CLINIC UPDATES Ocular Pharmaceutical Developments Dr Coral Pucci








- Slides: 31

CLINIC UPDATES Ocular Pharmaceutical Developments: Dr. Coral Pucci, UMSL 1/16/2022

Dr. Coral Pucci UMSL Dr. Coral Pucci attended the University of Missouri-St. Louis College of Optometry when she earned her Doctorate of Optometry. She then completed a residency in Primary Care with emphasis in Ocular Disease at the Marion Veterans Administration Hospital in Illinois. Following residency, she returned to UMSL as an Assistant Clinical Professor, where she precepts primary care clinics. Dr. Pucci is the course coordinator for the Pharmacology courses, and also teaches in the ocular disease and binocular vision labs. 1/16/2022 2

FINANCIAL DISCLOSURES No financial interests or affiliations to disclose. All products are reported without bias for the purpose of education only. 1/16/2022

Glaucoma Medications FDA Approvals • Vyzulta • FDA approved November 2017 • Rhopressa • FDA approved December 2017 • Xelpros • FDA approved September 2018 • Rocklatan • 1/16/2022 FDA approved March 2019 4

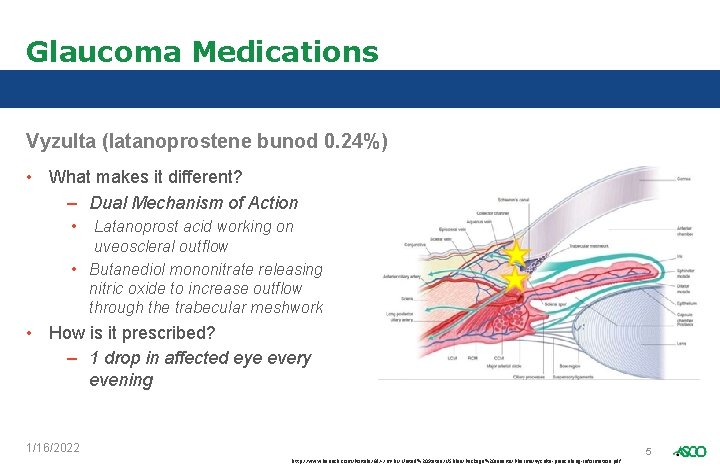
Glaucoma Medications Vyzulta (latanoprostene bunod 0. 24%) • What makes it different? – Dual Mechanism of Action • Latanoprost acid working on uveoscleral outflow • Butanediol mononitrate releasing nitric oxide to increase outflow through the trabecular meshwork • How is it prescribed? – 1 drop in affected eye every evening 1/16/2022 5 http: //www. bausch. com/Portals/69/-/m/BL/United%20 States/USFiles/Package%20 Inserts/Pharma/vyzulta-prescribing-information. pdf

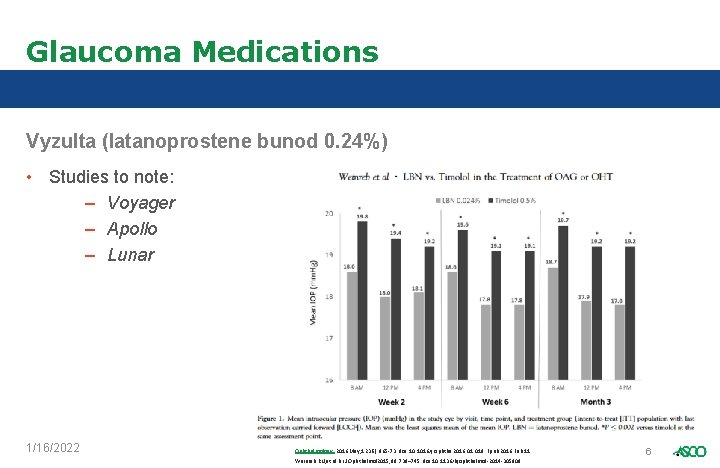
Glaucoma Medications Vyzulta (latanoprostene bunod 0. 24%) • Studies to note: – Voyager – Apollo – Lunar 1/16/2022 Ophthalmology. 2016 May; 123(5): 965 -73. doi: 10. 1016/j. ophtha. 2016. 019. Epub 2016 Feb 11 Weinreb RN, et al. Br J Ophthalmol 2015; 99: 738– 745. doi: 10. 1136/bjophthalmol-2014 -305908 6

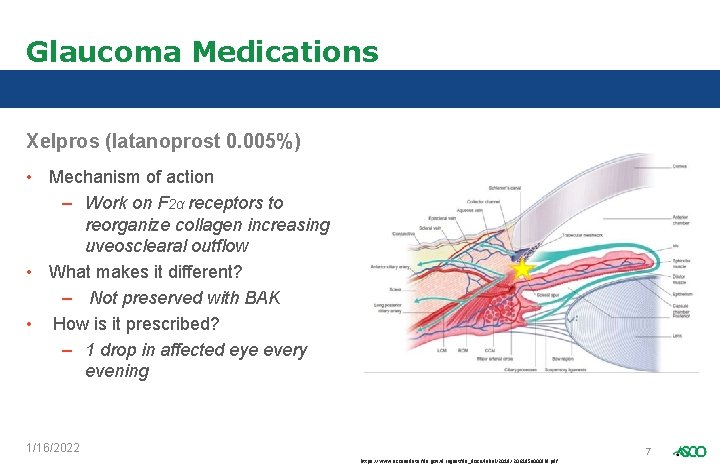
Glaucoma Medications Xelpros (latanoprost 0. 005%) • Mechanism of action – Work on F 2α receptors to reorganize collagen increasing uveosclearal outflow • What makes it different? – Not preserved with BAK • How is it prescribed? – 1 drop in affected eye every evening 1/16/2022 7 https: //www. accessdata. fda. gov/drugsatfda_docs/label/2018/206185 s 000 lbl. pdf

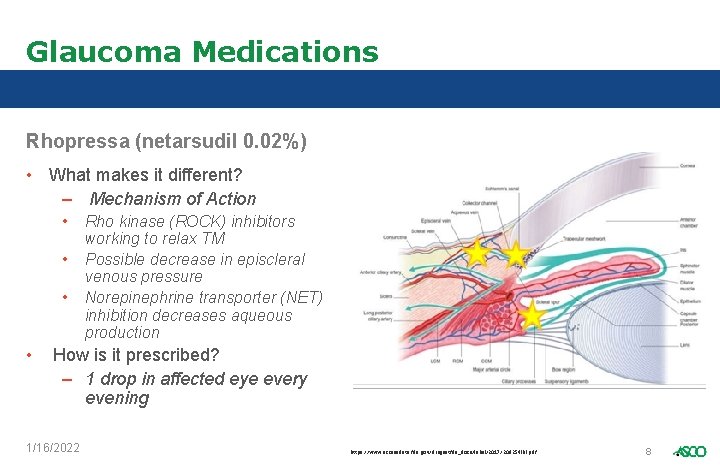
Glaucoma Medications Rhopressa (netarsudil 0. 02%) • What makes it different? – Mechanism of Action • • Rho kinase (ROCK) inhibitors working to relax TM Possible decrease in episcleral venous pressure Norepinephrine transporter (NET) inhibition decreases aqueous production How is it prescribed? – 1 drop in affected eye every evening 1/16/2022 https: //www. accessdata. fda. gov/drugsatfda_docs/label/2017/208254 lbl. pdf 8

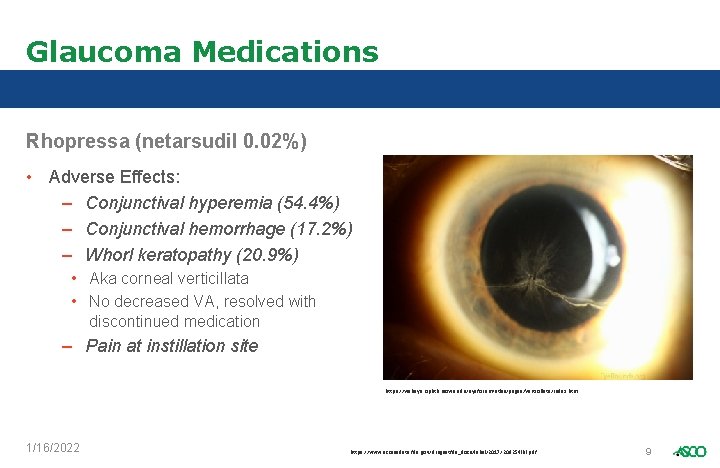
Glaucoma Medications Rhopressa (netarsudil 0. 02%) • Adverse Effects: – Conjunctival hyperemia (54. 4%) – Conjunctival hemorrhage (17. 2%) – Whorl keratopathy (20. 9%) • Aka corneal verticillata • No decreased VA, resolved with discontinued medication – Pain at instillation site https: //webeye. ophth. uiowa. edu/eyeforum/atlas/pages/Verticillata/index. htm 1/16/2022 https: //www. accessdata. fda. gov/drugsatfda_docs/label/2017/208254 lbl. pdf 9

Glaucoma Medications Rocklatan (netarsudil 0. 02% and latanoprost 0. 005%) • What makes it different? – First combination drop to include new glaucoma medications 1/16/2022 10

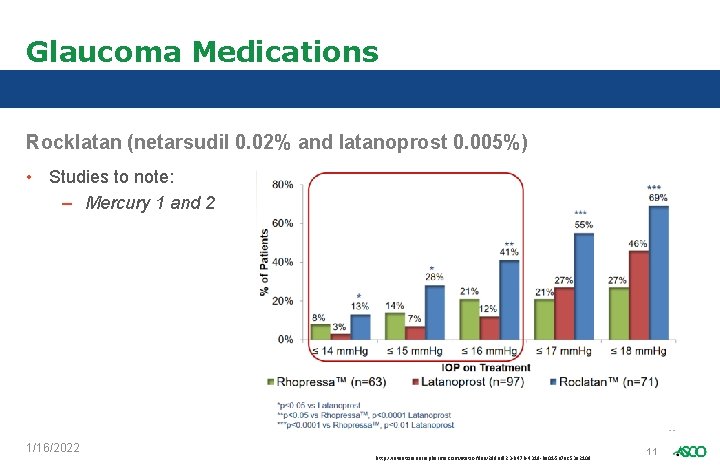
Glaucoma Medications Rocklatan (netarsudil 0. 02% and latanoprost 0. 005%) • Studies to note: – Mercury 1 and 2 1/16/2022 http: //investors. aeriepharma. com/static-files/29 defd 23 -b 47 b-4319 -ba 01 -5 a 7 ac 53 e 2108 11

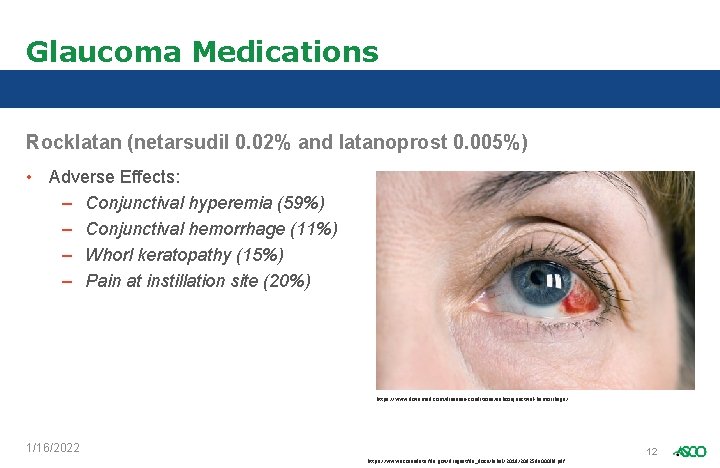
Glaucoma Medications Rocklatan (netarsudil 0. 02% and latanoprost 0. 005%) • Adverse Effects: – Conjunctival hyperemia (59%) – Conjunctival hemorrhage (11%) – Whorl keratopathy (15%) – Pain at instillation site (20%) https: //www. dovemed. com/diseases-conditions/subconjunctival-hemorrhage/ 1/16/2022 https: //www. accessdata. fda. gov/drugsatfda_docs/label/2019/208259 s 000 lbl. pdf 12

Antivirals FDA Approvals • Avaclyr • FDA approved March 2019 http: //medilinks. blogspot. com/2012/01/photos-for-herpes-simplex-keratitis-hsk. html 1/16/2022 13

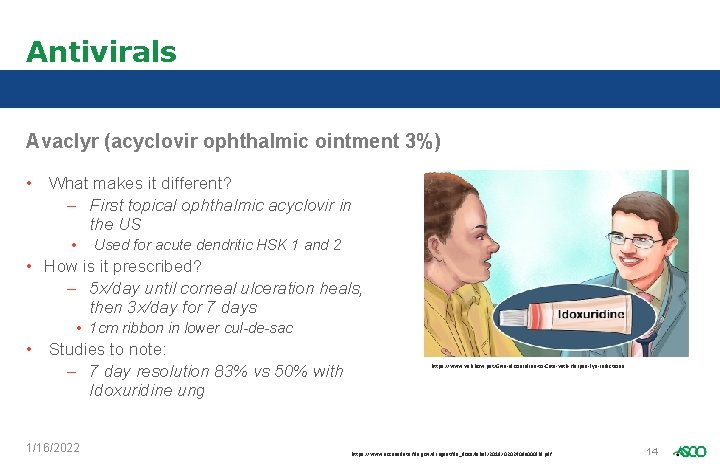
Antivirals Avaclyr (acyclovir ophthalmic ointment 3%) • What makes it different? – First topical ophthalmic acyclovir in the US • Used for acute dendritic HSK 1 and 2 • How is it prescribed? – 5 x/day until corneal ulceration heals, then 3 x/day for 7 days • 1 cm ribbon in lower cul-de-sac • Studies to note: – 7 day resolution 83% vs 50% with Idoxuridine ung 1/16/2022 https: //www. wikihow. pet/Give-Idoxuridine-to-Cats-with-Herpes-Eye-Infections https: //www. accessdata. fda. gov/drugsatfda_docs/label/2019/0202408 s 000 lbl. pdf 14

Steroids FDA Approvals • Inveltys • FDA approved August 2018 • Dextenza • FDA approved December 2018 • Lotemax SM • FDA approved February 2019 • Generic Loteprednol • 1/16/2022 FDA approved April 2019 15

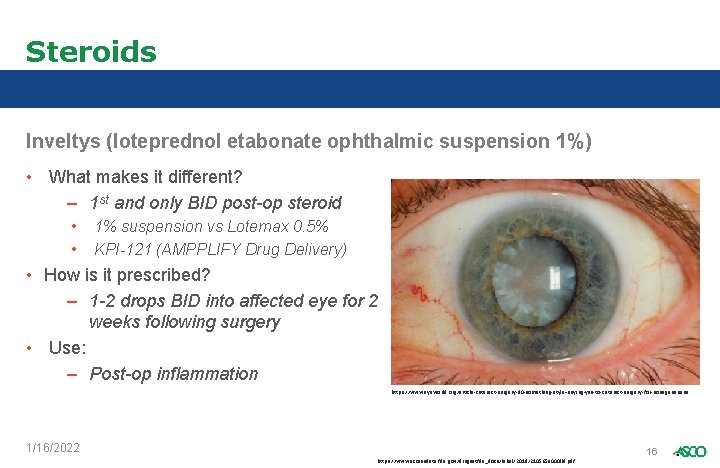
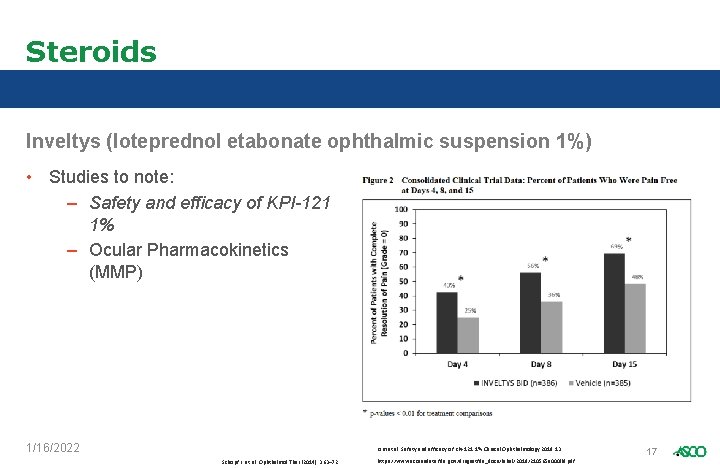
Steroids Inveltys (loteprednol etabonate ophthalmic suspension 1%) • What makes it different? – 1 st and only BID post-op steroid • • 1% suspension vs Lotemax 0. 5% KPI-121 (AMPPLIFY Drug Delivery) • How is it prescribed? – 1 -2 drops BID into affected eye for 2 weeks following surgery • Use: – Post-op inflammation https: //www. eyeworld. org/article-cataract-surgery-90 -something-style--saying-yes-to-cataract-surgery-for-nonagenarians 1/16/2022 16 https: //www. accessdata. fda. gov/drugsatfda_docs/label/2018/210565 s 000 lbl. pdf

Steroids Inveltys (loteprednol etabonate ophthalmic suspension 1%) • Studies to note: – Safety and efficacy of KPI-121 1% – Ocular Pharmacokinetics (MMP) 1/16/2022 Kim et al. Safety and efficacy of KPI-121 1%. Clinical Ophthalmology 2019: 13 Schopf L. et al. Ophthalmol Ther (2014) 3: 63– 72 https: //www. accessdata. fda. gov/drugsatfda_docs/label/2018/210565 s 000 lbl. pdf 17

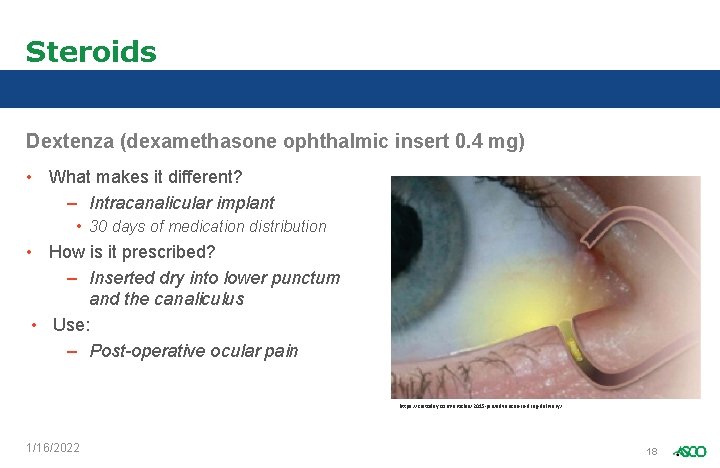
Steroids Dextenza (dexamethasone ophthalmic insert 0. 4 mg) • What makes it different? – Intracanalicular implant • 30 days of medication distribution • How is it prescribed? – Inserted dry into lower punctum and the canaliculus • Use: – Post-operative ocular pain https: //crstoday. com/articles/2015 -jan/advances-in-drug-delivery/ 1/16/2022 18

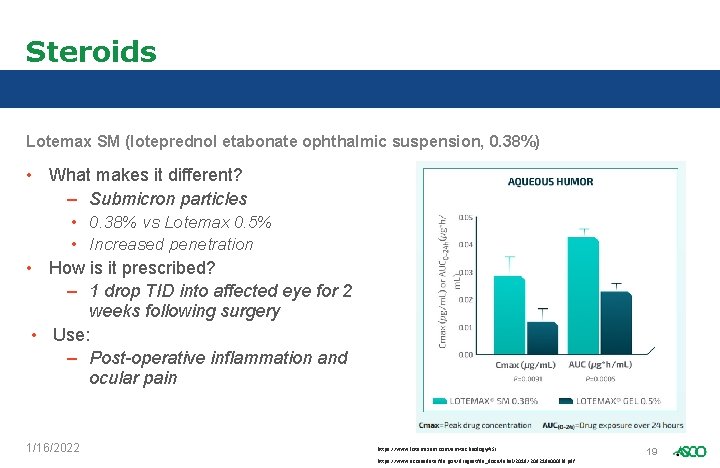
Steroids Lotemax SM (loteprednol etabonate ophthalmic suspension, 0. 38%) • What makes it different? – Submicron particles • 0. 38% vs Lotemax 0. 5% • Increased penetration • How is it prescribed? – 1 drop TID into affected eye for 2 weeks following surgery • Use: – Post-operative inflammation and ocular pain 1/16/2022 https: //www. lotemaxsm. com/sm-technology#ISI https: //www. accessdata. fda. gov/drugsatfda_docs/label/2019/208219 s 000 lbl. pdf 19

Steroids Generic loteprednol (loteprednol etabonate ophthalmic suspension, 0. 5%) • What makes it different? – First approved generic • How is it prescribed? – 1 drop QID in affected eye • Use: – Post-operative inflammation and ocular pain – Inflammation of conjunctiva and anterior segment 1/16/2022 20

Dry Eye Medications FDA Approvals • Cequa • FDA approved August 2018 • Lumify • 1/16/2022 FDA approved December 2017 21

Dry Eye Medications Cequa (cyclosporine ophthalmic solution 0. 09%) • What makes it different? – Nanomicellar technology – 0. 09% vs Restasis 0. 05% cyclosporine • How is it prescribed? – 1 drop BID into each eye 12 hours apart • Use: – To increase tear production https: //www. nytimes. com/2017/07/31/well/no-crying-over-dry-eyes. html 1/16/2022 22 https: //www. accessdata. fda. gov/drugsatfda_docs/label/2018/210913 s 000 lbl. pdf

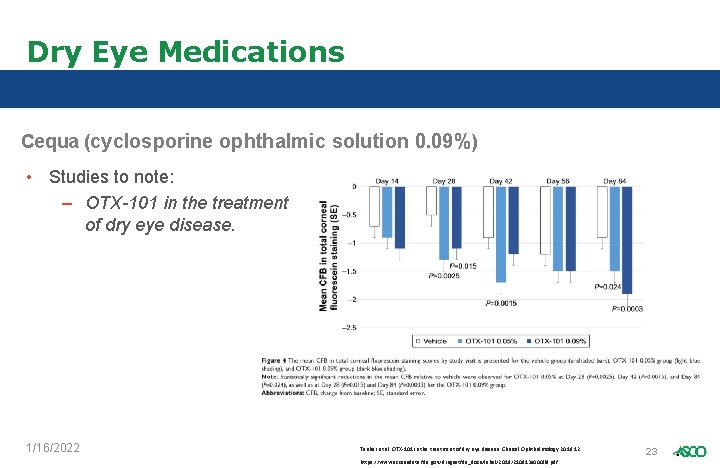
Dry Eye Medications Cequa (cyclosporine ophthalmic solution 0. 09%) • Studies to note: – OTX-101 in the treatment of dry eye disease. 1/16/2022 Tauber et al. OTX-101 in the treatment of dry eye disease. Clinical Ophthalmology 2018: 12 https: //www. accessdata. fda. gov/drugsatfda_docs/label/2018/210913 s 000 lbl. pdf 23

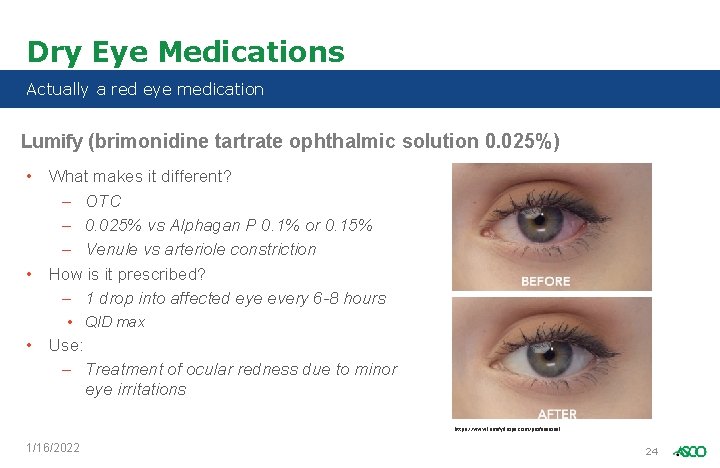
Dry Eye Medications Actually a red eye medication Lumify (brimonidine tartrate ophthalmic solution 0. 025%) • What makes it different? – OTC – 0. 025% vs Alphagan P 0. 1% or 0. 15% – Venule vs arteriole constriction • How is it prescribed? – 1 drop into affected eye every 6 -8 hours • QID max • Use: – Treatment of ocular redness due to minor eye irritations https: //www. lumifydrops. com/professional 1/16/2022 24

Allergy Medications FDA Approvals • Zerviate • 1/16/2022 FDA approved May 2017 25

Allergy Medications Zerviate (cetirizine ophthalmic solution 0. 24%) • What makes it different? – First topical ophthalmic cetirizine • How is it prescribed? – 1 drop BID into each eye • Separated by 8 hours • Use: – Ocular itching associated with allergic conjunctivitis 1/16/2022 26 https: //www. accessdata. fda. gov/drugsatfda_docs/label/2017/208694 s 000 lbl. pdf

Other Medications FDA Approvals • Oxervate • 1/16/2022 FDA approved August 2018 27

Other Medications Oxervate (cenegermin-bkbj ophthalmic solution 0. 002%) • What makes it different? – First medication for neurotrophic keratitis • Use of recombinant human nerve growth factor • How is it prescribed? – 1 drop 6 x/day into affected eye • One 8 week treatment course • Use: – Neurotrophic keratitis 1/16/2022 28 https: //www. accessdata. fda. gov/drugsatfda_docs/label/2018/761094 s 000 lbl. pdf

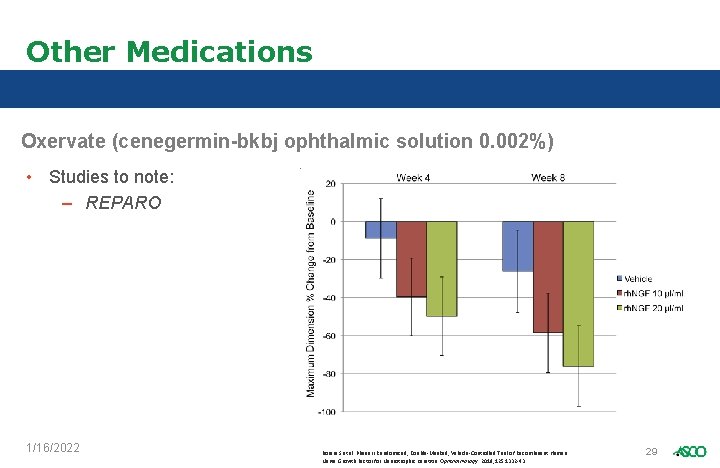
Other Medications Oxervate (cenegermin-bkbj ophthalmic solution 0. 002%) • Studies to note: – REPARO 1/16/2022 Bonini S et al. Phase II Randomized, Double-Masked, Vehicle-Controlled Trial of Recombinant Human Nerve Growth Factor for Neurotrophic Keratitis. Ophthalmology. 2018; 125: 1332 -43. 29

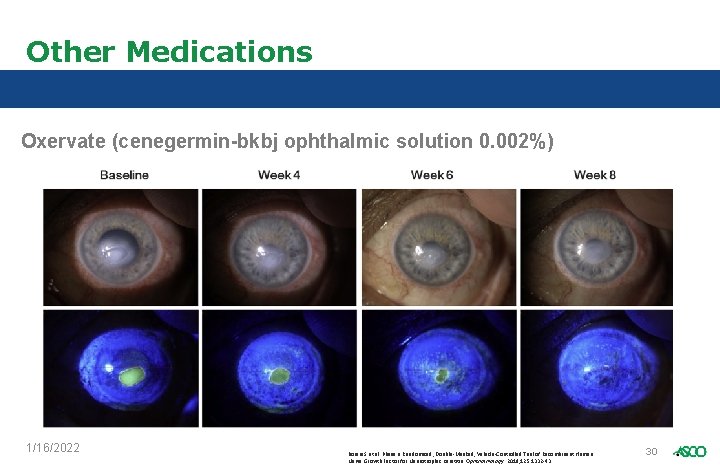
Other Medications Oxervate (cenegermin-bkbj ophthalmic solution 0. 002%) 1/16/2022 Bonini S et al. Phase II Randomized, Double-Masked, Vehicle-Controlled Trial of Recombinant Human Nerve Growth Factor for Neurotrophic Keratitis. Ophthalmology. 2018; 125: 1332 -43. 30

Thank you The future of ophthalmic pharmaceuticals looks bright! Email: Puccic@umsl. edu 1/16/2022 31