CTSN Trials of Mitral Valve Repair and Replacement









- Slides: 38

CTSN Trials of Mitral Valve Repair and Replacement Michael Mack, M. D. Baylor Scott & White Health Dallas, TX

Conflict of Interest Disclosure • Co- PI of COAPT Trial of Abbott Vascular • Co-PI of the PARTNER 3 Trial of Edwards Lifesciences • Executive Committee Intrepid Trial of Medtronic

What is CTSN? • Cardiothoracic Surgical Trials Network • Goal – Evaluate surgical approaches to cardiovascular disease • Support- $50 M (2013 -2018); $70 M (2019 -2025) – NIH • NHLBI • NINDS – CIHR- Canadian Institute of Health Research • PI’s – Richard Weisel-Toronto – Patrick O’Gara-Boston

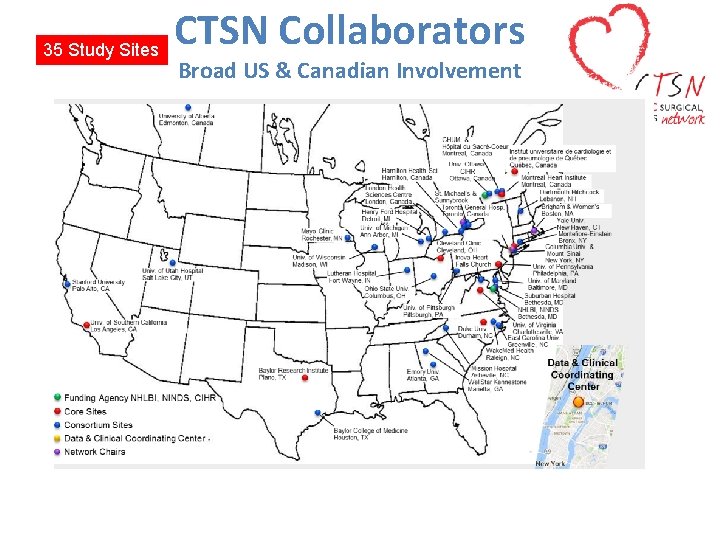
35 Study Sites CTSN Collaborators Broad US & Canadian Involvement

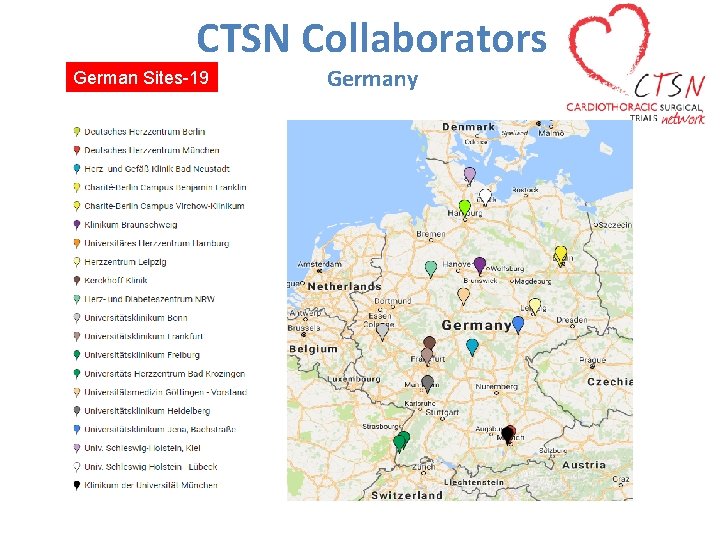
CTSN Collaborators German Sites-19 Germany

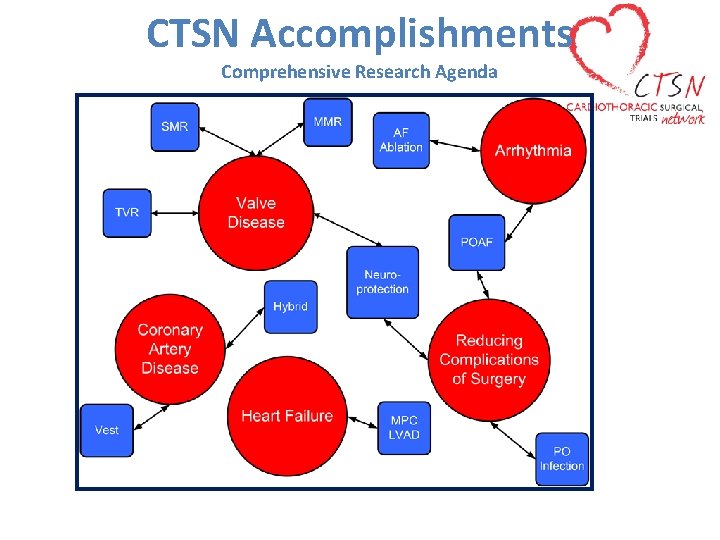
CTSN Accomplishments Comprehensive Research Agenda

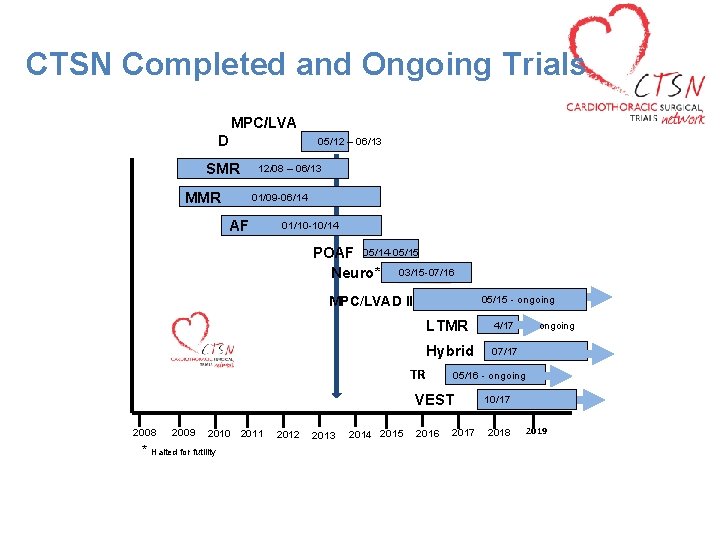
CTSN Completed and Ongoing Trials MPC/LVA D 05/12 – 06/13 SMR 12/08 – 06/13 MMR 01/09 -06/14 AF 01/10 -10/14 POAF 05/14 -05/155 Neuro* 03/15 -07/16 MPC/LVAD II 05/15 - ongoing LTMR 4/17 ongoing Hybrid 07/17 TR 05/16 - ongoing VEST 2010 * Halted for futility 2008 2009 2011 2012 2013 2014 2015 2016 2017 10/17 2018 2019

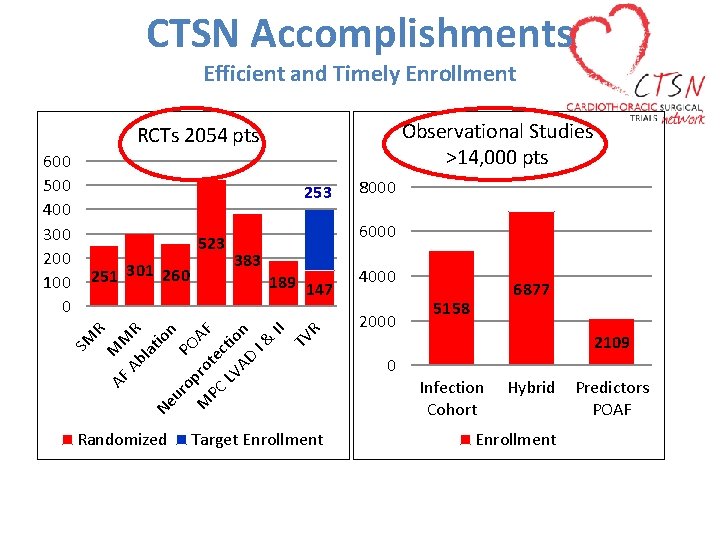
CTSN Accomplishments Efficient and Timely Enrollment Observational Studies >14, 000 pts RCTs 2054 pts 253 523 383 189 147 AF MM Ab R la tio Ne n ur op PO M ro AF PC te LV ctio AD n I& II TV R 251 301 260 SM Randomized 8000 6000 R 600 500 400 300 200 100 0 Target Enrollment 4000 2000 0 6877 5158 2109 Infection Cohort Hybrid Enrollment Predictors POAF

CTSN Impact Publications

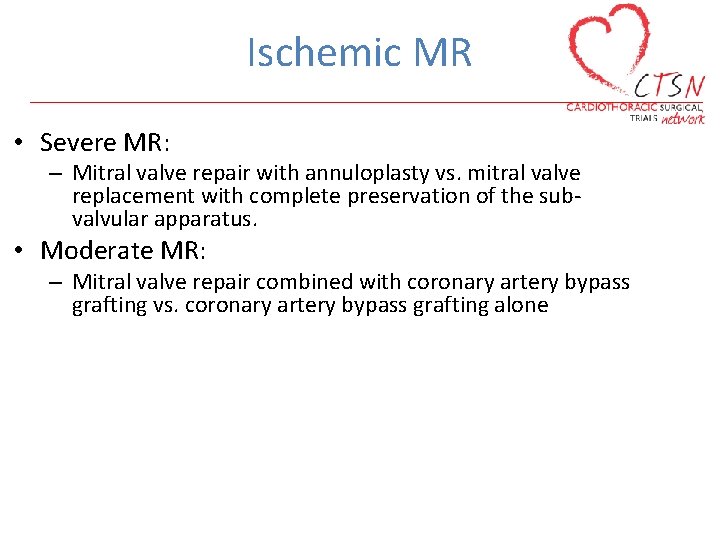
Ischemic MR • Severe MR: – Mitral valve repair with annuloplasty vs. mitral valve replacement with complete preservation of the subvalvular apparatus. • Moderate MR: – Mitral valve repair combined with coronary artery bypass grafting vs. coronary artery bypass grafting alone

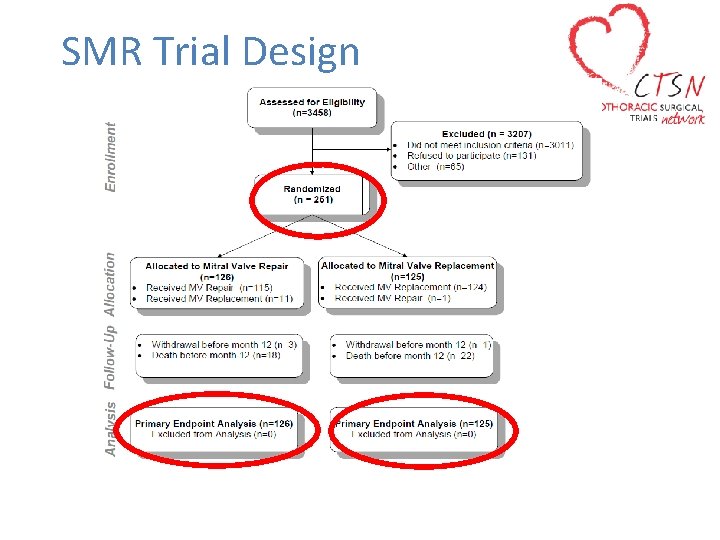
SMR Trial Design

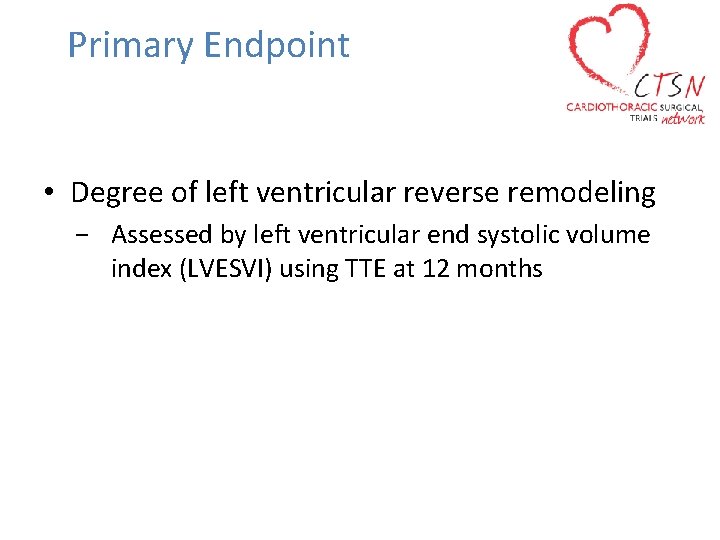
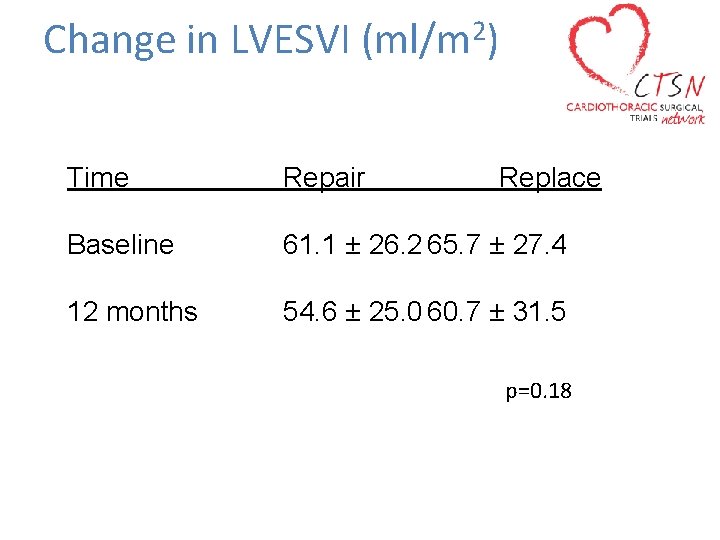
Primary Endpoint • Degree of left ventricular reverse remodeling − Assessed by left ventricular end systolic volume index (LVESVI) using TTE at 12 months


Change in LVESVI 2 (ml/m ) Time Repair Replace Baseline 61. 1 ± 26. 2 65. 7 ± 27. 4 12 months 54. 6 ± 25. 0 60. 7 ± 31. 5 p=0. 18

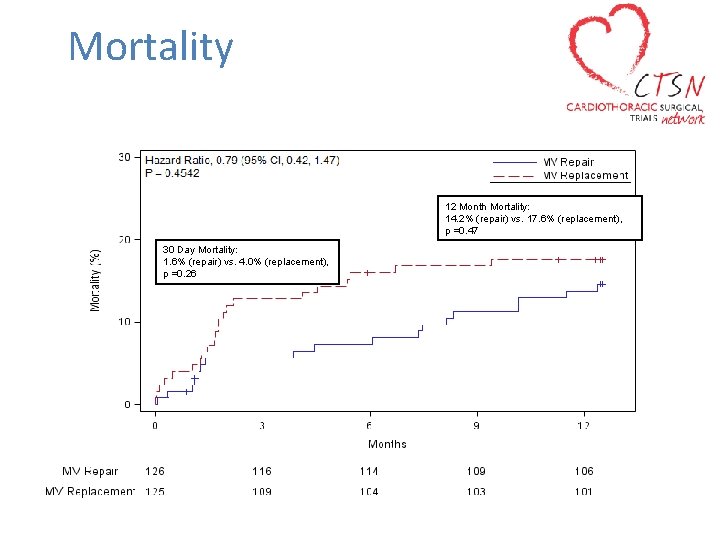
Mortality 12 Month Mortality: 14. 2% (repair) vs. 17. 6% (replacement), p =0. 47 30 Day Mortality: 1. 6% (repair) vs. 4. 0% (replacement), p =0. 26

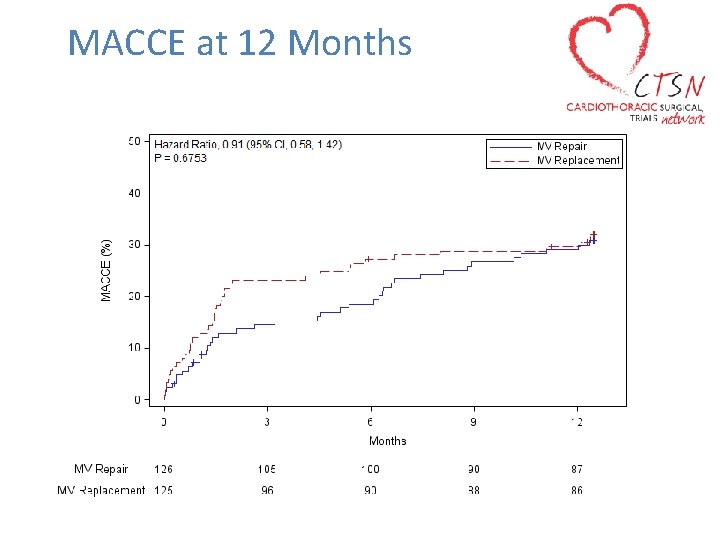
MACCE at 12 Months

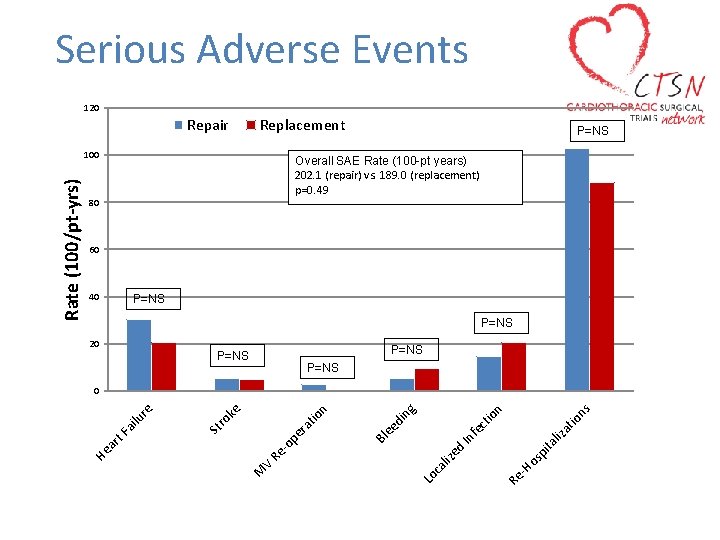
Serious Adverse Events 120 Repair Replacement Overall SAE Rate (100 -pt years) 202. 1 (repair) vs. 189. 0 (replacement) p=0. 49 80 60 40 P=NS 20 P=NS ns ita os p -H Re Lo ca liz ed In liz fe at io ct io n ng di ee pe r -o Re V M Bl at io n ro ke St ar t. F ai lu re 0 He Rate (100/pt-yrs) 100 P=NS

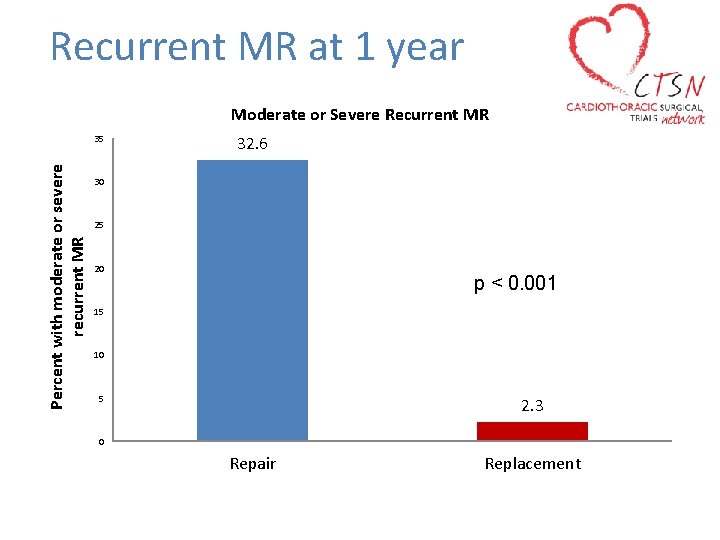
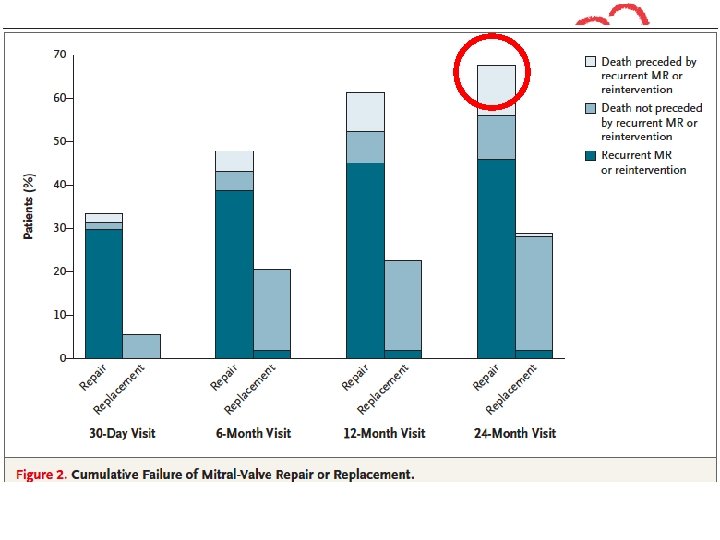
Recurrent MR at 1 year Moderate or Severe Recurrent MR Percent with moderate or severe recurrent MR 35 32. 6 30 25 20 p < 0. 001 15 10 5 2. 3 0 Repair Replacement

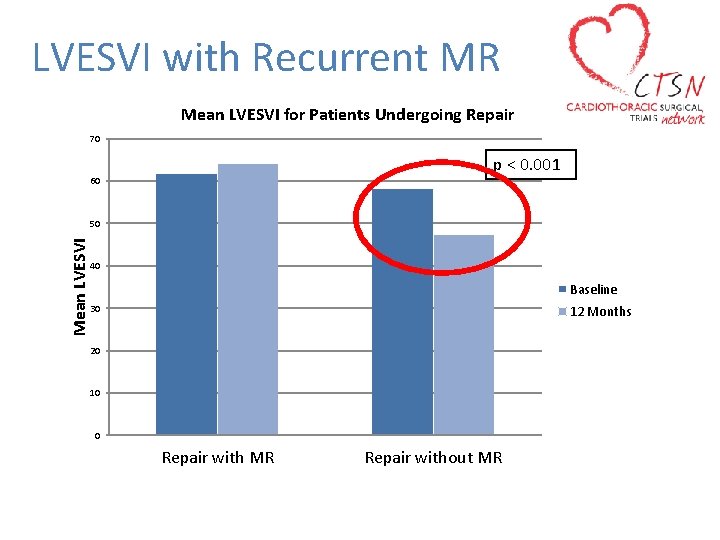
LVESVI with Recurrent MR Mean LVESVI for Patients Undergoing Repair 70 p < 0. 001 60 Mean LVESVI 50 40 Baseline 30 12 Months 20 10 0 Repair with MR Repair without MR

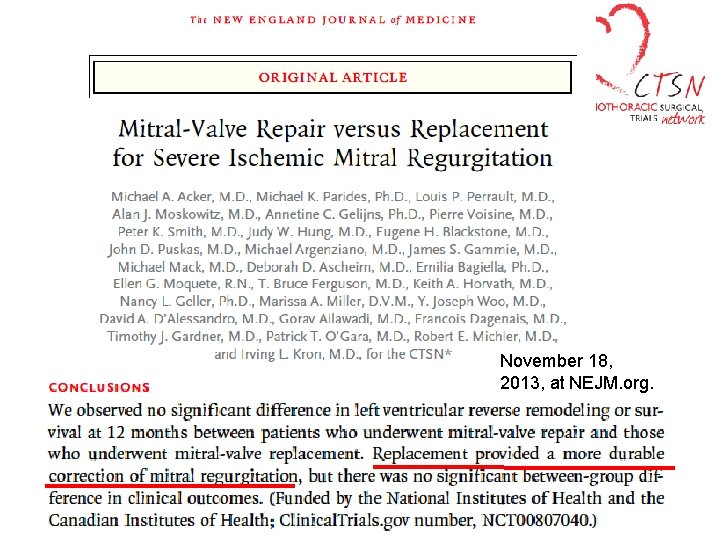
November 18, 2013, at NEJM. org.

• J Thorac Cardiovasc Surg 2015; 149: 752 -61)

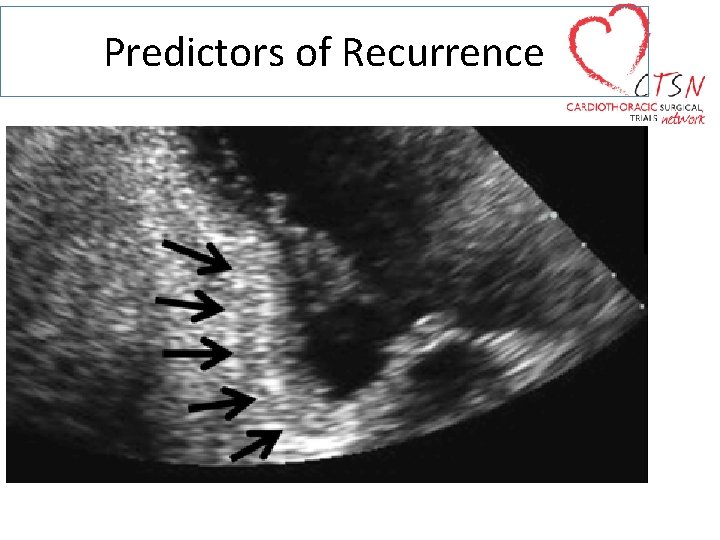
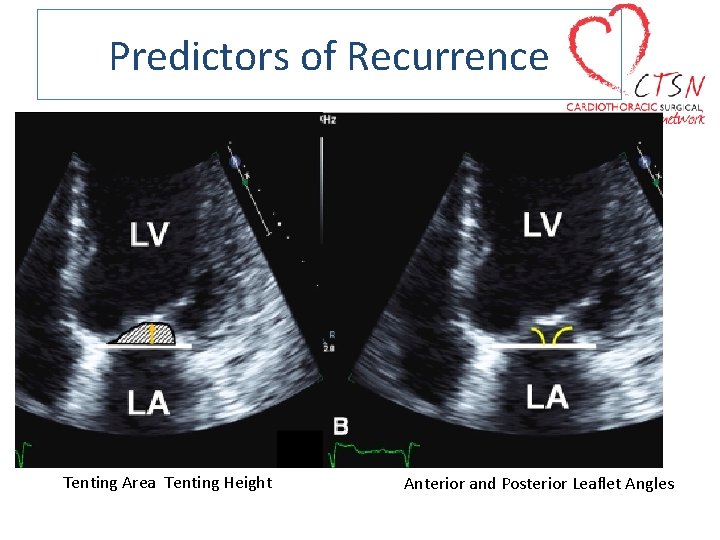
Predictors of Recurrence

Predictors of Recurrence Tenting Area Tenting Height Anterior and Posterior Leaflet Angles

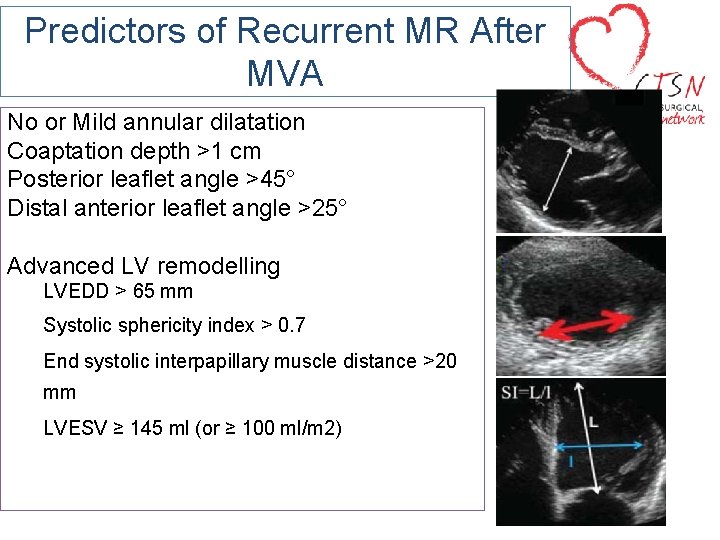
Predictors of Recurrent MR After MVA No or Mild annular dilatation Coaptation depth >1 cm Posterior leaflet angle >45° Distal anterior leaflet angle >25° Advanced LV remodelling LVEDD > 65 mm Systolic sphericity index > 0. 7 End systolic interpapillary muscle distance >20 mm LVESV ≥ 145 ml (or ≥ 100 ml/m 2)



Rates of Mitral Valve Repair and Replacement for Severe IMR in the US: What is the Impact of the CTSN Trial on Clinical Practice? • Thourani VH, Vemulapalli S, Gelijns AC, Moskowitz AJ, Acker M, Woo J, Zhang S, Gammie JS, O’Gara PT, Ailawadi G, Moon M, Jung S, Badhwar V, Adams D, Suri RM, Bolling S, Gillinov AM, Michler RE, Smith PK, Perrault LP, Goldstein D, Argenziano M, Bavaria J, Bafi A, Jacobs J, Miller M, Weisel RD, and Mack MJ 55 th Annual Scientific Meeting of the Society of Thoracic Surgeons January 29, 2018

Objectives • Assess relative rates of MV repair vs replacement for severe IMR before and after publication of CTSN trial outcomes • Explore whether change in surgical practice is associated with changes in 1 -year clinical outcomes

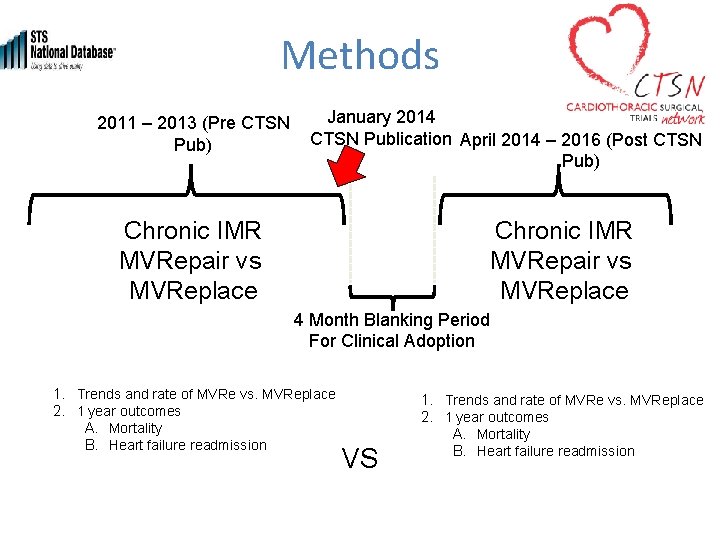
Methods January 2014 2011 – 2013 (Pre CTSN Publication April 2014 – 2016 (Post CTSN Pub) Chronic IMR MVRepair vs MVReplace 4 Month Blanking Period For Clinical Adoption 1. Trends and rate of MVRe vs. MVReplace 2. 1 year outcomes A. Mortality B. Heart failure readmission VS 1. Trends and rate of MVRe vs. MVReplace 2. 1 year outcomes A. Mortality B. Heart failure readmission

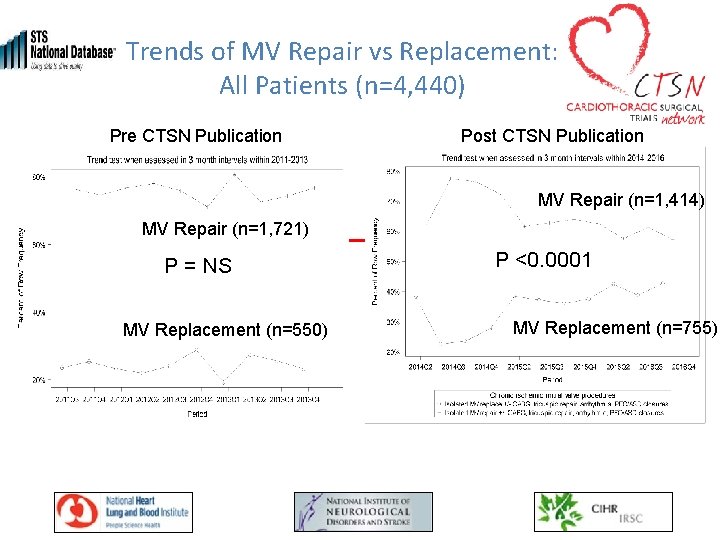
Trends of MV Repair vs Replacement: All Patients (n=4, 440) Pre CTSN Publication Post CTSN Publication MV Repair (n=1, 414) MV Repair (n=1, 721) P = NS MV Replacement (n=550) P <0. 0001 MV Replacement (n=755)

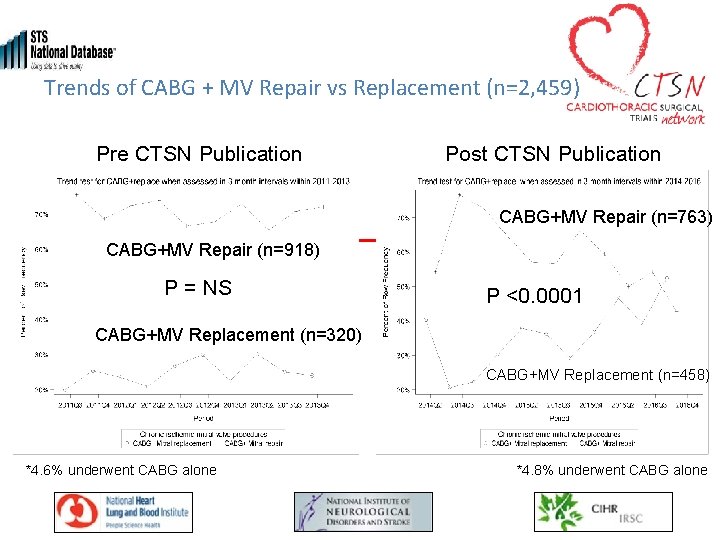
Trends of CABG + MV Repair vs Replacement (n=2, 459) Pre CTSN Publication Post CTSN Publication CABG+MV Repair (n=763) CABG+MV Repair (n=918) P = NS P <0. 0001 CABG+MV Replacement (n=320) CABG+MV Replacement (n=458) *4. 6% underwent CABG alone *4. 8% underwent CABG alone

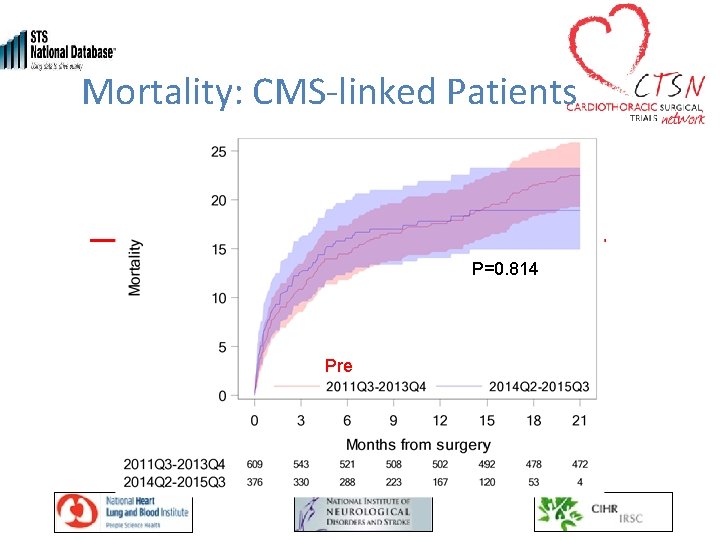
Mortality: CMS-linked Patients P=0. 814 Pre Post

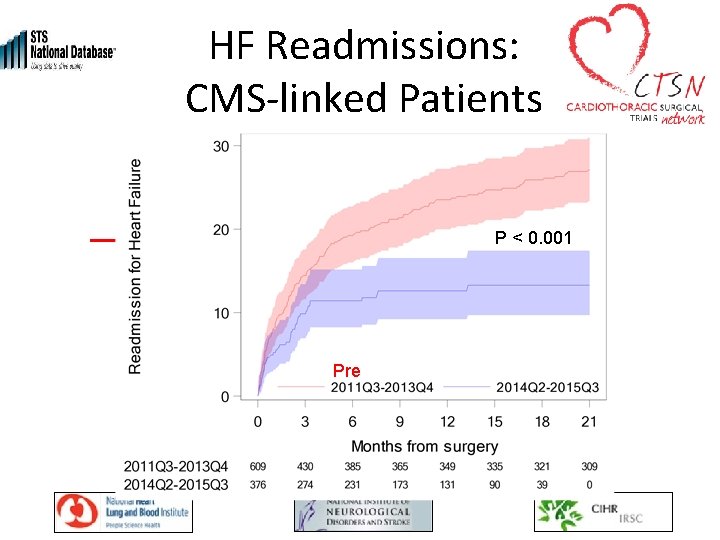
HF Readmissions: CMS-linked Patients P < 0. 001 Pre Post

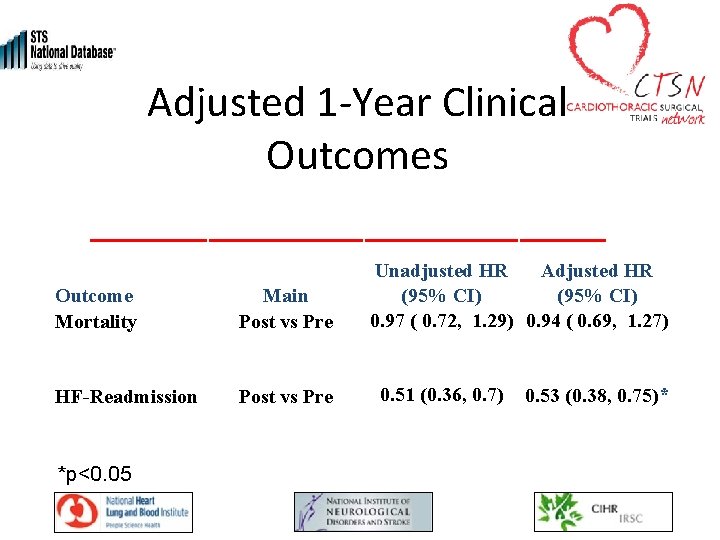
Adjusted 1 -Year Clinical Outcomes Outcome Mortality Main Post vs Pre HF-Readmission Post vs Pre *p<0. 05 Unadjusted HR Adjusted HR (95% CI) 0. 97 ( 0. 72, 1. 29) 0. 94 ( 0. 69, 1. 27) 0. 51 (0. 36, 0. 7) 0. 53 (0. 38, 0. 75)*

Conclusions • There is significant early impact of CTSN publication on surgical practice patterns with a decrease, but not elimination of MV repair for severe IMR • No temporal difference in mortality within the 2 time periods • Temporal reduction in HF readmissions • Exceeding background impact of improvements in patient (including HF) management • Presumably from an improvement in patient selection as potentially learned from the CTSN trial

Transcatheter MV Repair: Device Landscape 2018 Gregg Stone Edge-to-edge • Mitra. Clip*** • Mitra. Flex MV replacement (cont) • Edwards Cardi. AQ* • Mitral. Heal • Edwards Fortis* • HT Consultant Saturn Coronary sinus annuloplasty • Neovasc Tiara* • Lutter valve • Cardiac Dimensions Carillon** • Abbott Tendyne* • Transcatheter Technologies • Cerclage annuloplasty • Medtronic Intrepid* Tresillo • High. Life* • Venus Direct annuloplasty and • MValve* • Verso basal ventriculoplasty • Caison* • Transmural Systems • Mitralign TAMR** • NCSI Navi. Gate • Valtech Cardioband** Other approaches • St. Jude • GDS Accucinch* • Neo. Chord DS 1000** • Micro Interventional • Harpoon neochords* • Millipede IRIS* • Valtech Cardio. Valve • MVRx ARTO* • Babic chords* • Valve. Xchange • Mardil BACE* • Middle Peak Medical* • Mitr. Assist • Mitraspan* • St. Jude leaflet plication* • Braile Quattuor • Valcare Amend* • Cardiosolutions Mitra • Cephea • Micardia en. Cor Spacer* • Direct Flow • Cardiac Implants RDS • Valtech Vchordal • Sinomed Accufit • Quantum. Cor (RF) • Mitralix

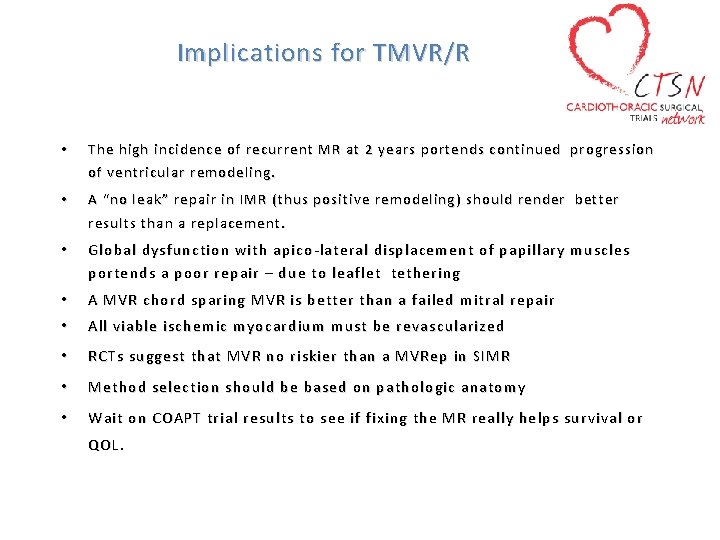
Implications for TMVR/R • The high incidence of recurrent MR at 2 years portends continued progression of ventricular remodeling. • A “no leak” repair in IMR (thus positive remodeling) should render better results than a replacement. • Global dysfunction with apico-lateral displacement of papillary muscles portends a poor repair – due to leaflet tethering • A MVR chord sparing MVR is better than a failed mitral repair • All viable ischemic myocardium must be revascularized • RCTs suggest that MVR no riskier than a MVRep in SIMR • Method selection should be based on pathologic anatomy • Wait on COAPT trial results to see if fixing the MR really helps survival or QOL.

d e t n e s Tr e r P e l B l i W 018 s t l u 2 s T e C R T l ia
 Apical pulse and mitral valve
Apical pulse and mitral valve Pht mitral valve
Pht mitral valve Pht mitral valve
Pht mitral valve Tendyne
Tendyne Ctsn scitt
Ctsn scitt Ctsn scitt
Ctsn scitt Base excision repair vs mismatch repair
Base excision repair vs mismatch repair Base excision repair
Base excision repair Na o cl
Na o cl Mechanical servo valve
Mechanical servo valve Mitral facies
Mitral facies Mitral facies
Mitral facies Severe ms heart
Severe ms heart Pathophysiology of valvular heart disease
Pathophysiology of valvular heart disease Site:slidetodoc.com
Site:slidetodoc.com Tendinous cords
Tendinous cords Mitral stenosis chest x ray
Mitral stenosis chest x ray Heart disease
Heart disease Atheromatous thoracic aorta
Atheromatous thoracic aorta Wilkins score ms
Wilkins score ms Aortal stenoz
Aortal stenoz Diyastolik rulman nedir
Diyastolik rulman nedir Insuficiencia mitral
Insuficiencia mitral Valvuloplastia mitral percutánea
Valvuloplastia mitral percutánea Valvula mitral en paracaidas
Valvula mitral en paracaidas Mitral stenosis pulmonary hypertension
Mitral stenosis pulmonary hypertension Mitral klapan yetishmovchiligi
Mitral klapan yetishmovchiligi Malar flush pathophysiology
Malar flush pathophysiology Heart sound
Heart sound Adlov
Adlov Estenose mitral
Estenose mitral Frémissement cataire diastolique
Frémissement cataire diastolique Valva mitral
Valva mitral Valva mitral
Valva mitral Aortic regurgitation murmur
Aortic regurgitation murmur Do we our life done
Do we our life done Phs human subjects and clinical trials information
Phs human subjects and clinical trials information Audits and inspections of clinical trials
Audits and inspections of clinical trials Design and analysis of cross over trials
Design and analysis of cross over trials