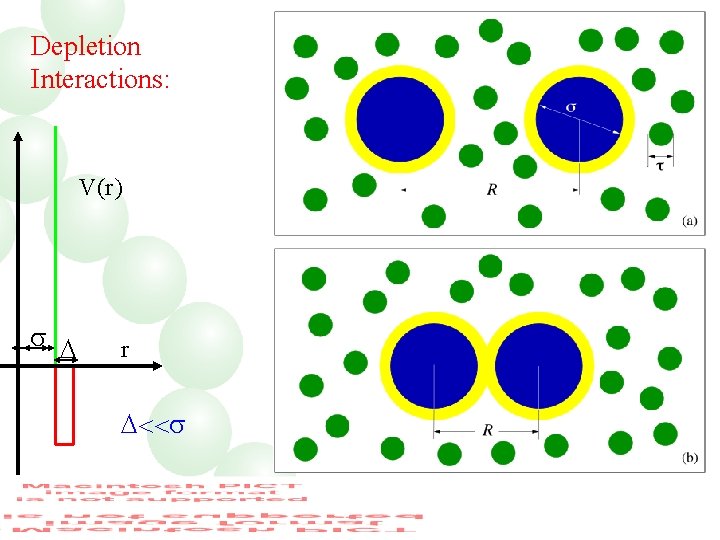
Depletion Interactions: V(r ) s D r D<<s

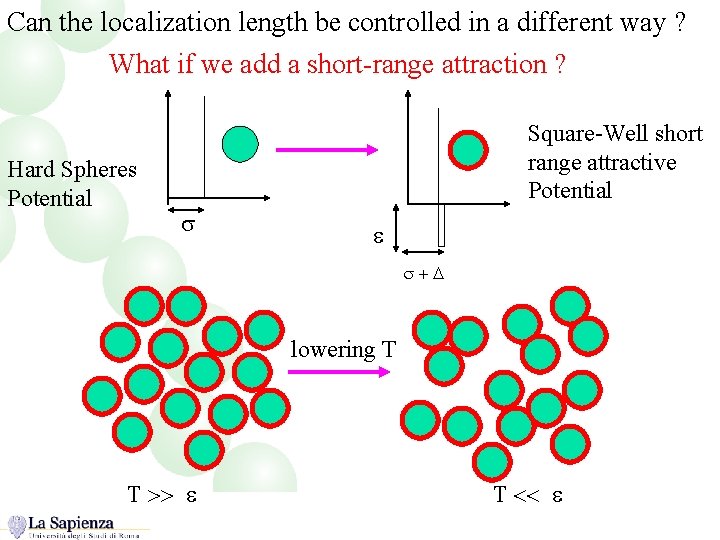
Can the localization length be controlled in a different way ? What if we add a short-range attraction ? Hard Spheres Potential Square-Well short range attractive Potential s e s+D lowering T T >> e T << e

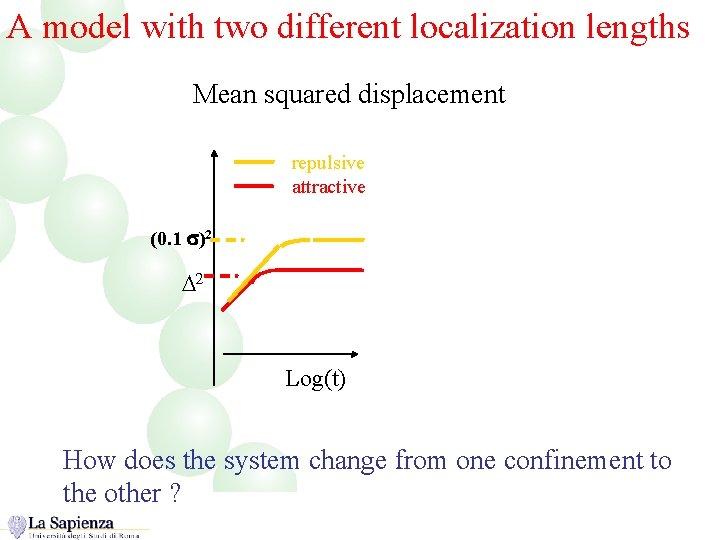
A model with two different localization lengths Mean squared displacement repulsive attractive (0. 1 s)2 D 2 Log(t) How does the system change from one confinement to the other ?

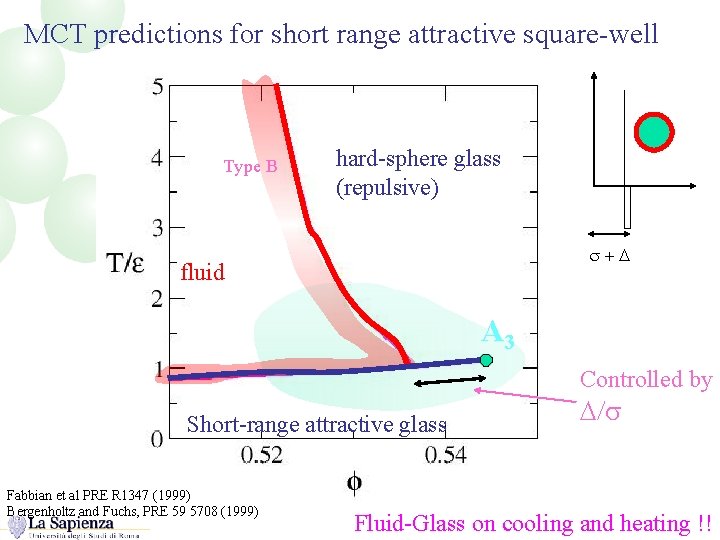
MCT predictions for short range attractive square-well Type B hard-sphere glass (repulsive) s+D fluid A 3 Controlled by Short-range attractive glass Fabbian et al PRE R 1347 (1999) Bergenholtz and Fuchs, PRE 59 5708 (1999) D/s Fluid-Glass on cooling and heating !!

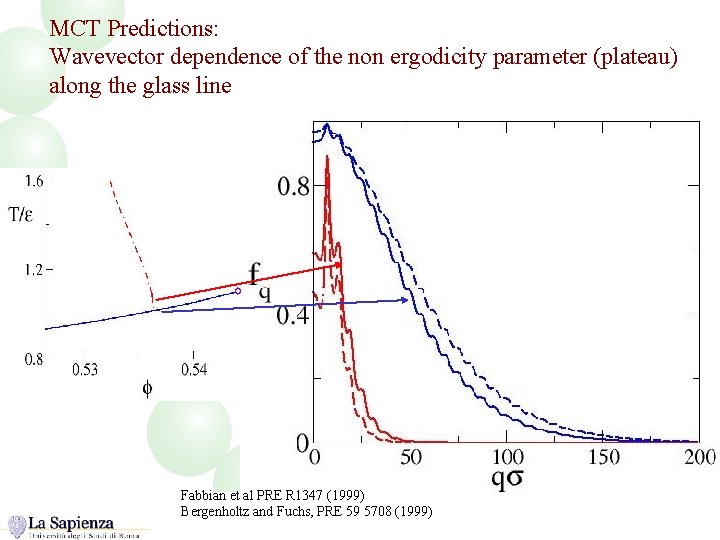
MCT Predictions: Wavevector dependence of the non ergodicity parameter (plateau) along the glass line Fabbian et al PRE R 1347 (1999) Bergenholtz and Fuchs, PRE 59 5708 (1999)

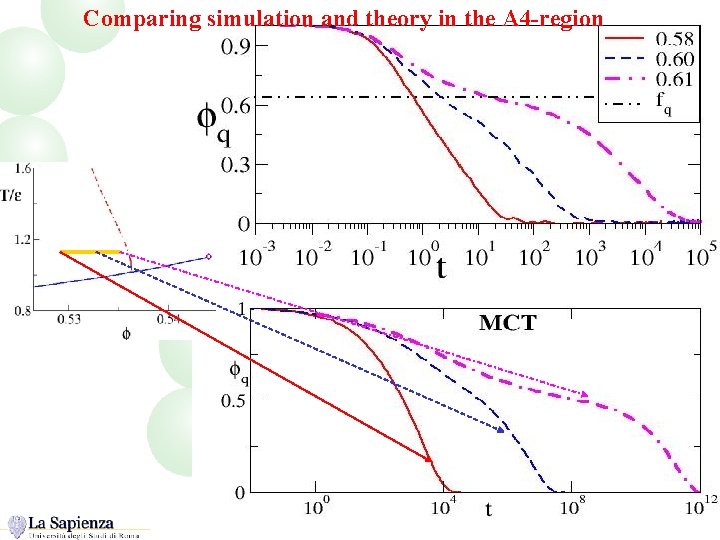
Comparing simulation and theory in the A 4 -region

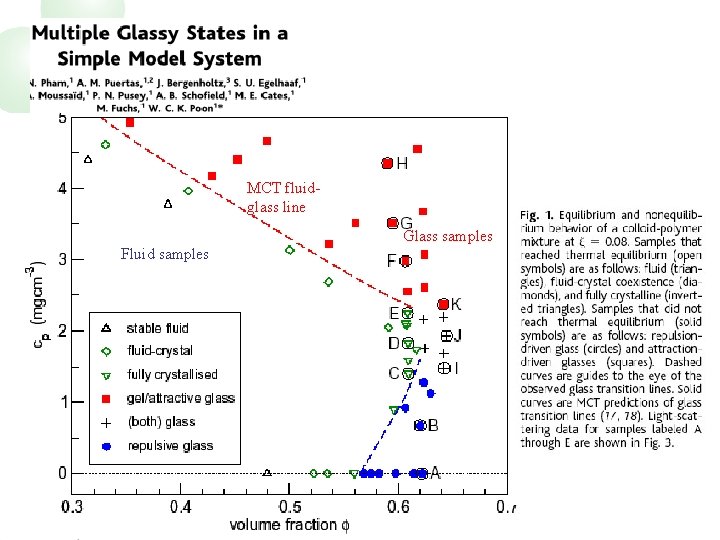
Temperature MCT fluidglass line Glass samples Fluid samples


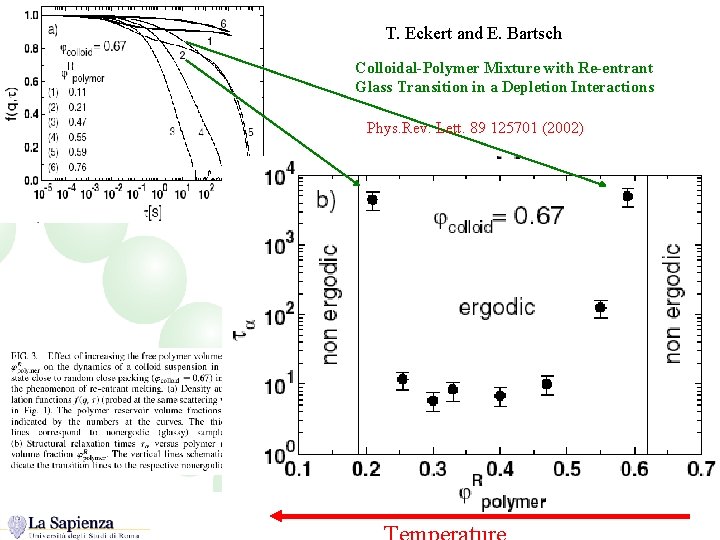
T. Eckert and E. Bartsch Colloidal-Polymer Mixture with Re-entrant Glass Transition in a Depletion Interactions Phys. Rev. Lett. 89 125701 (2002)

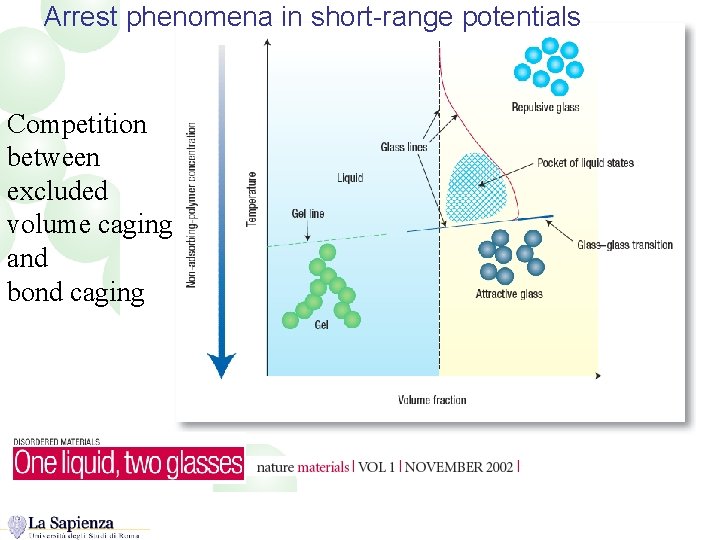
Arrest phenomena in short-range potentials Competition between excluded volume caging and bond caging

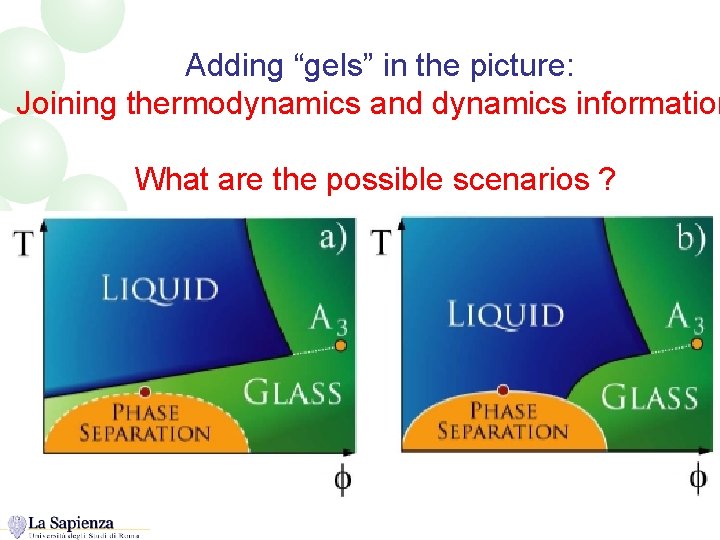
Adding “gels” in the picture: Joining thermodynamics and dynamics information What are the possible scenarios ?

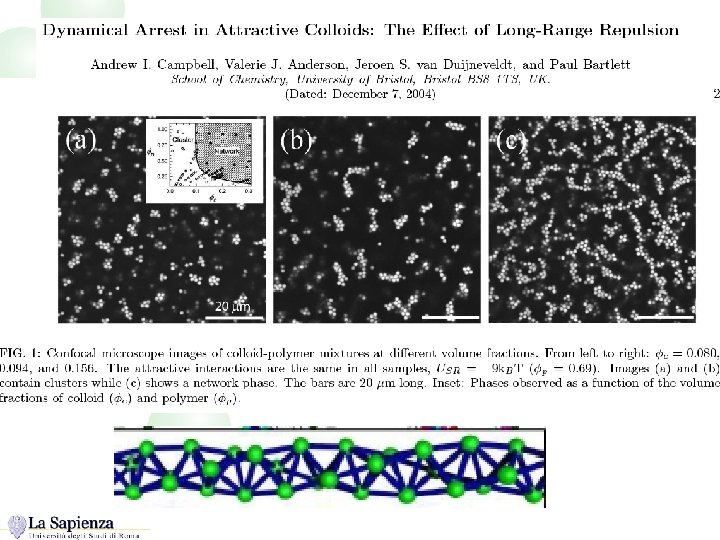
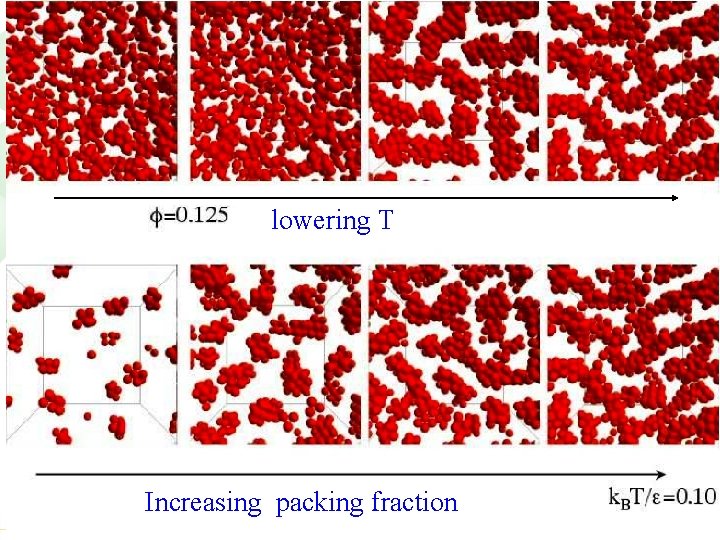
For HS+attraction, arrest at low (gelation) is the result of a phase separation process interrupted by the glass transition Nature, in press CONFOCAL IMAGES (THE REAL STUFF!)


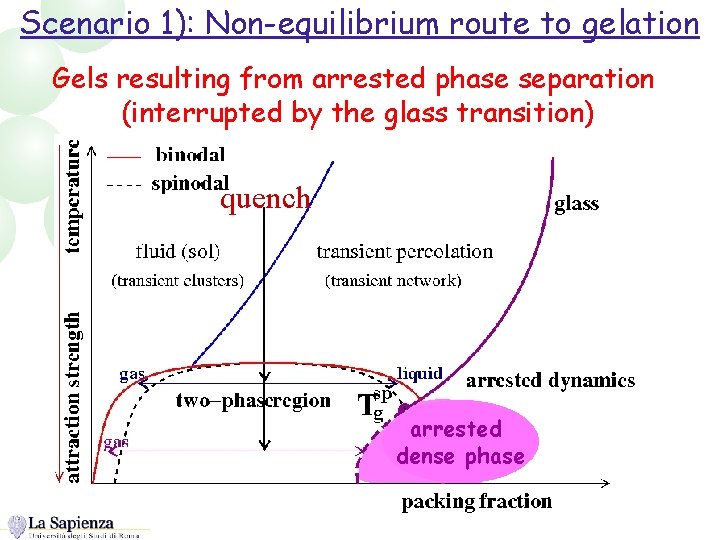
Scenario 1): Non-equilibrium route to gelation Gels resulting from arrested phase separation (interrupted by the glass transition) quench arrested dense phase

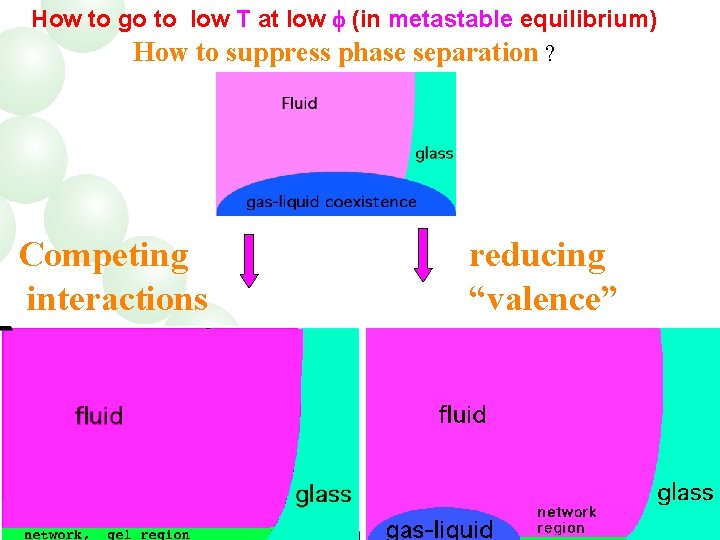
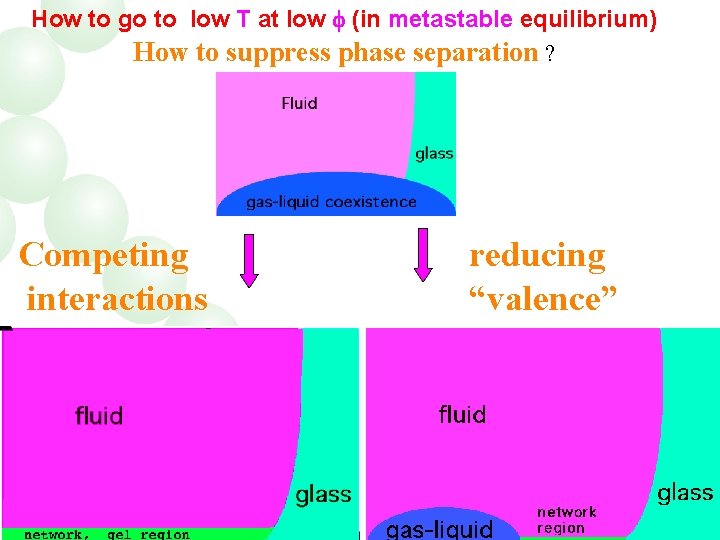
How to go to low T at low (in metastable equilibrium) How to suppress phase separation ? Competing interactions reducing “valence”

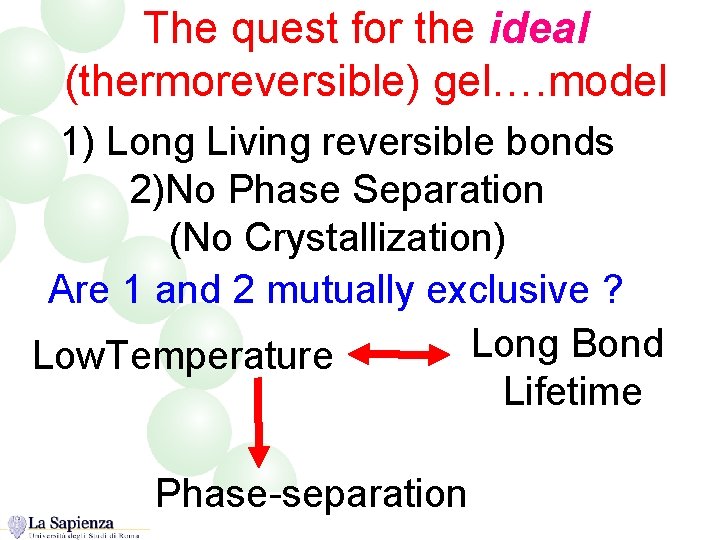
The quest for the ideal (thermoreversible) gel…. model 1) Long Living reversible bonds 2)No Phase Separation (No Crystallization) Are 1 and 2 mutually exclusive ? Long Bond Low. Temperature Lifetime Phase-separation

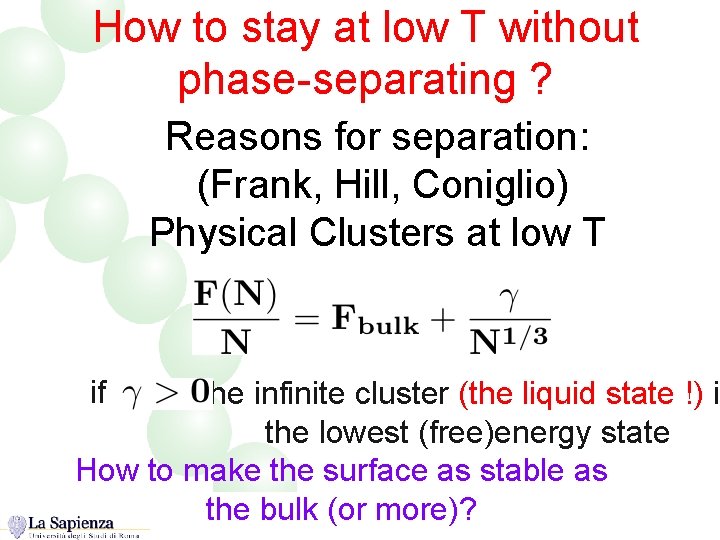
How to stay at low T without phase-separating ? Reasons for separation: (Frank, Hill, Coniglio) Physical Clusters at low T if the infinite cluster (the liquid state !) is the lowest (free)energy state How to make the surface as stable as the bulk (or more)?

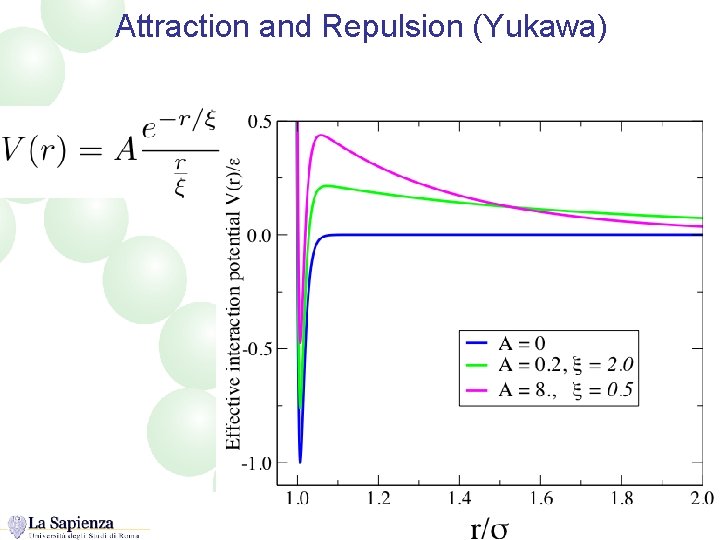
Attraction and Repulsion (Yukawa)

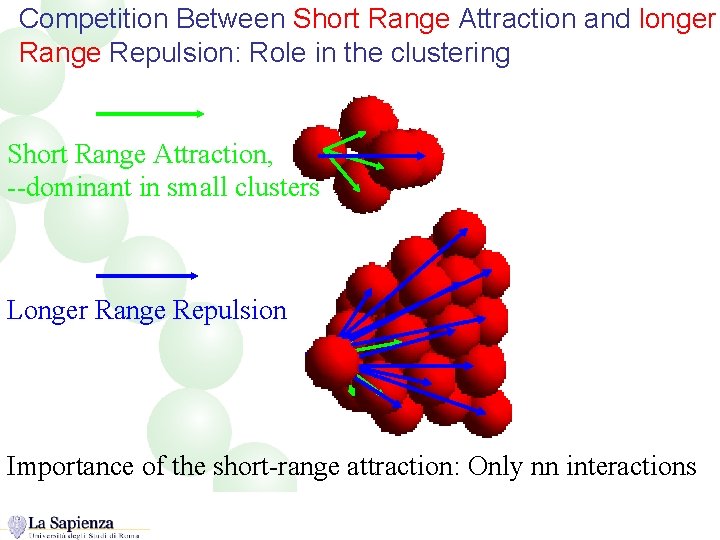
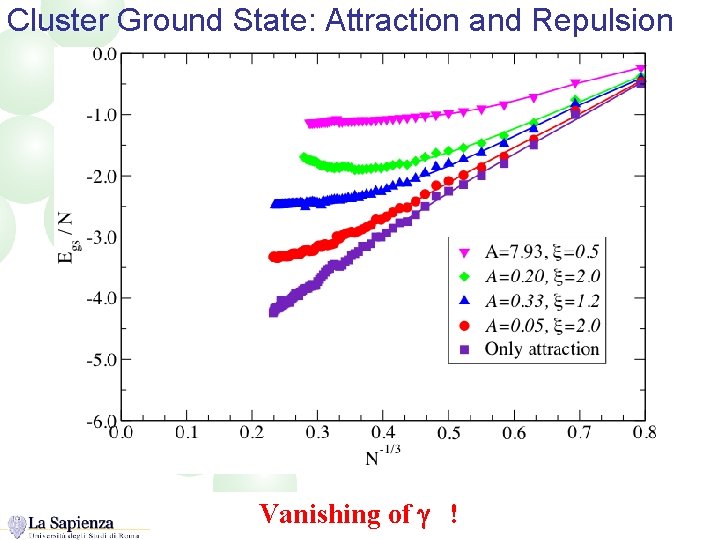
Competition Between Short Range Attraction and longer Range Repulsion: Role in the clustering Short Range Attraction, --dominant in small clusters Longer Range Repulsion Importance of the short-range attraction: Only nn interactions

Cluster Ground State: Attraction and Repulsion Vanishing of g !

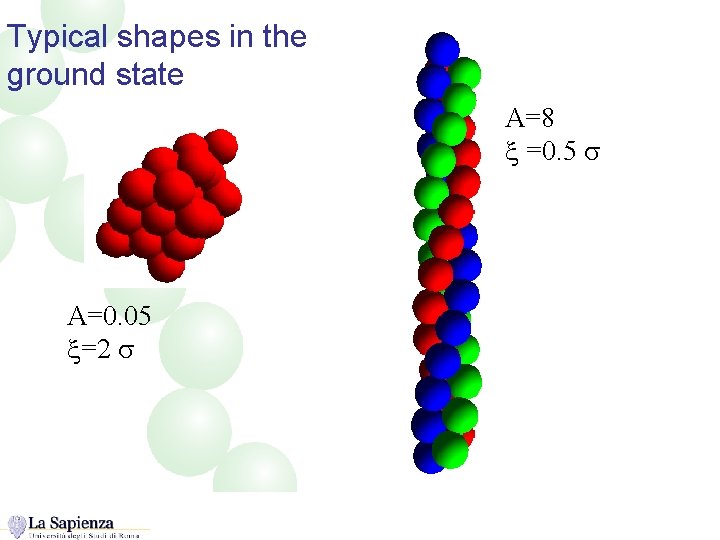
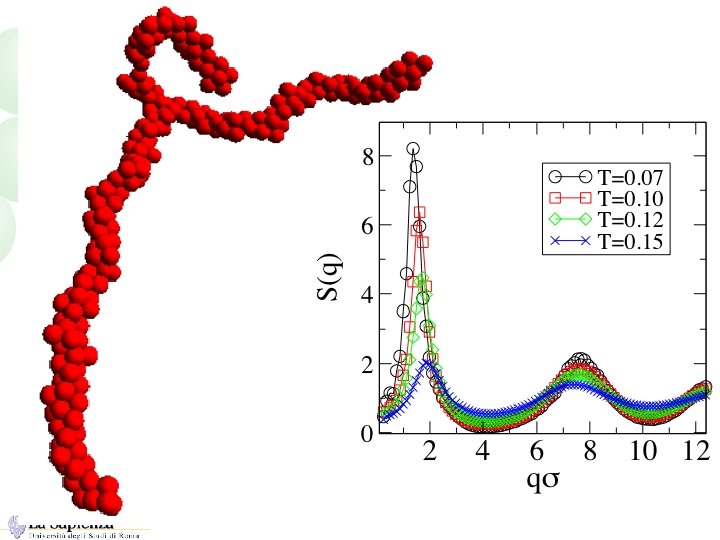
Typical shapes in the ground state A=8 x =0. 5 s A=0. 05 x=2 s

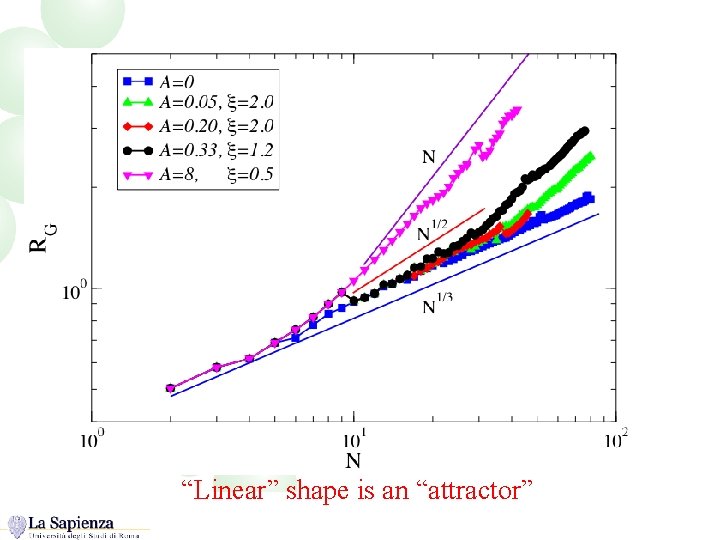
Size dependence of the cluster shape “Linear” shape is an “attractor”




T=0. 15 T=0. 10

Proteins as colloids…

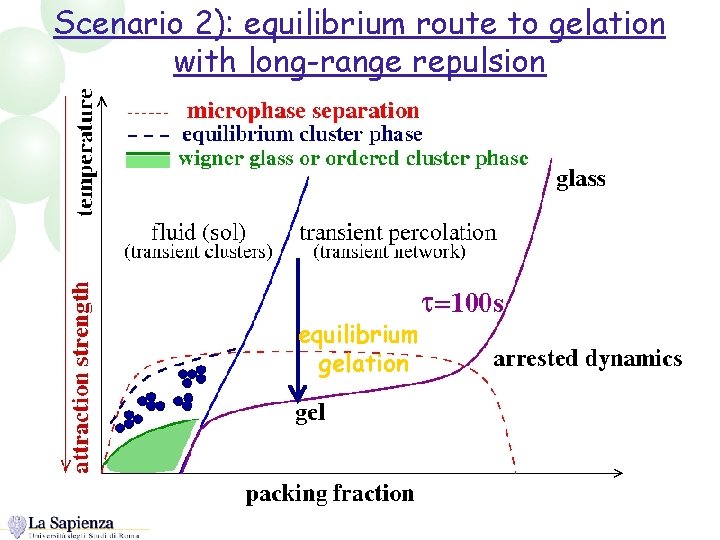
Scenario 2): equilibrium route to gelation with long-range repulsion equilibrium gelation

How to go to low T at low (in metastable equilibrium) How to suppress phase separation ? Competing interactions reducing “valence”


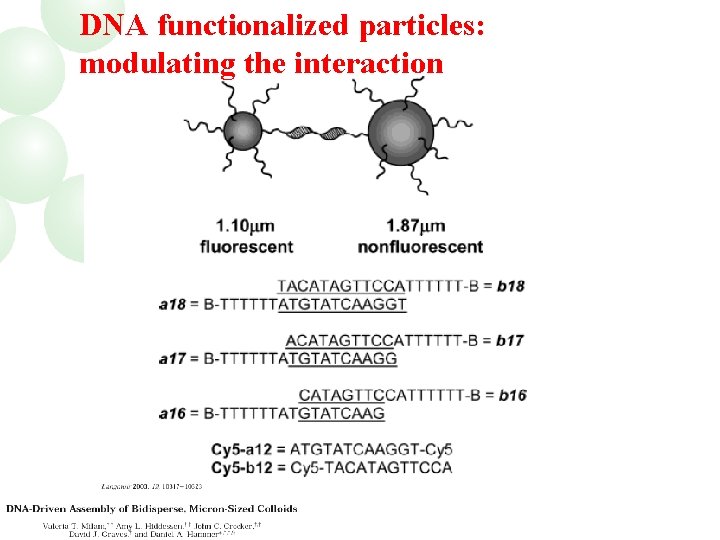
DNA functionalized particles: modulating the interaction

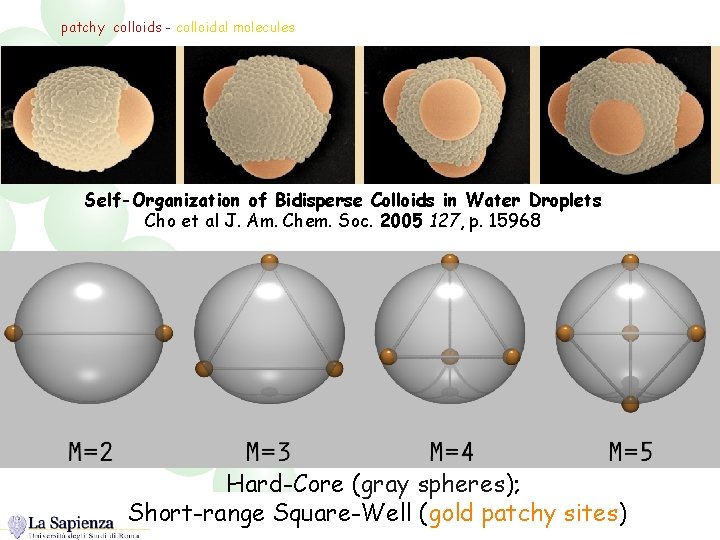
patchy colloids - colloidal molecules Self-Organization of Bidisperse Colloids in Water Droplets Cho et al J. Am. Chem. Soc. 2005 127, p. 15968 Hard-Core (gray spheres); Short-range Square-Well (gold patchy sites)

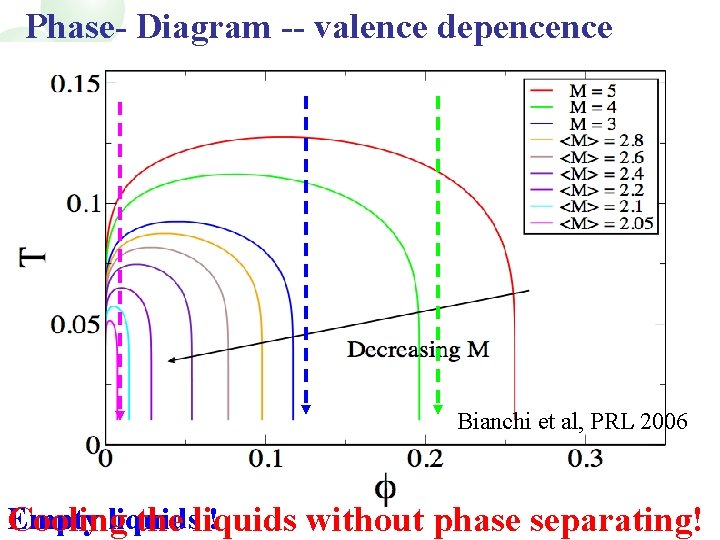
Phase- Diagram -- valence depencence Bianchi et al, PRL 2006 Empty liquids ! Cooling the liquids without phase separating!

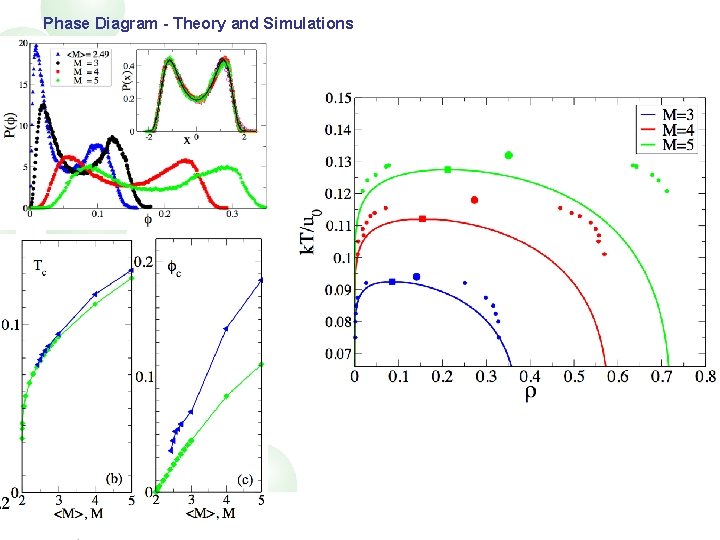
Phase Diagram - Theory and Simulations

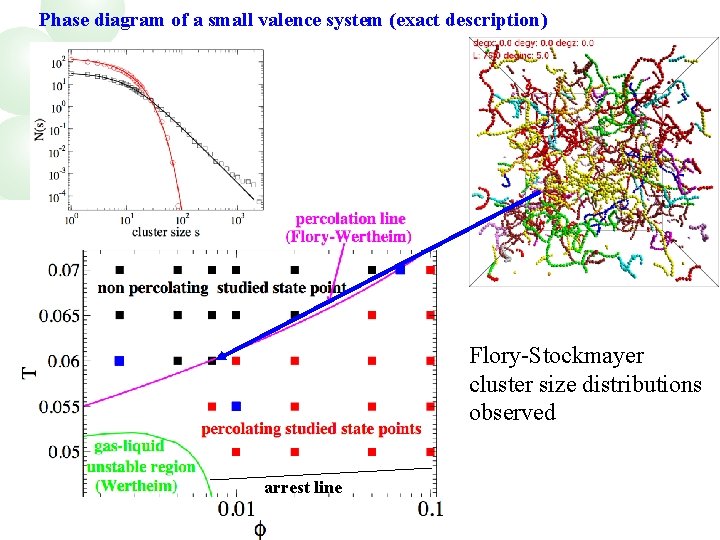
Phase diagram of a small valence system (exact description) Flory-Stockmayer cluster size distributions observed arrest line

A snapshot of <M>=2. 025 An “empty liquid” configuration N 2=5670 N 3=330 T=0. 05, =0. 01

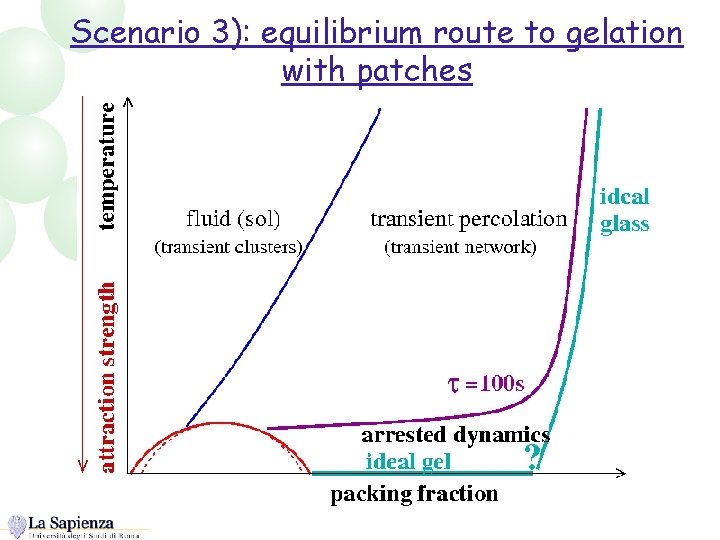
Scenario 3): equilibrium route to gelation with patches

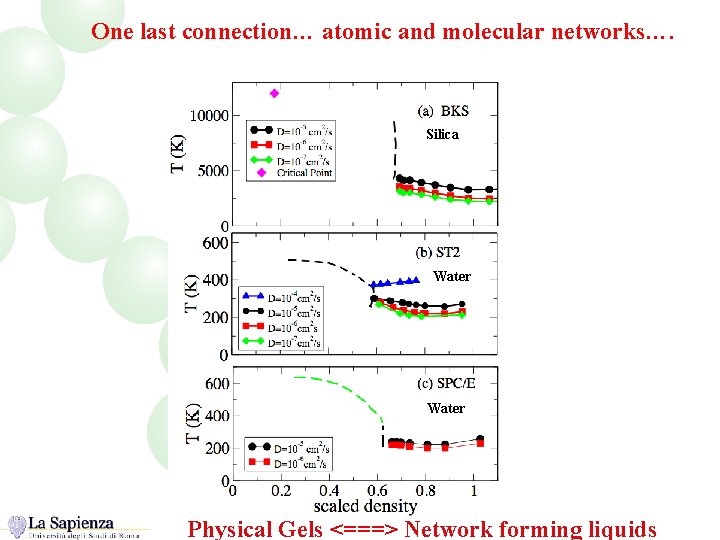
One last connection… atomic and molecular networks…. Silica Water Physical Gels <===> Network forming liquids

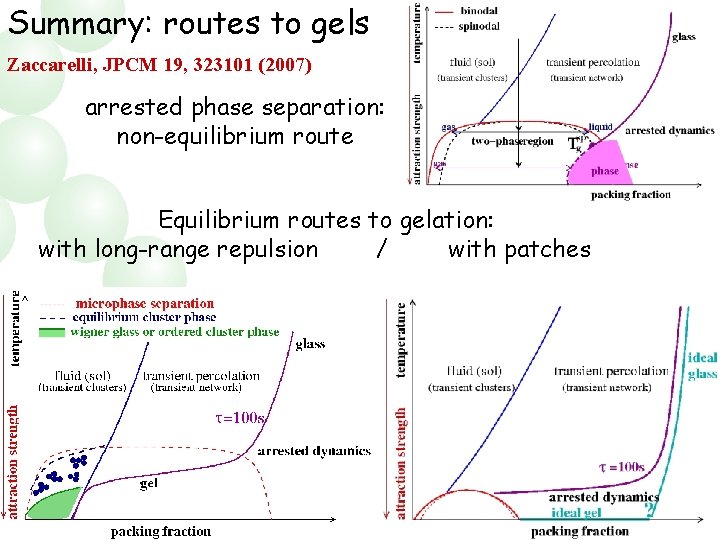
Summary: routes to gels Zaccarelli, JPCM 19, 323101 (2007) arrested phase separation: non-equilibrium route Equilibrium routes to gelation: with long-range repulsion / with patches

In collaboration with…… Piero Tartaglia Emanuela Zaccarelli Ivan Saika-Voivod (now Canada) Emanuela Bianchi Julio Largo (now Spain) Angel Moreno (now Spain) Stefano Mossa (now France ESRF) Sergey Buldyrev (New York)

Conclusions…. (open questions) Glass-glass transitions Empty liquids Competing interactions Network-forming liquids --- equilibrium gels (no Kauzmann) Self-assembly and network formation (loops) Surface geometry (Janus particles)

From isolated to interacting clusters Role of T and f: On cooling (or on increasing attraction), monomers tend to cluster…. In the region of the phase diagram where the attractive potential would generate a phase separation…. repulsion slows down (or stop) aggregation. The range of the attractive interactions plays a role. How do clusters interact ?

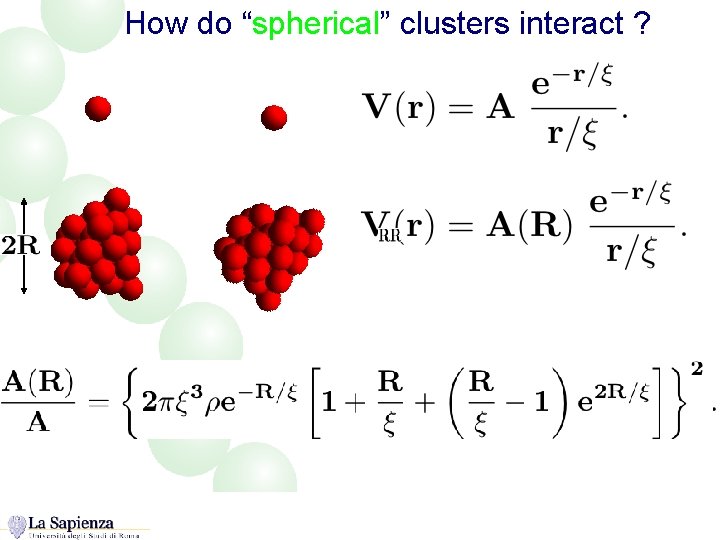
How do “spherical” clusters interact ?

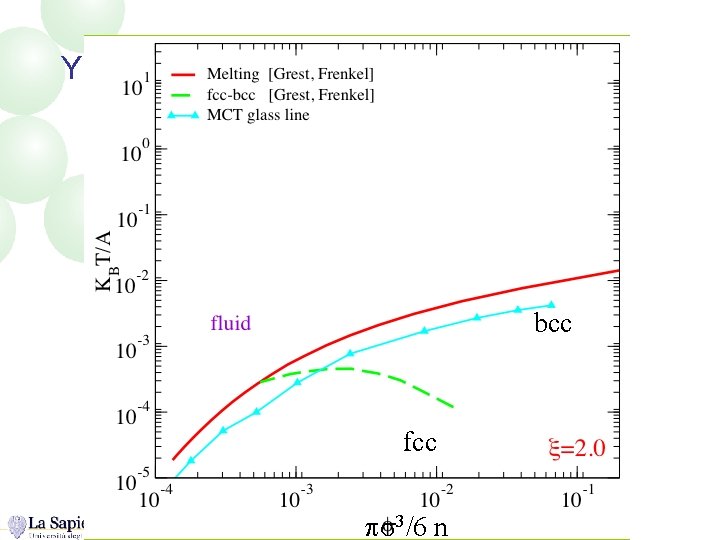
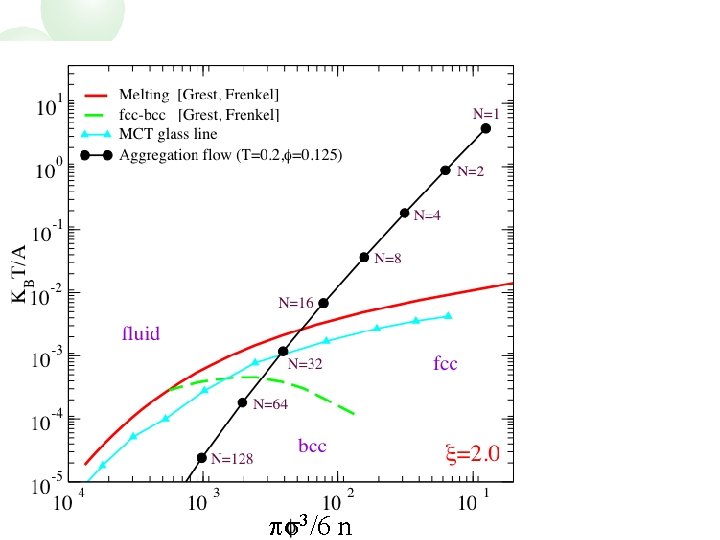
Yukawa Phase Diagram bcc fcc ps 3/6 n

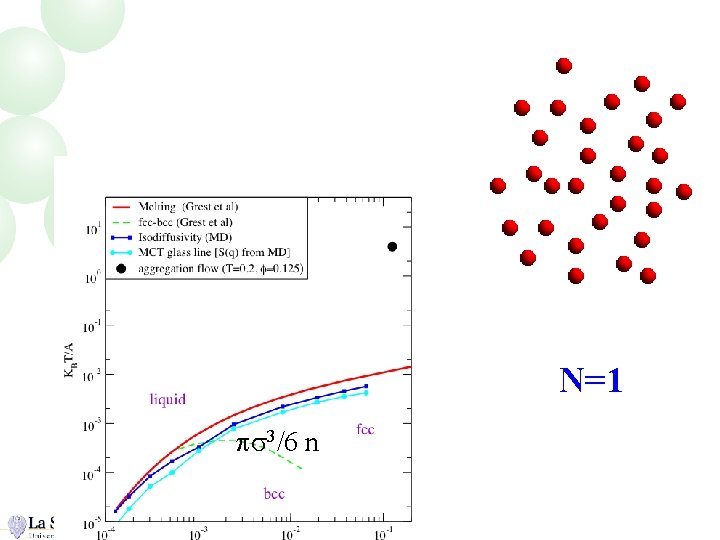
N=1 ps 3/6 n

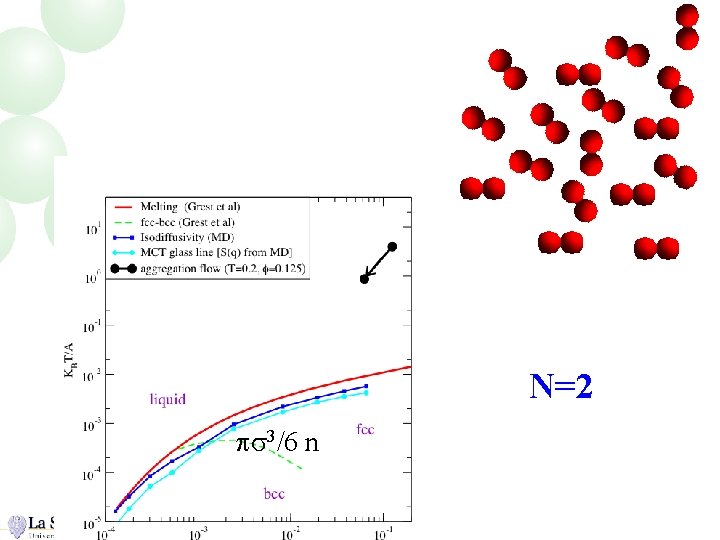
N=2 ps 3/6 n

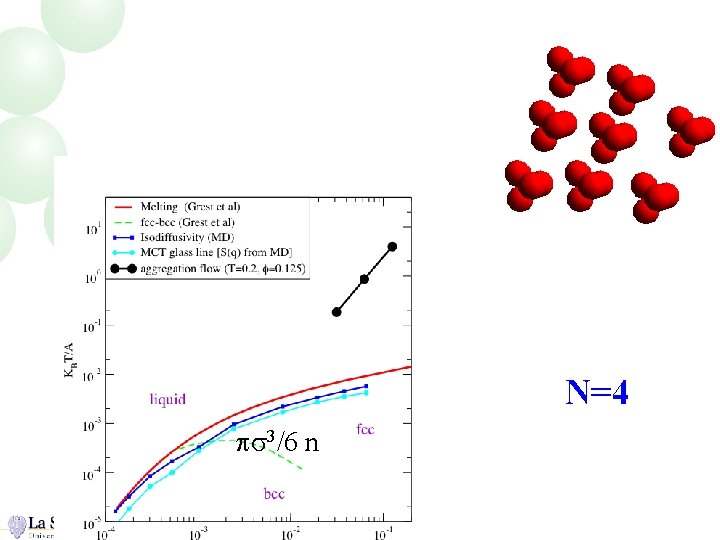
N=4 ps 3/6 n

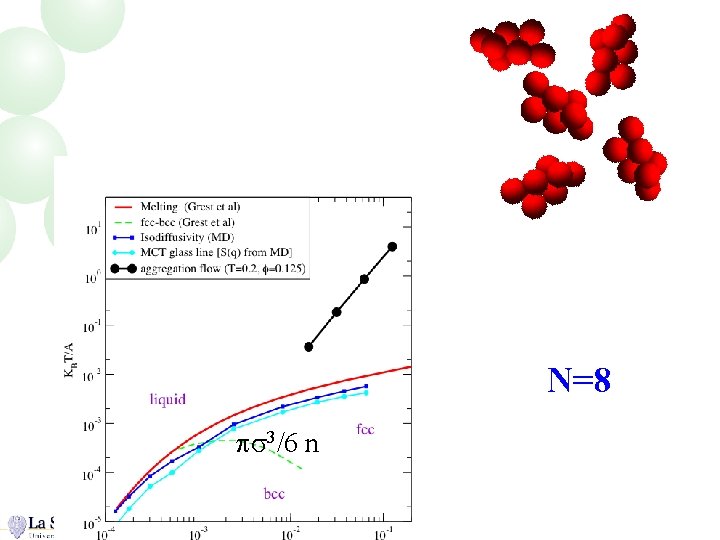
N=8 ps 3/6 n

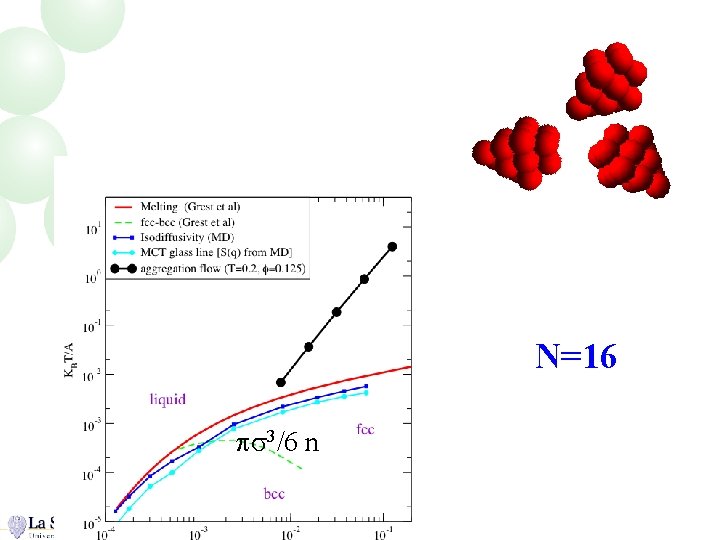
N=16 ps 3/6 n

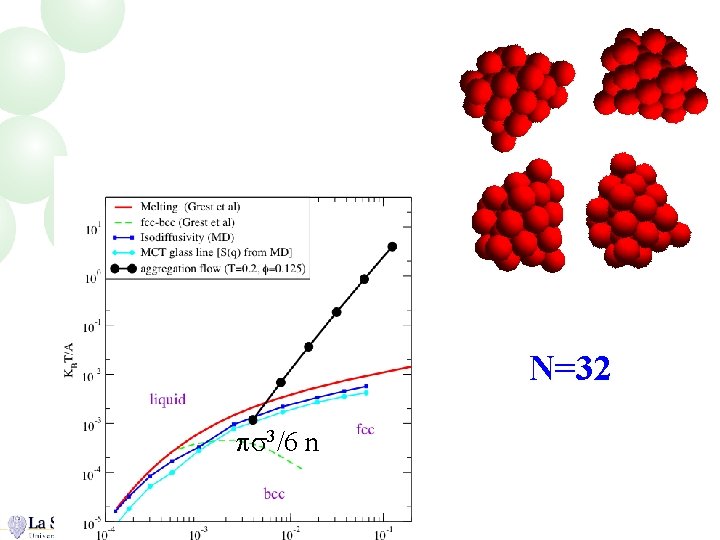
N=32 ps 3/6 n

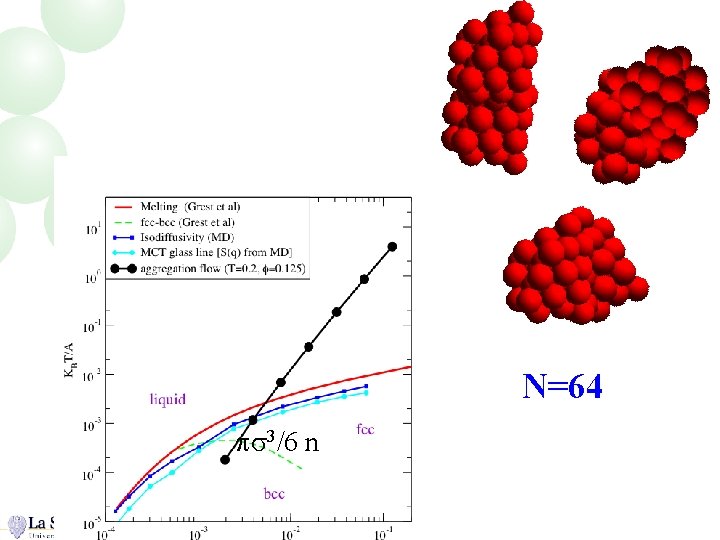
N=64 ps 3/6 n

Yukawa Phase Diagram ps 3/6 n

lowering T Increasing packing fraction