Control of ECF osmolality and volume MAIN DIFFERENCES

![Osmolar concentration of plasma: 290 mosm/L - 142 m. Eq/L [Na+] Tonicity – Osmolality Osmolar concentration of plasma: 290 mosm/L - 142 m. Eq/L [Na+] Tonicity – Osmolality](https://slidetodoc.com/presentation_image/f9ba490dbc8fdeffc2d8f5ca8fcc8c93/image-5.jpg)

















- Slides: 52

Control of ECF osmolality and volume

MAIN DIFFERENCES BETWEEN ICF AND ECF • More Na+ in ECF • More K+ in ICF • More Cl- in ECF • More PO 4, HCO 3, and Pr- in ICF These differences are maintained by transport processes in the cell membrane

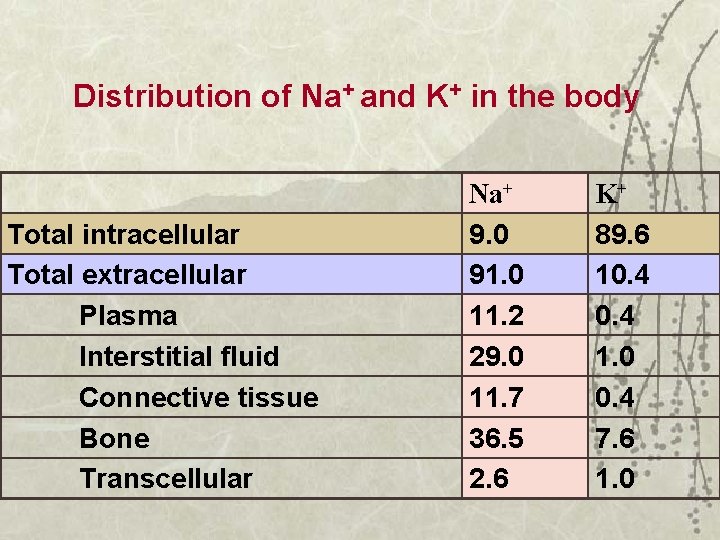
Distribution of Na+ and K+ in the body Total intracellular Total extracellular Plasma Interstitial fluid Connective tissue Bone Transcellular Na+ 9. 0 91. 0 11. 2 29. 0 11. 7 36. 5 2. 6 K+ 89. 6 10. 4 1. 0 0. 4 7. 6 1. 0

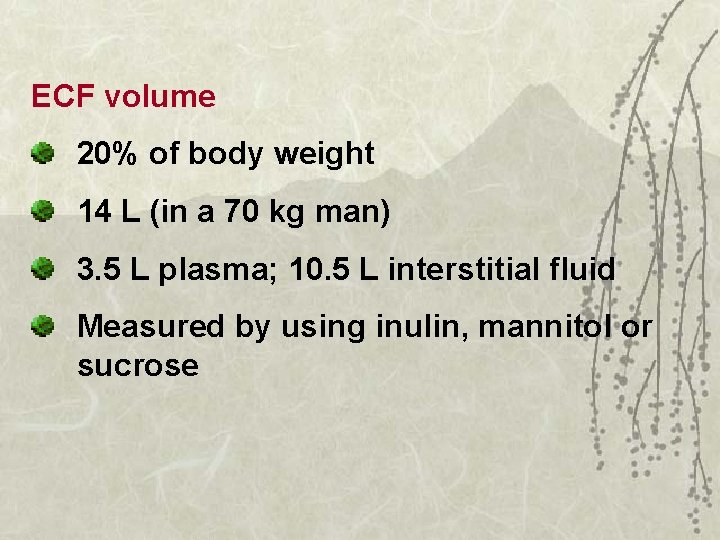
ECF volume 20% of body weight 14 L (in a 70 kg man) 3. 5 L plasma; 10. 5 L interstitial fluid Measured by using inulin, mannitol or sucrose
![Osmolar concentration of plasma 290 mosmL 142 m EqL Na Tonicity Osmolality Osmolar concentration of plasma: 290 mosm/L - 142 m. Eq/L [Na+] Tonicity – Osmolality](https://slidetodoc.com/presentation_image/f9ba490dbc8fdeffc2d8f5ca8fcc8c93/image-5.jpg)
Osmolar concentration of plasma: 290 mosm/L - 142 m. Eq/L [Na+] Tonicity – Osmolality of a solution in relation to plasma - isotonic, hypertonic, hypotonic 0. 9% saline is isotonic 270 mosm/L is contributed by Na+, Cland HCO 3 Plasma proteins contribute less than 2 mosm/L (28 mm Hg oncotic pressure)

Ranges of salt and water intake and excretion: a. Salt intake from 50 mg to 25 g/day b. Water excretion from 400 ml to 25 l/day

Total body sodium is relatively constant. Freely filtered Reabsorbed but not secreted Therefore, Na+ excretion = Na+ filtered – Na+ reabsorbed = (GFR X Pna) - Na+ reabsorbed Pna is relatively constant Therefore control is exerted by GFR Na+ reabsorption

Sensors: 1. Extrarenal baroreceptors 2. Carotid sinuses 3. Arteries 4. Great veins 5. Atria 6. 2. Renal juxtaglomerular apparatus 7. Efferents: 8. Renal sympathetic nerves 9. Macula densa renin angiotensin II aldosterone

Control of GFR: 1. Angiotensin II efferent arteriolar constriction PGC 2. Renal sympathetic nerves Na+ adrenergic receptors Constriction of afferent and efferent arterioles PGC

Osmoreceptor -ADH mechanisms

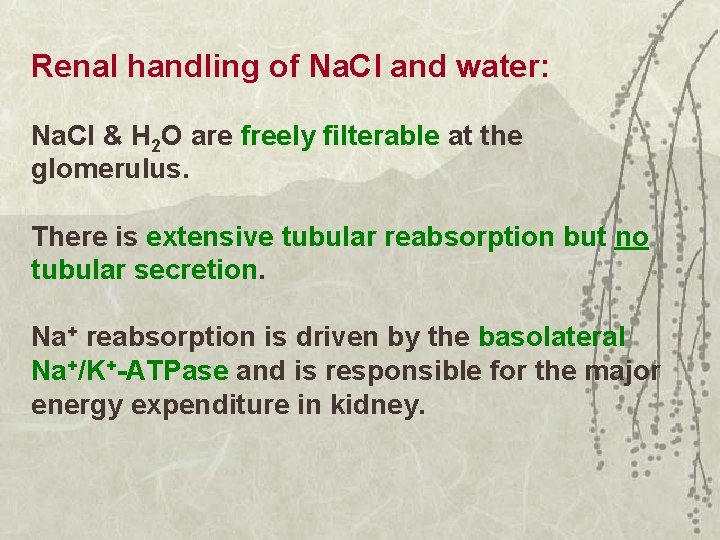
Renal handling of Na. Cl and water: Na. Cl & H 2 O are freely filterable at the glomerulus. There is extensive tubular reabsorption but no tubular secretion. Na+ reabsorption is driven by the basolateral Na+/K+-ATPase and is responsible for the major energy expenditure in kidney.

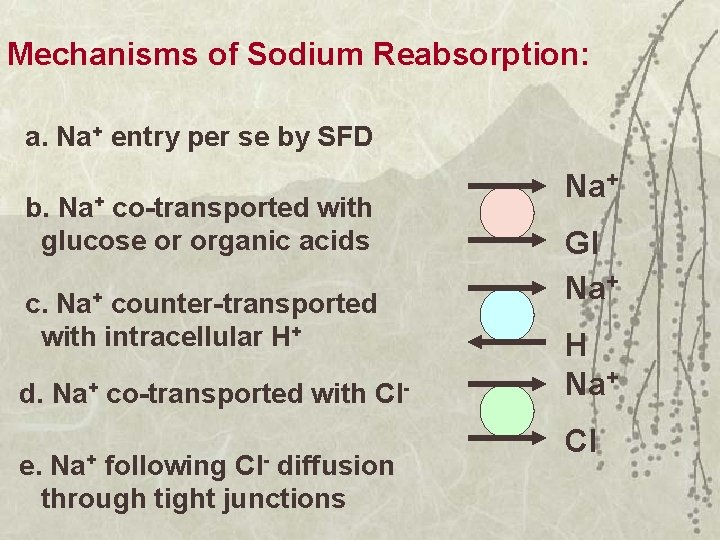
Mechanisms of Sodium Reabsorption: a. Na+ entry per se by SFD b. Na+ co-transported with glucose or organic acids c. Na+ counter-transported with intracellular H+ d. Na+ co-transported with Cl. Na+ Cl- e. following diffusion through tight junctions Na+ Gl Na+ H Na+ Cl

Proximal Tubule: The PT is highly permeable to water. Reabsorbs ~ 65% of filtered sodium (active transport) and water plus organic nutrients etc. Water reabsorption is passive, along osmotic gradients and keeps pace with solute. Therefore, the [Na+] remains virtually constant through the PT, whereas the mass of Na+ is reduced by 65%.

Movement of water is facilitated by the presence of water channels - aquaporin 1, in the apical membranes of proximal tubule epithelial cells Late in the PT, some Na+ is also reabsorbed by simple diffusion and solvent drag. Cl- initially lags behind and the concentration gradient is established by water reabsorption. Accordingly, in the middle and late PT, Cl- is the major anion coupled with Na+.


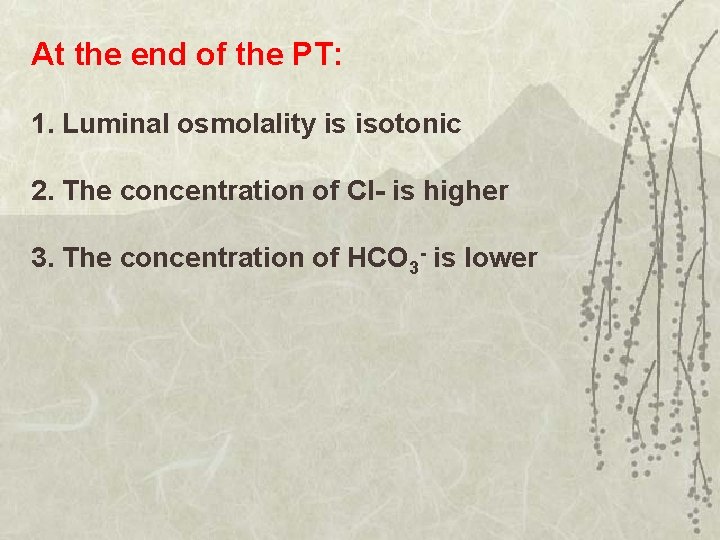
At the end of the PT: 1. Luminal osmolality is isotonic 2. The concentration of Cl- is higher 3. The concentration of HCO 3 - is lower

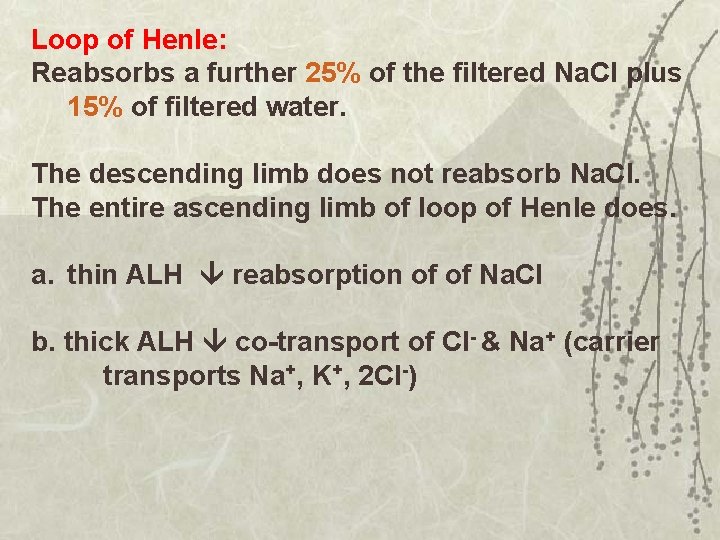
Loop of Henle: Reabsorbs a further 25% of the filtered Na. Cl plus 15% of filtered water. The descending limb does not reabsorb Na. Cl. The entire ascending limb of loop of Henle does. a. thin ALH reabsorption of of Na. Cl b. thick ALH co-transport of Cl- & Na+ (carrier transports Na+, K+, 2 Cl-)


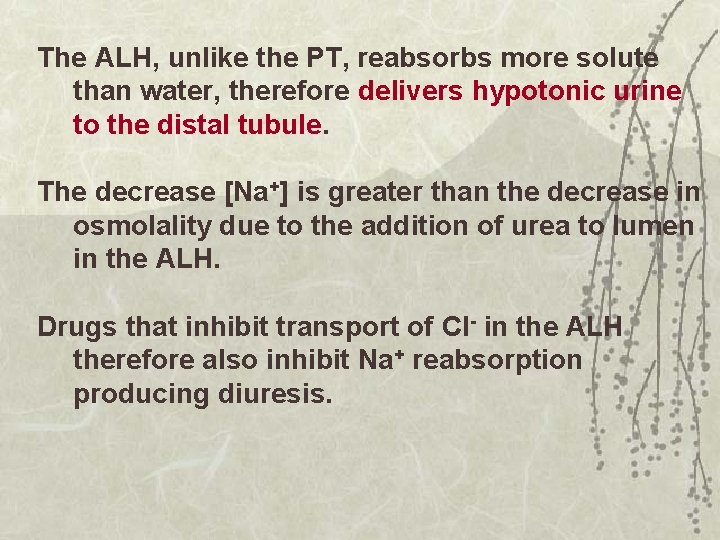
The ALH, unlike the PT, reabsorbs more solute than water, therefore delivers hypotonic urine to the distal tubule. The decrease [Na+] is greater than the decrease in osmolality due to the addition of urea to lumen in the ALH. Drugs that inhibit transport of Cl- in the ALH therefore also inhibit Na+ reabsorption producing diuresis.

Distal Tubule & Collecting Duct: Na. Cl reabsorption continues along the DT & CT so that the final urine contains ~ 1% of the filtered mass. H 2 O permeability of the early DT is extremely low and not subject to physiological control. Accordingly almost no water is reabsorbed in the early distal segment.

H 2 O permeability of the late DT: Water permeability of distal tubule and initial collecting tubule, is also extremely low. However under the influence of ADH it becomes highly water permeable. Further removal of solute in the EDT presents the LDT with markedly hypotonic urine containing even less Na+ Removal of Na+ continues in the LDT and collecting system, so that the final urine may contain virtually no Na+.

Anti-diuretic hormone: ADH (antidiuretic hormone), vasopressin or arginine vasopressin (AVP) is the major regulator of urine osmolality and urine volume. ADH is a nonapeptide produced by neurons in the supraoptic and paraventricular nuclei of the hypothalamus. The axon terminals of these neurons reside in the posterior pituitary. ADH is stored in these axon terminals.

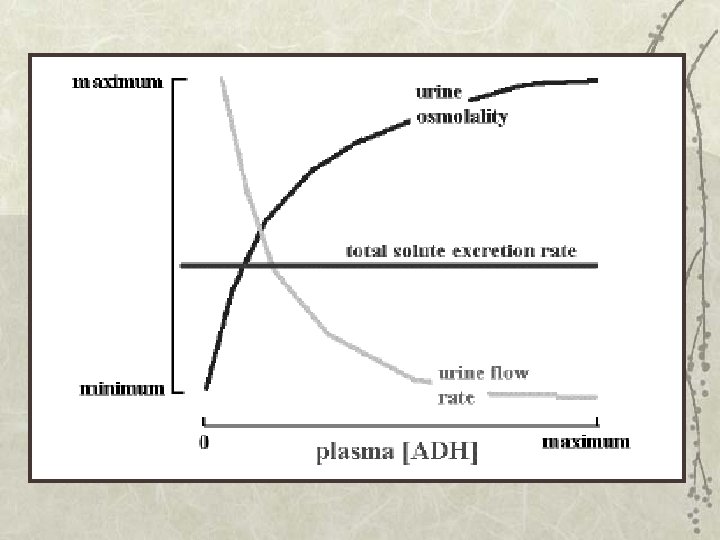
When ADH is released from the posterior pituitary it causes the kidney to produce urine that is high in osmolality and low in volume. In the absence of ADH the kidney tends to produce a large volume of urine with low osmolality. Total solute excretion is relatively constant over a wide range of urine flow rates and osmolalities.


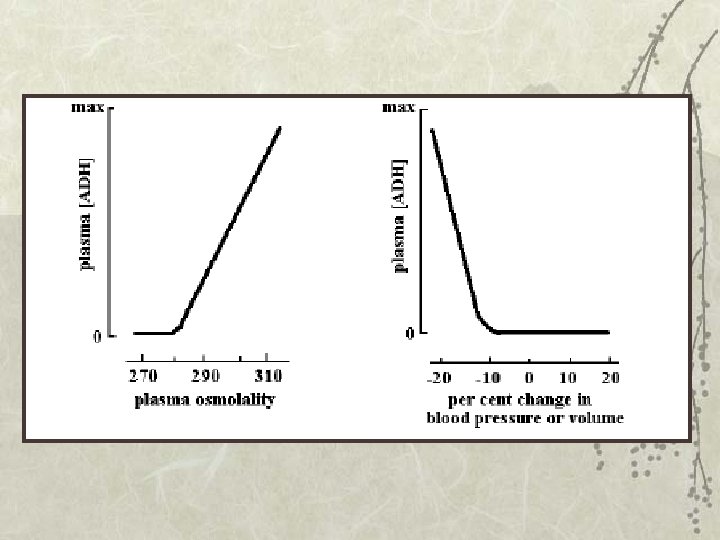
Control of ADH release: 1. Increased osmolality of ECF is a powerful stimulus for ADH release: a 1% change in osmolality induces significant increase in ADH release. Hypothalamic supra-optic and paraventricular nuclei respond to increased osmolality of ECF by producing ADH. As a result of this high sensitivity, responses to increased osmolality occur rapidly.

Control of ADH release: 2. Volume: In a volume-depleted individual, the release of ADH is more sensitive to increased osmolality. In a volume-expanded state, ADH release is less sensitive to increases in osmolality. 3. Decreased blood pressure or blood volume also enhance ADH release, but not with such high sensitivity: 5 to 10% changes are required to alter ADH secretion.


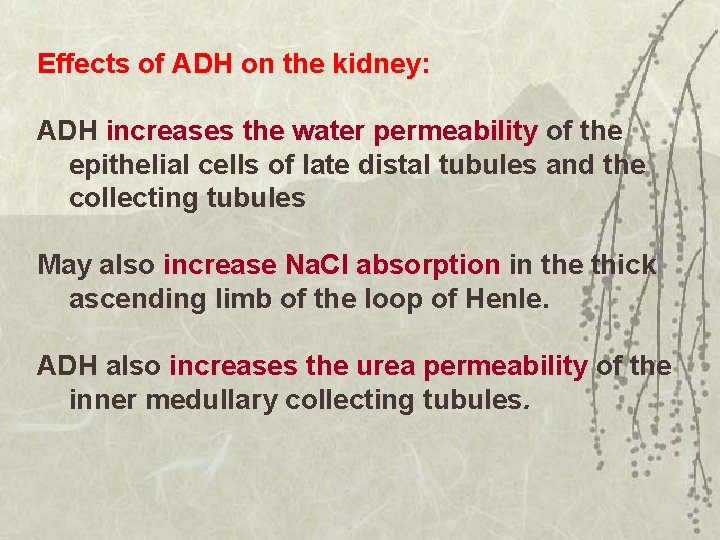
Effects of ADH on the kidney: ADH increases the water permeability of the epithelial cells of late distal tubules and the collecting tubules May also increase Na. Cl absorption in the thick ascending limb of the loop of Henle. ADH also increases the urea permeability of the inner medullary collecting tubules.

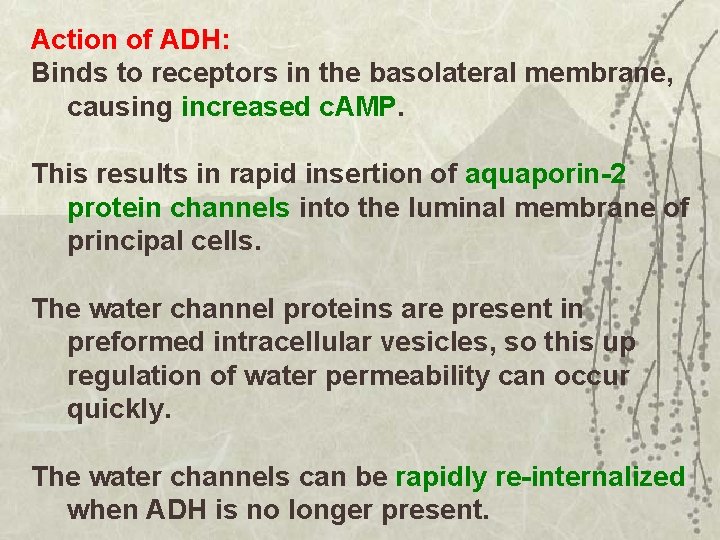
Action of ADH: Binds to receptors in the basolateral membrane, causing increased c. AMP. This results in rapid insertion of aquaporin-2 protein channels into the luminal membrane of principal cells. The water channel proteins are present in preformed intracellular vesicles, so this up regulation of water permeability can occur quickly. The water channels can be rapidly re-internalized when ADH is no longer present.

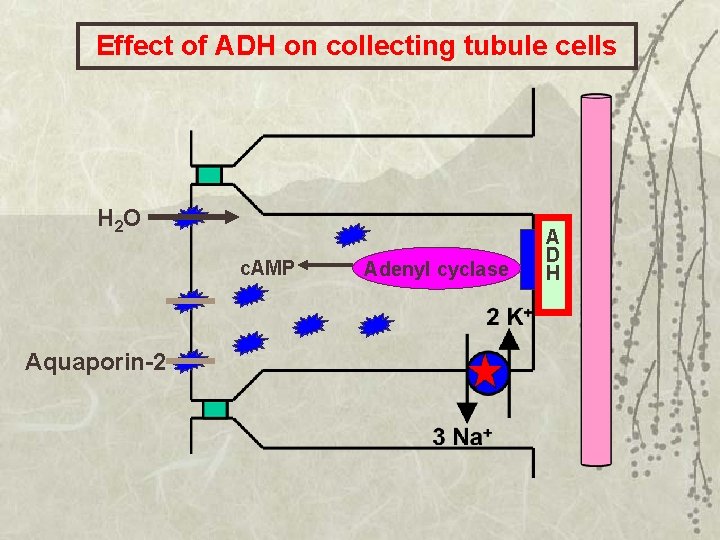
Effect of ADH on collecting tubule cells H 2 O c. AMP Aquaporin-2 Adenyl cyclase A D H

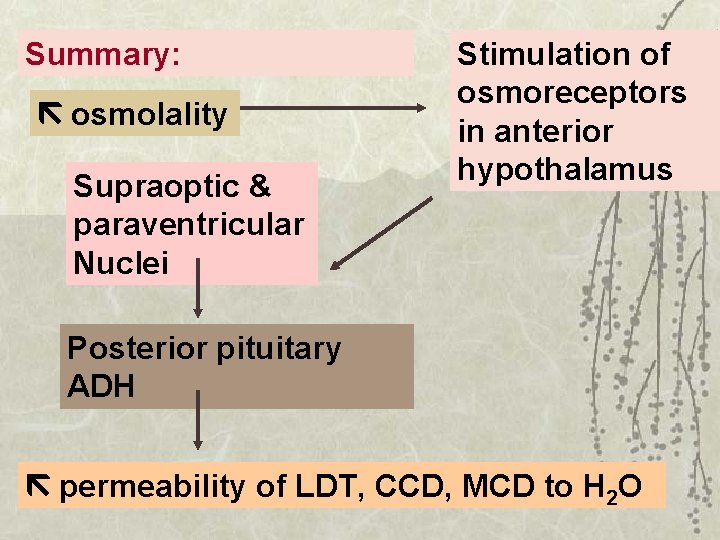
Summary: osmolality Supraoptic & paraventricular Nuclei Stimulation of osmoreceptors in anterior hypothalamus Posterior pituitary ADH permeability of LDT, CCD, MCD to H 2 O

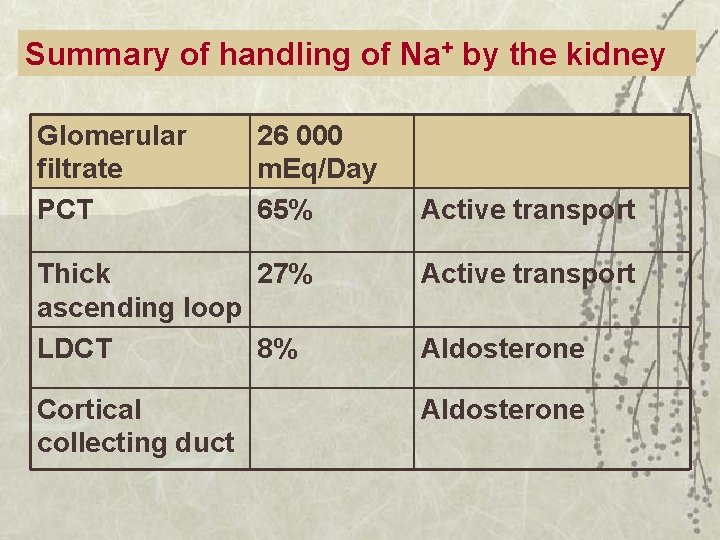
Summary of handling of Na+ by the kidney Glomerular filtrate PCT 26 000 m. Eq/Day 65% Active transport Thick 27% ascending loop LDCT 8% Active transport Cortical collecting duct Aldosterone

Thirst mechanism

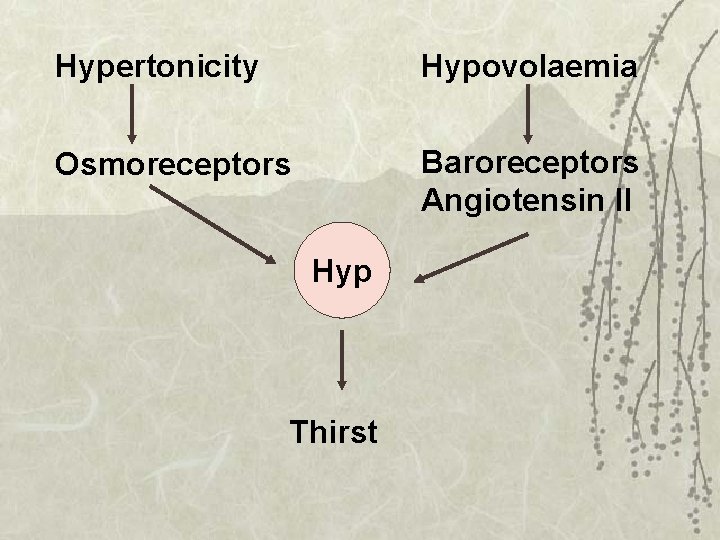
Thirst (conscious desire for water): Under hypothalamic osmoreceptor control Water intake is regulated by - increased plasma osmolality - decreased ECF volume - psychological factors

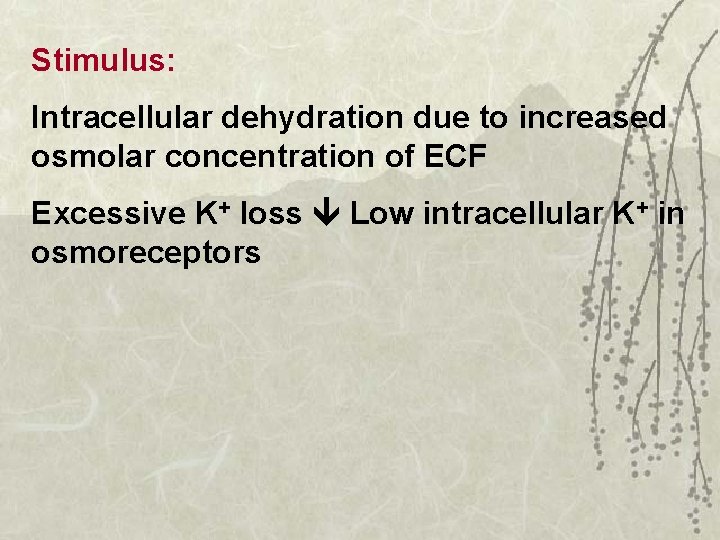
Stimulus: Intracellular dehydration due to increased osmolar concentration of ECF Excessive K+ loss Low intracellular K+ in osmoreceptors

Mechanism is activated by The arterial baroreceptor reflex BP The volume receptors- low pressure receptors in atria; CVP Angiotensin II Increased Na+ in CSF

Hypertonicity Hypovolaemia Osmoreceptors Baroreceptors Angiotensin II Hyp Thirst

Thirst center: Subfornical organ Organum vasculosum of the lamina terminalis

Other factors regulating water intake: Psycho-social Dryness of pharyngeal mucous membrane ? Gastrointestinal pharyngeal metering

Renin-angiotensin – aldosterone system

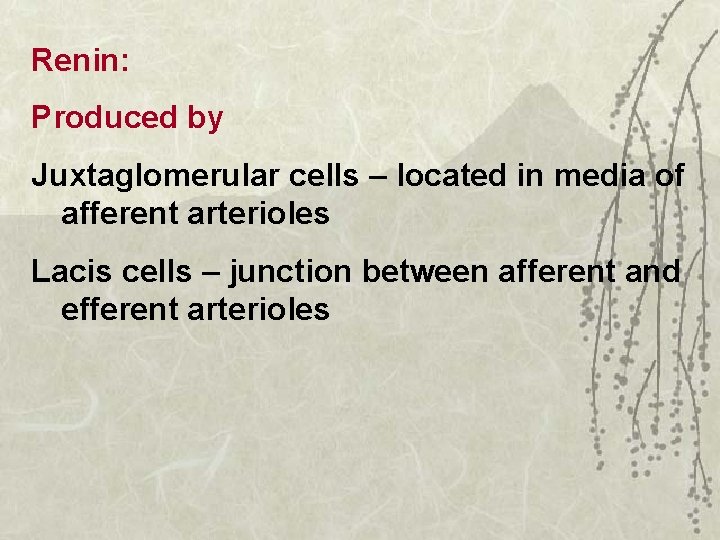
Renin: Produced by Juxtaglomerular cells – located in media of afferent arterioles Lacis cells – junction between afferent and efferent arterioles

Factors affecting renin secretion: Stimulatory Increased sympathetic activity via renal nerves Increased circulating catecholamines Prostaglandins Inhibitory Increased Na+ and Cl- reabsorption in macula densa Angiotensin II Vasopressin

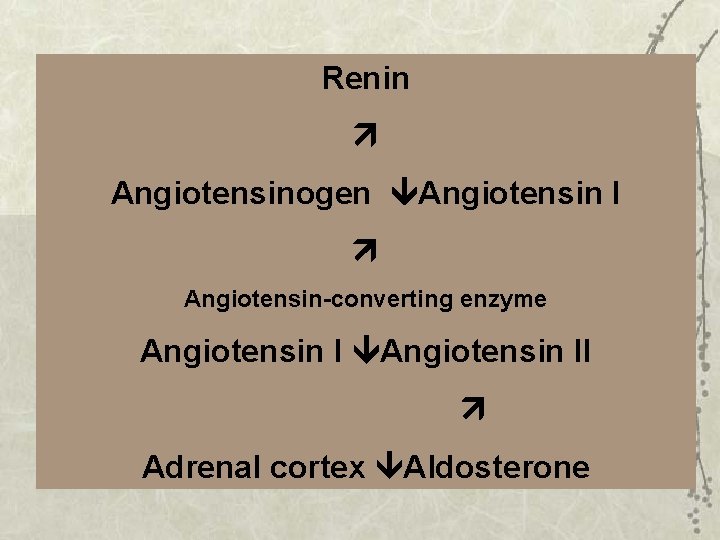
Renin Angiotensinogen Angiotensin I Angiotensin-converting enzyme Angiotensin II Adrenal cortex Aldosterone

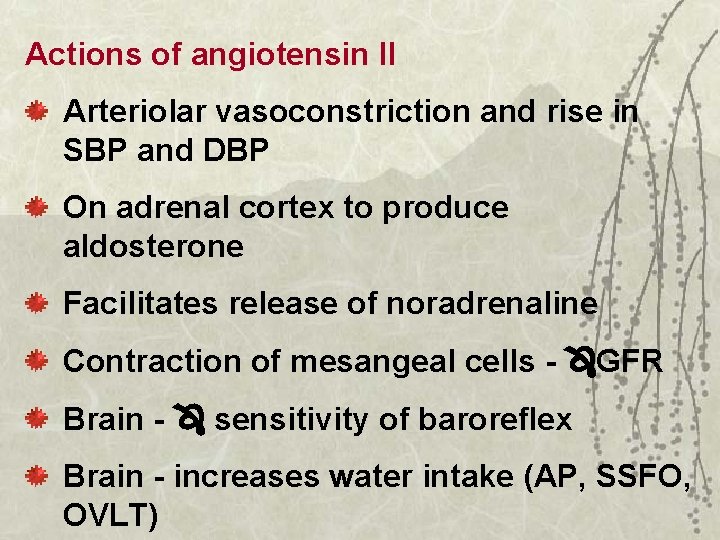
Actions of angiotensin II Arteriolar vasoconstriction and rise in SBP and DBP On adrenal cortex to produce aldosterone Facilitates release of noradrenaline Contraction of mesangeal cells - GFR Brain - sensitivity of baroreflex Brain - increases water intake (AP, SSFO, OVLT)

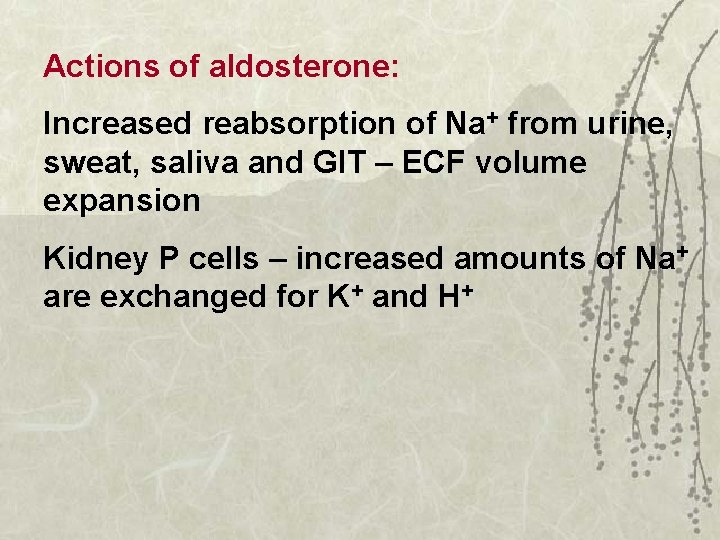
Actions of aldosterone: Increased reabsorption of Na+ from urine, sweat, saliva and GIT – ECF volume expansion Kidney P cells – increased amounts of Na+ are exchanged for K+ and H+

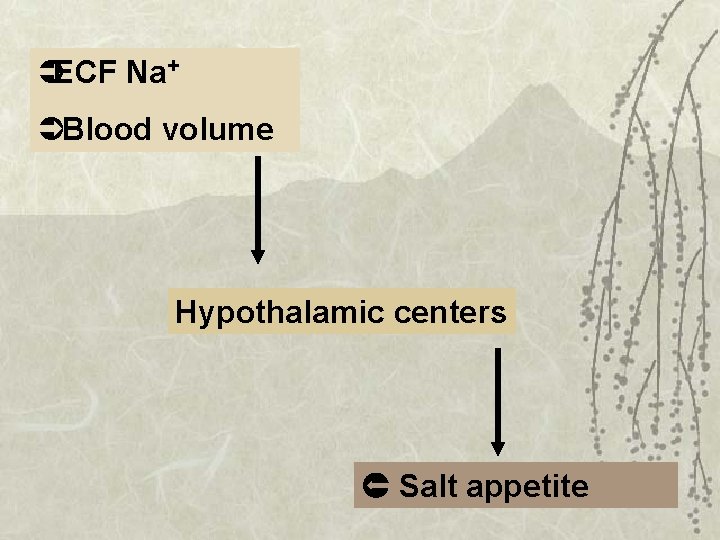
Salt appetite

ÜECF Na+ ÜBlood volume Hypothalamic centers Salt appetite

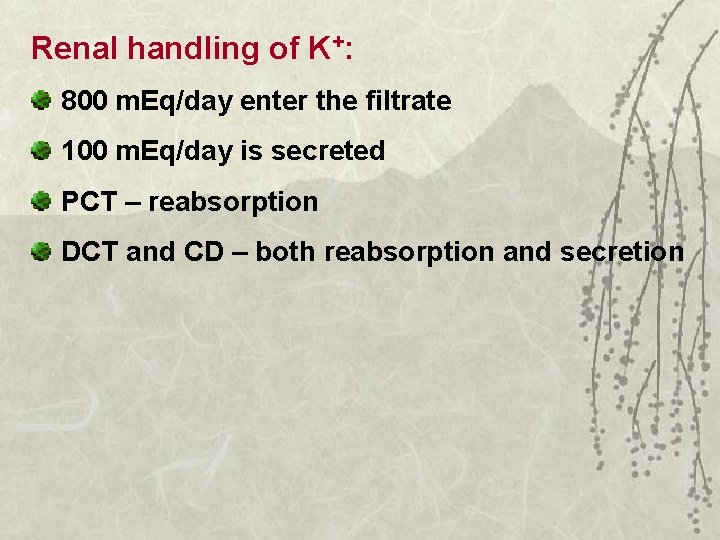
Potassium excretion

Renal handling of K+: 800 m. Eq/day enter the filtrate 100 m. Eq/day is secreted PCT – reabsorption DCT and CD – both reabsorption and secretion

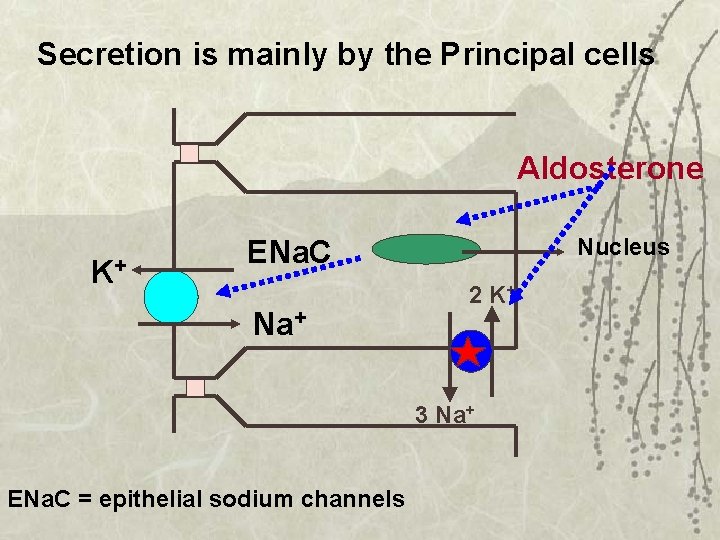
Secretion is mainly by the Principal cells Aldosterone K+ Nucleus ENa. C Na+ 2 K+ 3 Na+ ENa. C = epithelial sodium channels

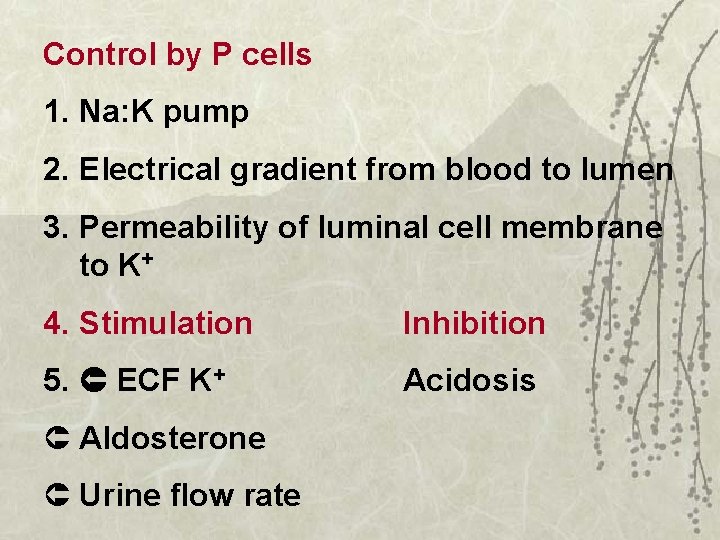
Control by P cells 1. Na: K pump 2. Electrical gradient from blood to lumen 3. Permeability of luminal cell membrane to K+ 4. Stimulation Inhibition 5. ECF K+ Acidosis Û Aldosterone Û Urine flow rate

The End