CRITERIA POLLUTANTS SIX MOST IMPORTANT POLLUTANTS It is










































- Slides: 48

CRITERIA POLLUTANTS (SIX MOST IMPORTANT POLLUTANTS):




• It is produced when carbonaceous fuels are burned under less than ideal conditions. • 77% of total CO emissions are from transportation sector. • All CO in urban areas comes from motor vehicles. • Incomplete combustion (yielding CO instead of CO 2) results when any of the following four variables are not kept sufficiently high: Ø Oxygen supply Ø Combustion temperature Ø Gas residence time at high temperature Ø Combustion Chamber Turbulence
















3. Photochemical Smog and ground level Ozone • Volatile organic compounds (VOCs) include unborn hydrocarbons that are emitted when fossil fuels are not completely combusted along with gaseous hydrocarbons that enter the atmosphere when solvents, fuels and other organics evaporate. • 1/3 of VOCs emissions result from transport sources. • 2/3 of VOCs emissions result from industrial sources. • Less than 2% of VOCs result from fossil fuel combustion in power plants and industrial boilers.

• When oxides of nitrogen, VOCs, and sunlight come together, they can initiate a complex set of reactions that produce a number of secondary pollutants known as photochemical oxidants. • Ground level Ozone (O 3) is the most abundant of the photochemical oxidants. • VOCs + NOX + Sunlight → Photochemical Smog (O 3 + etc. ) • Ground level Ozone (O 3) is harmful to our health. But stratospheric Ozone protects our health by shielding us from ultraviolet radiation from the sun. • It causes materials and vegetables damage. • It causes respiratory effects such as coughing, shortness of breath, headache, chest tightness and

4. Particulate matter • Atmospheric particulate matter consists of any dispersed matter, solid, or liquid. • Size range from 0. 005μm diameter to 100μm (roughly the size of a human hair). • Term “aerosol” is used for any tiny particulate, liquid, or solid, dispersed in the atmosphere. • Particles have very irregular shapes.

Sources • Sources are fuel combustion, industrial processing and transportation. • Transportation sources are emissions from buses and trucks. • 95% of particulate emissions are from wild fires, wind-driven soil erosion, dust from croplands, construction, mining activities, and the constant abrasion of paved and unpaved roadways.

Effects • Large particles that enter the respiratory system can be trapped by the hairs and lining of the nose. (Driven out by cough or sneeze). • Smaller particles can be captured by hair like cilia in the respiratory system. (Removed by swallowing or spitting). • Aerosols consisting (SO 4) contribute to respiratory distress, degradation of materials, and major cause of reduced visibility. • The bulk smoke from diesel engines and smoke stacks consists solid particles made up of “carbon atoms fused in benzene rings” can irritate lungs.

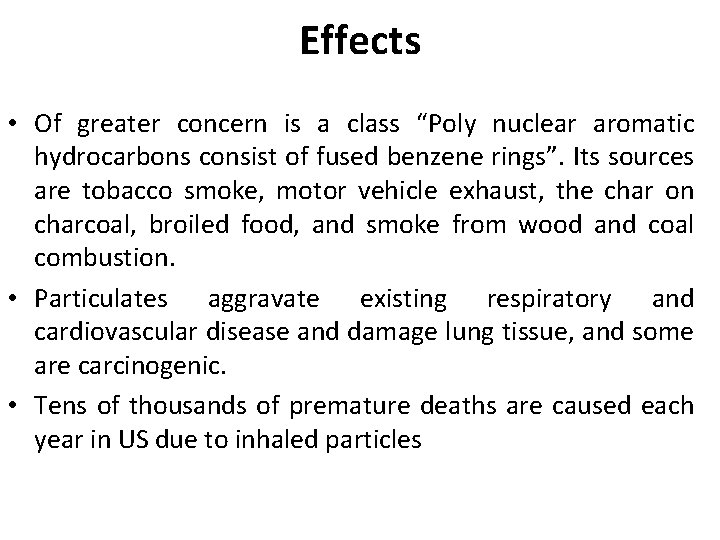
Effects • Of greater concern is a class “Poly nuclear aromatic hydrocarbons consist of fused benzene rings”. Its sources are tobacco smoke, motor vehicle exhaust, the char on charcoal, broiled food, and smoke from wood and coal combustion. • Particulates aggravate existing respiratory and cardiovascular disease and damage lung tissue, and some are carcinogenic. • Tens of thousands of premature deaths are caused each year in US due to inhaled particles

5. Oxides of Sulfur • Sulfur dioxide is highly water soluble than any other criteria pollutant. When it is inhaled it is absorbed in moist passages of the upper respiratory tract, the nose and upper airways, where it does less long term damage. • When Sulfur oxides reach far deeper into the lungs, the combination of particulate matter and sulfur oxides can act synergistically to cause excess mortality. (50, 000 premature deaths, 2% of total deaths per year in the US and Canada).

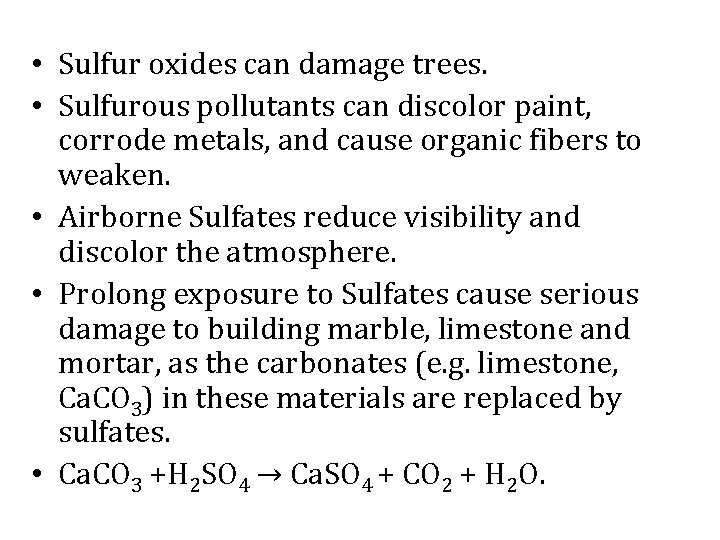
• Sulfur oxides can damage trees. • Sulfurous pollutants can discolor paint, corrode metals, and cause organic fibers to weaken. • Airborne Sulfates reduce visibility and discolor the atmosphere. • Prolong exposure to Sulfates cause serious damage to building marble, limestone and mortar, as the carbonates (e. g. limestone, Ca. CO 3) in these materials are replaced by sulfates. • Ca. CO 3 +H 2 SO 4 → Ca. SO 4 + CO 2 + H 2 O.

• The calcium Sulfate (Gypsum, Ca. SO 4) produced by this reaction is water soluble and easily washed away leaving a pitted, eroded surface. • In US, 90% of 22 million tons/ year of sulfurous emissions are the result of fossil fuel combustion in stationary sources. 85% of that is emitted from electric utility power plants (16 million tons/ year). • 3% sulfurous emissions come from highway vehicles. • Non combustion sulfurous emissions are from petroleum refining, copper smelting, and cement manufacture.

6. Lead: • Most lead emissions from motor vehicles burning gasoline containing the antiknock additive tetraethyl lead Pb (C 2 H 5) 4. • Ambient (outdoor) lead levels tend to be high in the vicinity (nearness) of industrial facilities such as metal smelters and plants that manufacture lead acid batteries. • Lead is emitted into the atmosphere in the form of inorganic particulates.

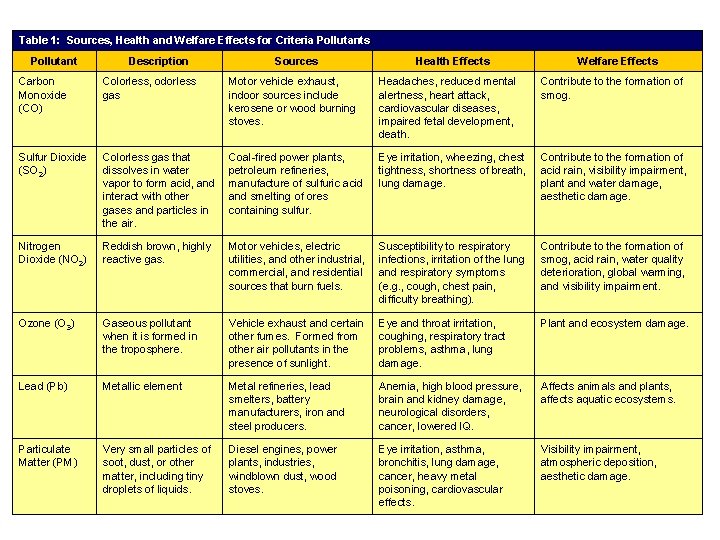
Table 1: Sources, Health and Welfare Effects for Criteria Pollutants. Pollutant Description Sources Health Effects Welfare Effects Carbon Monoxide (CO) Colorless, odorless gas Motor vehicle exhaust, indoor sources include kerosene or wood burning stoves. Headaches, reduced mental alertness, heart attack, cardiovascular diseases, impaired fetal development, death. Contribute to the formation of smog. Sulfur Dioxide (SO 2) Colorless gas that dissolves in water vapor to form acid, and interact with other gases and particles in the air. Coal-fired power plants, petroleum refineries, manufacture of sulfuric acid and smelting of ores containing sulfur. Eye irritation, wheezing, chest tightness, shortness of breath, lung damage. Contribute to the formation of acid rain, visibility impairment, plant and water damage, aesthetic damage. Nitrogen Dioxide (NO 2) Reddish brown, highly reactive gas. Motor vehicles, electric utilities, and other industrial, commercial, and residential sources that burn fuels. Susceptibility to respiratory infections, irritation of the lung and respiratory symptoms (e. g. , cough, chest pain, difficulty breathing). Contribute to the formation of smog, acid rain, water quality deterioration, global warming, and visibility impairment. Ozone (O 3) Gaseous pollutant when it is formed in the troposphere. Vehicle exhaust and certain other fumes. Formed from other air pollutants in the presence of sunlight. Eye and throat irritation, coughing, respiratory tract problems, asthma, lung damage. Plant and ecosystem damage. Lead (Pb) Metallic element Metal refineries, lead smelters, battery manufacturers, iron and steel producers. Anemia, high blood pressure, brain and kidney damage, neurological disorders, cancer, lowered IQ. Affects animals and plants, affects aquatic ecosystems. Particulate Matter (PM) Very small particles of soot, dust, or other matter, including tiny droplets of liquids. Diesel engines, power plants, industries, windblown dust, wood stoves. Eye irritation, asthma, bronchitis, lung damage, cancer, heavy metal poisoning, cardiovascular effects. Visibility impairment, atmospheric deposition, aesthetic damage.

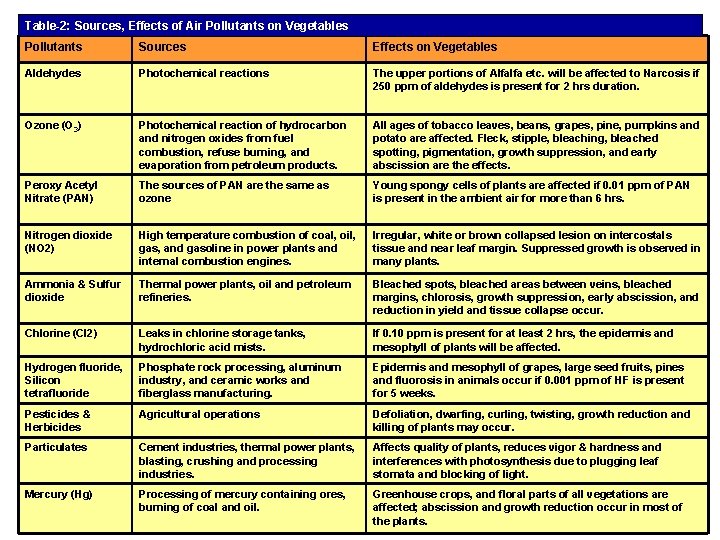
Table-2: Sources, Effects of Air Pollutants on Vegetables Pollutants Sources Effects on Vegetables Aldehydes Photochemical reactions The upper portions of Alfalfa etc. will be affected to Narcosis if 250 ppm of aldehydes is present for 2 hrs duration. Ozone (O 3) Photochemical reaction of hydrocarbon and nitrogen oxides from fuel combustion, refuse burning, and evaporation from petroleum products. All ages of tobacco leaves, beans, grapes, pine, pumpkins and potato are affected. Fleck, stipple, bleaching, bleached spotting, pigmentation, growth suppression, and early abscission are the effects. Peroxy Acetyl Nitrate (PAN) The sources of PAN are the same as ozone Young spongy cells of plants are affected if 0. 01 ppm of PAN is present in the ambient air for more than 6 hrs. Nitrogen dioxide (NO 2) High temperature combustion of coal, oil, gas, and gasoline in power plants and internal combustion engines. Irregular, white or brown collapsed lesion on intercostals tissue and near leaf margin. Suppressed growth is observed in many plants. Ammonia & Sulfur dioxide Thermal power plants, oil and petroleum refineries. Bleached spots, bleached areas between veins, bleached margins, chlorosis, growth suppression, early abscission, and reduction in yield and tissue collapse occur. Chlorine (Cl 2) Leaks in chlorine storage tanks, hydrochloric acid mists. If 0. 10 ppm is present for at least 2 hrs, the epidermis and mesophyll of plants will be affected. Hydrogen fluoride, Silicon tetrafluoride Phosphate rock processing, aluminum industry, and ceramic works and fiberglass manufacturing. Epidermis and mesophyll of grapes, large seed fruits, pines and fluorosis in animals occur if 0. 001 ppm of HF is present for 5 weeks. Pesticides & Herbicides Agricultural operations Defoliation, dwarfing, curling, twisting, growth reduction and killing of plants may occur. Particulates Cement industries, thermal power plants, blasting, crushing and processing industries. Affects quality of plants, reduces vigor & hardness and interferences with photosynthesis due to plugging leaf stomata and blocking of light. Mercury (Hg) Processing of mercury containing ores, burning of coal and oil. Greenhouse crops, and floral parts of all vegetations are affected; abscission and growth reduction occur in most of the plants.

TOXIC AIR POLLUTANTS • These are carcinogenic, teratogenic, neurotoxic, which cause reproductive dysfunction, or which are acutely are chronically toxic. • There are hundreds of such chemicals. • Examples are asbestos, benzene, beryllium, coke-oven emissions, inorganic arsenic, mercury, radionuclides, and vinyl chloride.

AIR POLLUTION IN BIG CITIES • Principal source is motor vehicles. • Leaded fuels are burned. • High %age of the vehicles is diesel-powered trucks and buses with no emission controls. • Many streets are unpaved. • Traffic congestion, which intensifies emissions, is overwhelming.

AIR POLLUTION IN BIG CITIES • The resulting concentrations of Pb, CO, NOX, O 3 and suspended particulate matter (SPM) are higher. • Coal-fired power plants and other industrial facilities within city limits and levels of SOX, NOX and particulate matter are high. • Combustion of coal and biomass fuels for cooking and heating leads to extremely high pollutant concentrations indoors, where many people spend most of their time.

INDOOR AIR QUALITY • Combustion that takes place inside of homes and other buildings to cook, heat water, and provide space heating and cooling can produce elevated levels of CO, Nitrogen oxides, hydrocarbons, and respirable particulates. • Cigarette smoke emits CO, benzene, acrobin, and other aldehydes, and particulates, as well as about 4000 other chemicals.

INDOOR AIR QUALITY • Some pollutants are unique to the indoor environment such as following; • Asbestos: Used for fireproofing and insulation. • Radon gas: Seeps out of the houses and collects in houses. • Biological Pollutants: Such as house dust mites, fungi, and other microorganisms.

INDOOR AIR QUALITY ASBESTOS: • Asbestos is a common name for a group of naturally occurring fibrous silicate minerals that are made up of thin but strong durable fibers. Because of its tensile strength and heat resistant properties, asbestos has been used extensively in building materials such as ceilings, insulation and tiles, automobile clutch and transmission parts and heat resistant fabrics.

INDOOR AIR QUALITY ASBESTOS: • Asbestos is a mineral and is relatively stable in the environment unless they are disturbed. Natural weathering or upon damage or disturbance of asbestos-containing products, the microscopic fibers become airborne and can be inhaled into the lungs or ingested with contaminated food where they can cause significant health problems.

Asbestos

Asbestos

INDOOR AIR QUALITY Asbestos: • It is Hazardous air pollutants. Asbestos is dangerous when it is crushed, crumbled or disturbed because fibers can be released into the air. • It is used to be a common building material found in structural fireproofing, heatingsystem insulation, floor and ceiling tiles, roofing felts and shingles.

INDOOR AIR QUALITY Asbestos: • Used in consumer products such as fireplace gloves, and certain hair dryers. • If these materials damage, microscopic fibers may be dispersed into the indoor air environment. • Inhalation of these fibers can lead to diseases including asbestosis, lung cancer and mesothelioma.

INDOOR AIR QUALITY ASBESTOS: • Asbestos exposure along with tobacco smoke elevates the lung cancer risk by approximately fivefold.

INDOOR AIR QUALITY RADON: It is second leading cause of lung cancer after smoking. • Sources are diffusion from soil, ground water and building materials such as brick, concrete, and tiles. • Radon itself is inert but its short-lived decay products-Polonium, lead, and bismuth-are chemically active and easily become attached to inhaled particles that can lodge in the lung.

INDOOR AIR QUALITY RADON: • Indoor air can be exchanged with outdoor air by any combination of following three mechanisms. • Radon is a naturally-occurring, invisible and odorless radioactive gas. • Radon gas causes more deaths every year than fires and carbon monoxide combined.


INDOOR AIR QUALITY • (1) Infiltration: Natural air exchange, when doors and windows are closed. Air exchange happens by leakage through various cracks and holes in building. • (2) Natural Ventilation: Air exchange, when windows or doors are purposely opened to increase air circulation. • (3) Forced Ventilation: Air exchange using fans or blowers by mechanical handling systems.
 Trees
Trees Primary pollutants and secondary pollutants
Primary pollutants and secondary pollutants Difference between secondary and primary pollutants
Difference between secondary and primary pollutants Newspaper article format
Newspaper article format From most important to least important in writing
From most important to least important in writing Least important to most important
Least important to most important Brand elements choice criteria
Brand elements choice criteria Classify each polygon
Classify each polygon Rumelt's criteria for evaluating strategies example
Rumelt's criteria for evaluating strategies example Secondary pollutants examples
Secondary pollutants examples What are secondary pollutants
What are secondary pollutants Primary vs secondary pollution
Primary vs secondary pollution Benjamin cummings
Benjamin cummings Inorganic gaseous pollutants of air
Inorganic gaseous pollutants of air Secondary air pollutants
Secondary air pollutants Primary vs secondary pollutants
Primary vs secondary pollutants Stock pollutants
Stock pollutants Inorganic gases
Inorganic gases Primary and secondary pollutants difference
Primary and secondary pollutants difference What are the secondary air pollutants
What are the secondary air pollutants The meaning of environmental pollution
The meaning of environmental pollution Before and after global warming
Before and after global warming Major air pollutants
Major air pollutants Air pollutants
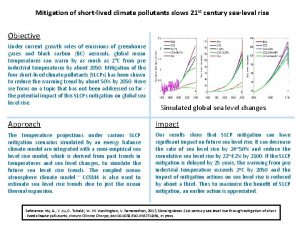
Air pollutants Short lived climate pollutants
Short lived climate pollutants Indoor air pollution sources
Indoor air pollution sources Why do thermal inversion layers trap pollutants
Why do thermal inversion layers trap pollutants 2 most important commandments
2 most important commandments Conclusion of salamanca statement
Conclusion of salamanca statement Most important agent of socialization
Most important agent of socialization What is resocialization
What is resocialization Different parts of body
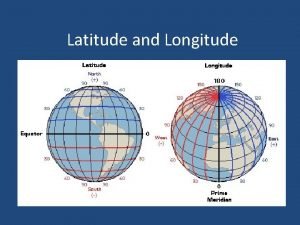
Different parts of body Earth horizontal lines
Earth horizontal lines International students inc
International students inc The most important thing in life is to be successful essay
The most important thing in life is to be successful essay Chemistry of shampoo
Chemistry of shampoo What were mansa musa's most important achievements
What were mansa musa's most important achievements Braylin
Braylin Water is one of the most important
Water is one of the most important The most important component
The most important component Most important organelle
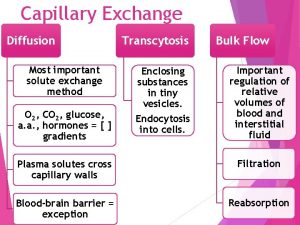
Most important organelle Capillary exchange
Capillary exchange For all pots p there is a lid l such that
For all pots p there is a lid l such that Elements of plot
Elements of plot Nomenclature of ether
Nomenclature of ether A customer is the most important visitor
A customer is the most important visitor Whats the most important lab safety rule
Whats the most important lab safety rule Where the agricultural sangam was developed
Where the agricultural sangam was developed The most important graph in the world
The most important graph in the world