Updates From NOTION The First AllComer TAVR Trial

- Slides: 22

Updates From NOTION: The First All-Comer TAVR Trial Lars Sondergaard, MD, DMSc Professor of Cardiology Rigshospitalet Copenhagen, Denmark

Disclosure Statement of Financial Interest Within the past 12 months, I or my spouse/partner have had a financial interest/arrangement or affiliation with the organization(s) listed below. Affiliation/Financial Relationship • • Grant/Research Support Consulting Fees/Honoraria Company • • BSci, SJM, Symetis BSci, Medtronic, SJM, Symetis

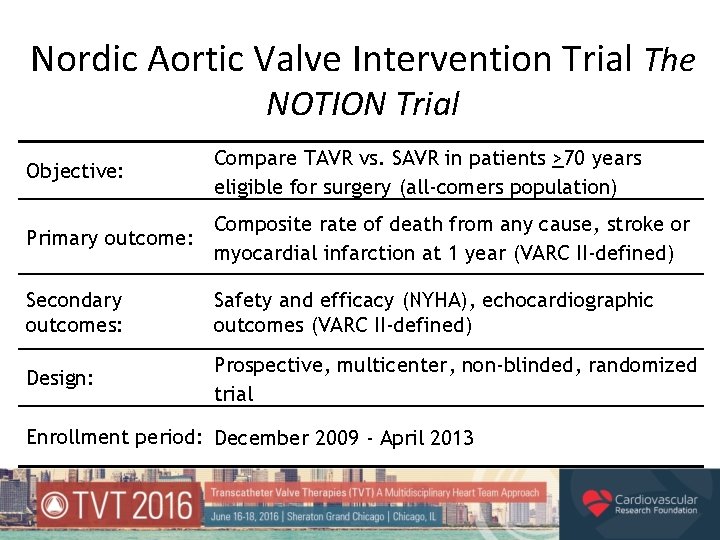
Nordic Aortic Valve Intervention Trial The NOTION Trial Objective: Compare TAVR vs. SAVR in patients >70 years eligible for surgery (all-comers population) Composite rate of death from any cause, stroke or Primary outcome: myocardial infarction at 1 year (VARC II-defined) Secondary outcomes: Safety and efficacy (NYHA), echocardiographic outcomes (VARC II-defined) Design: Prospective, multicenter, non-blinded, randomized trial Enrollment period: December 2009 - April 2013

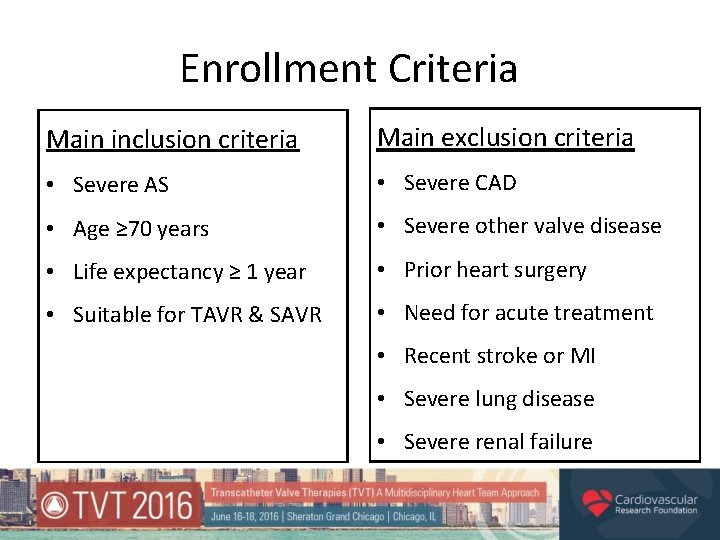
Enrollment Criteria Main inclusion criteria Main exclusion criteria • Severe AS • Severe CAD • Age ≥ 70 years • Severe other valve disease • Life expectancy ≥ 1 year • Prior heart surgery • Suitable for TAVR & SAVR • Need for acute treatment • Recent stroke or MI • Severe lung disease • Severe renal failure

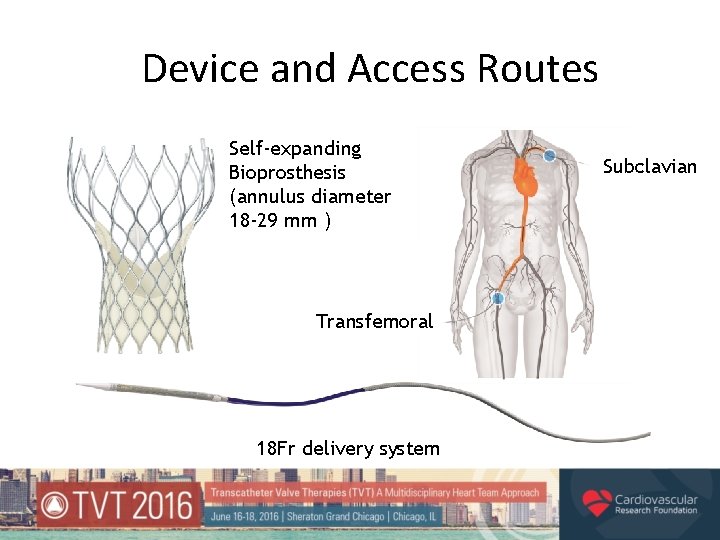
Device and Access Routes Self-expanding Bioprosthesis (annulus diameter 18 -29 mm ) Transfemoral 18 Fr delivery system Subclavian

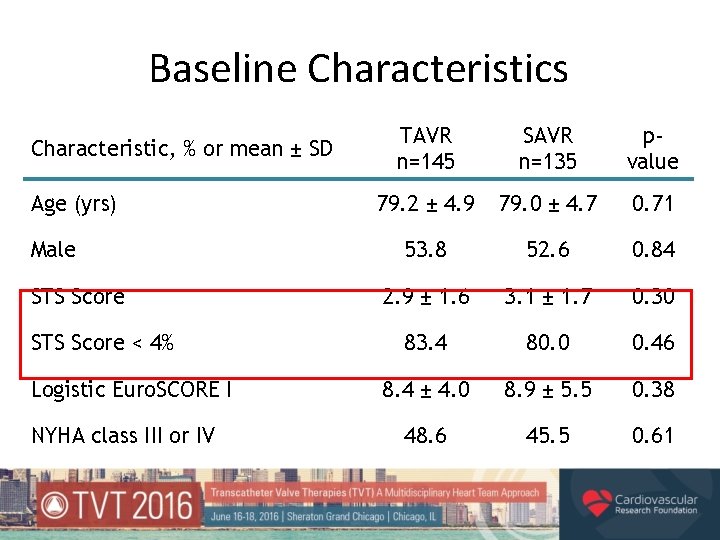
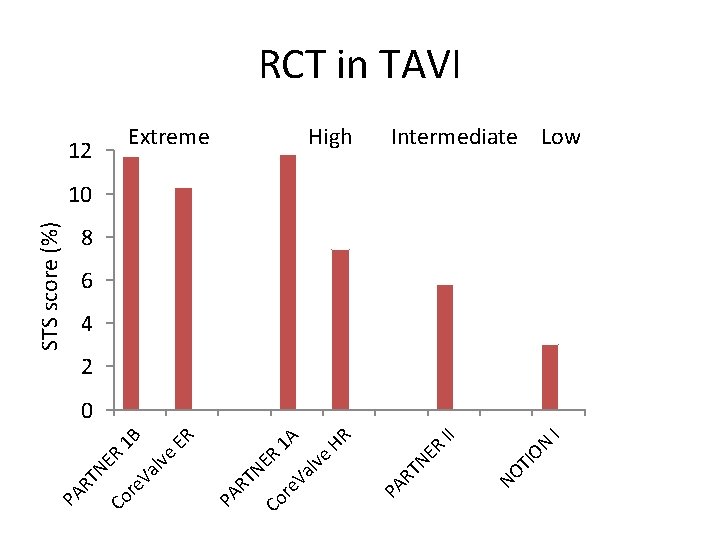
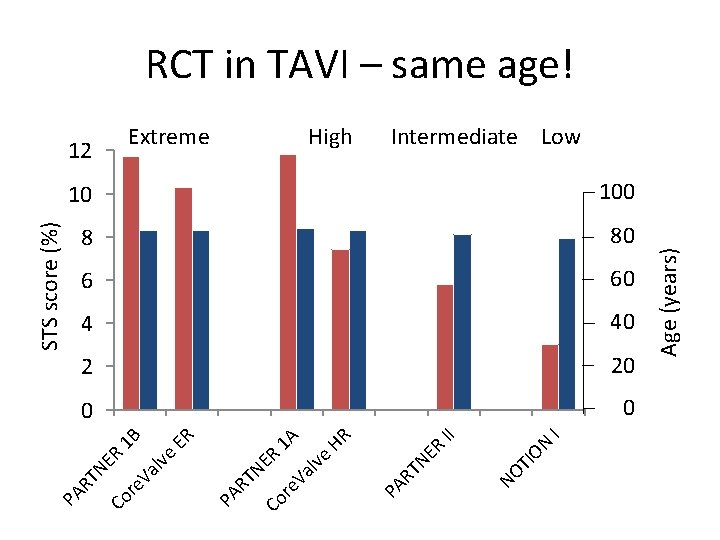
Baseline Characteristics Characteristic, % or mean ± SD Age (yrs) Male STS Score < 4% Logistic Euro. SCORE I NYHA class III or IV TAVR n=145 SAVR n=135 pvalue 79. 2 ± 4. 9 79. 0 ± 4. 7 0. 71 53. 8 52. 6 0. 84 2. 9 ± 1. 6 3. 1 ± 1. 7 0. 30 83. 4 80. 0 0. 46 8. 4 ± 4. 0 8. 9 ± 5. 5 0. 38 48. 6 45. 5 0. 61

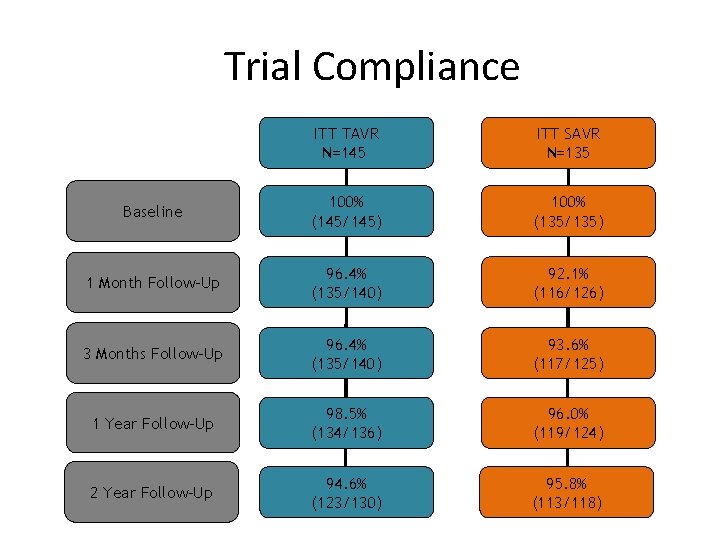
Trial Compliance ITT TAVR N=145 ITT SAVR N=135 Baseline 100% (145/145) 100% (135/135) 1 Month Follow-Up 96. 4% (135/140) 92. 1% (116/126) 3 Months Follow-Up 96. 4% (135/140) 93. 6% (117/125) 1 Year Follow-Up 98. 5% (134/136) 96. 0% (119/124) 2 Year Follow-Up 94. 6% (123/130) 95. 8% (113/118)

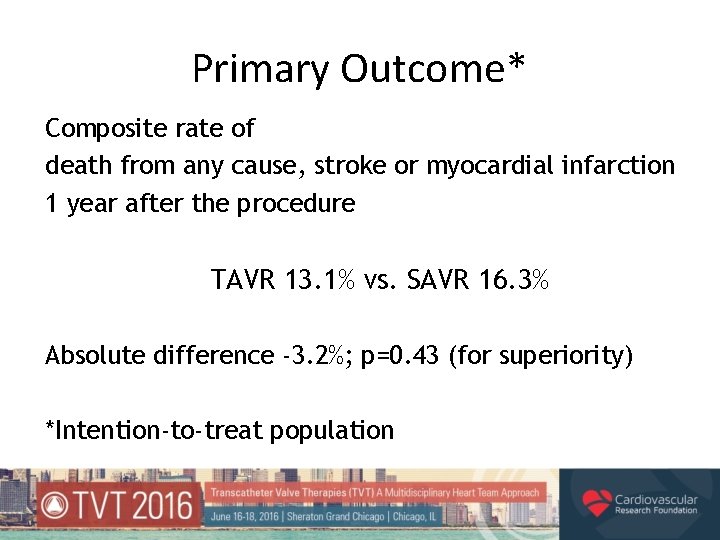
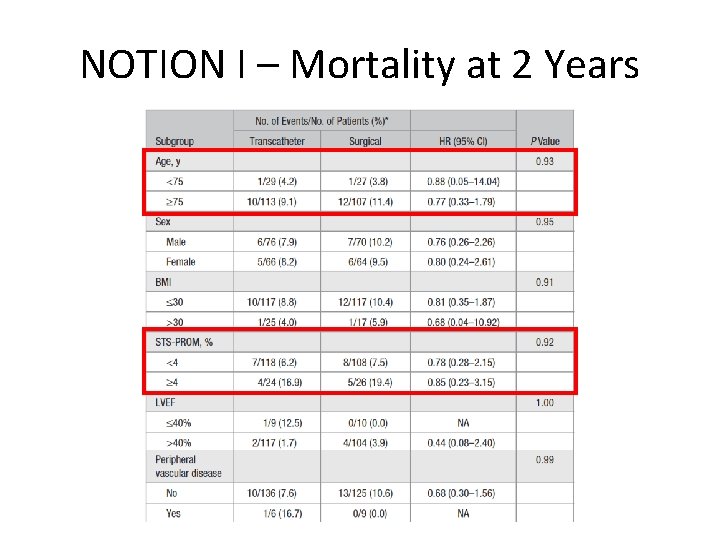
Primary Outcome* Composite rate of death from any cause, stroke or myocardial infarction 1 year after the procedure TAVR 13. 1% vs. SAVR 16. 3% Absolute difference -3. 2%; p=0. 43 (for superiority) *Intention-to-treat population

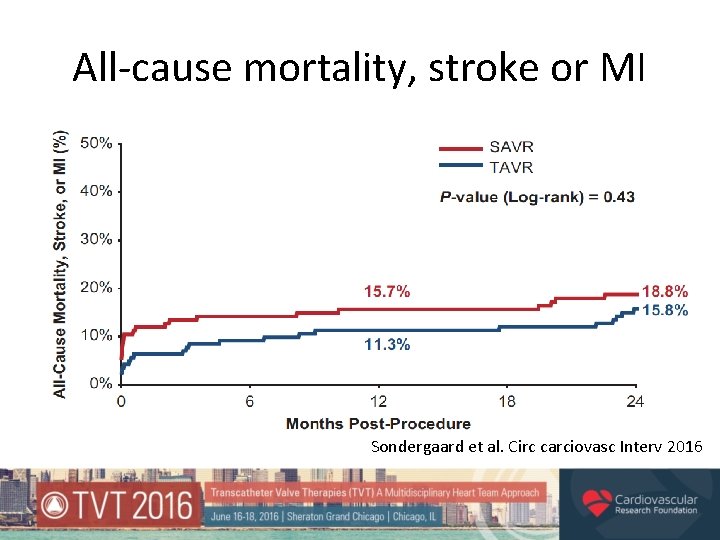
All-cause mortality, stroke or MI Sondergaard et al. Circ carciovasc Interv 2016

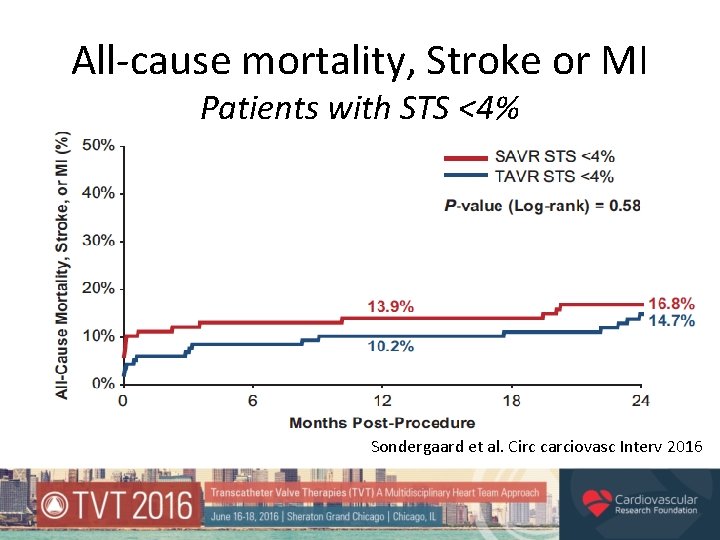
All-cause mortality, Stroke or MI Patients with STS <4% Sondergaard et al. Circ carciovasc Interv 2016

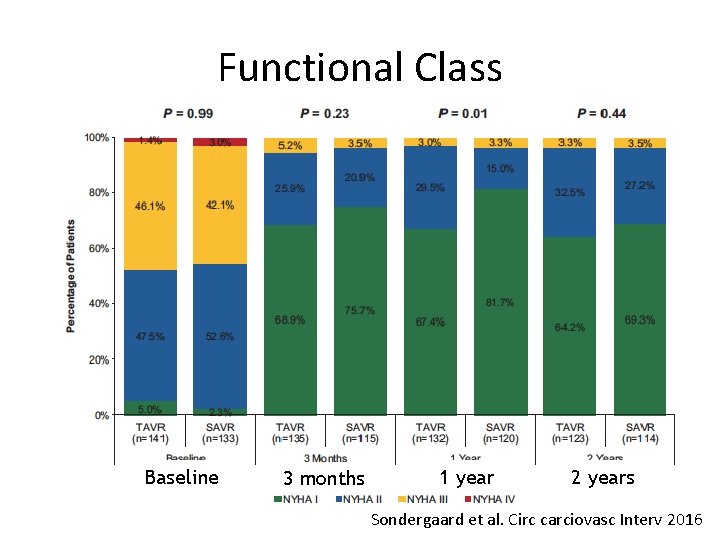
Functional Class Baseline 3 months 1 year 2 years Sondergaard et al. Circ carciovasc Interv 2016

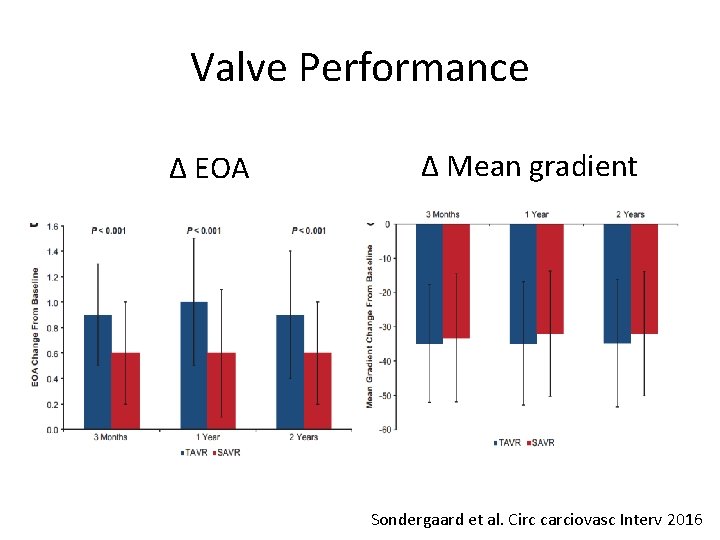
Valve Performance Δ EOA Δ Mean gradient Sondergaard et al. Circ carciovasc Interv 2016

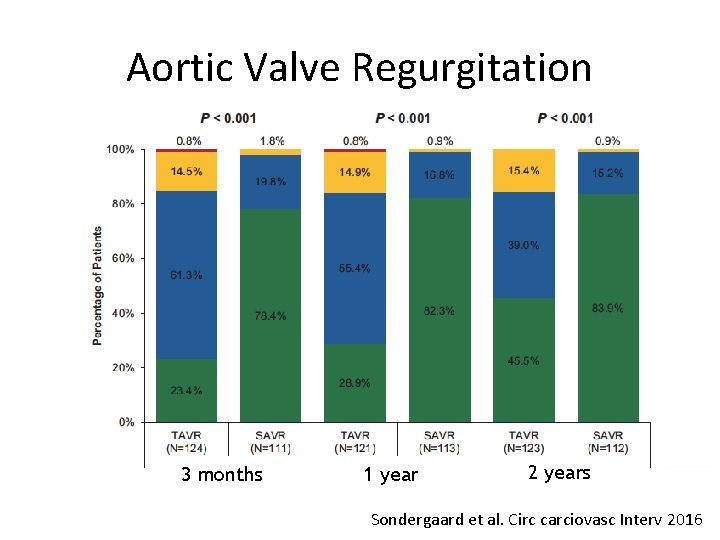
Aortic Valve Regurgitation 3 months 1 year 2 years Sondergaard et al. Circ carciovasc Interv 2016

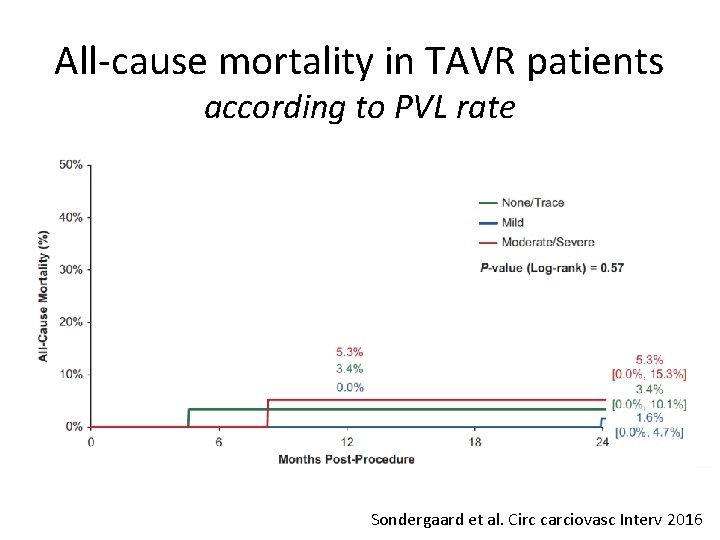
All-cause mortality in TAVR patients according to PVL rate Sondergaard et al. Circ carciovasc Interv 2016

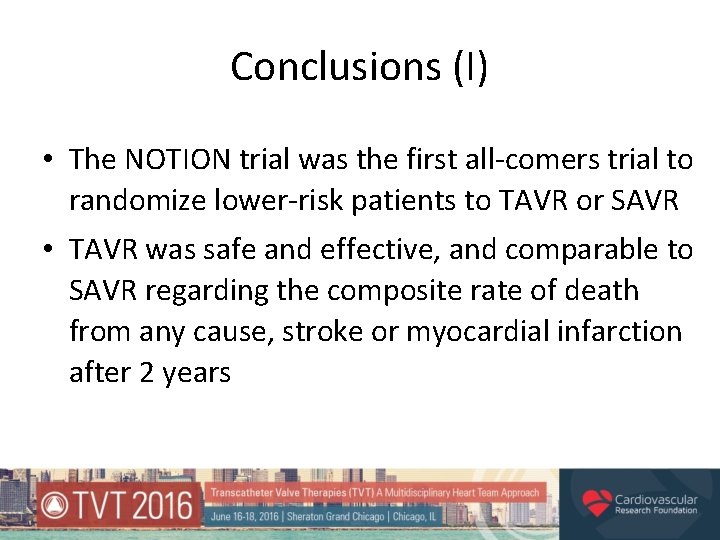
Conclusions (I) • The NOTION trial was the first all-comers trial to randomize lower-risk patients to TAVR or SAVR • TAVR was safe and effective, and comparable to SAVR regarding the composite rate of death from any cause, stroke or myocardial infarction after 2 years

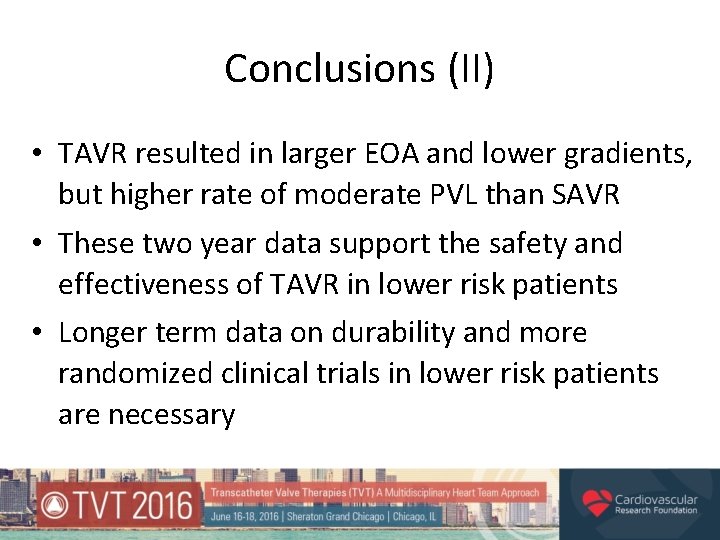
Conclusions (II) • TAVR resulted in larger EOA and lower gradients, but higher rate of moderate PVL than SAVR • These two year data support the safety and effectiveness of TAVR in lower risk patients • Longer term data on durability and more randomized clinical trials in lower risk patients are necessary

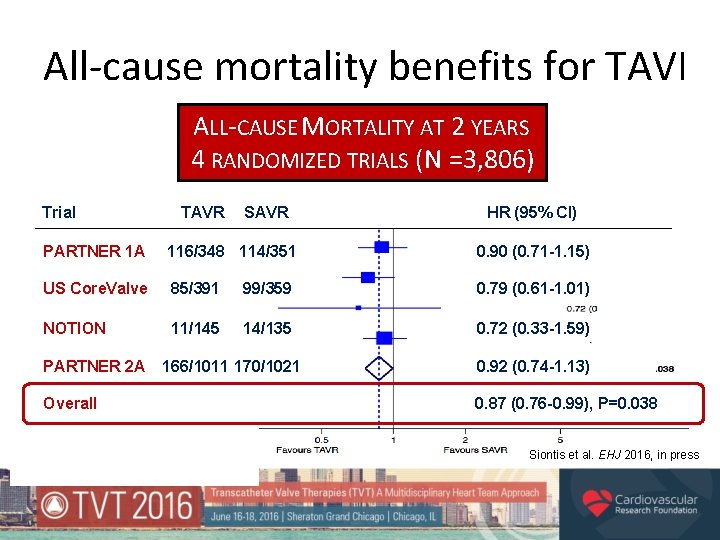
All-cause mortality benefits for TAVI ALL-CAUSE MORTALITY AT 2 YEARS 4 RANDOMIZED TRIALS (N =3, 806) Trial TAVR SAVR HR (95% CI) PARTNER 1 A 116/348 114/351 0. 90 (0. 71 -1. 15) US Core. Valve 85/391 99/359 0. 79 (0. 61 -1. 01) NOTION 11/145 14/135 0. 72 (0. 33 -1. 59) PARTNER 2 A Overall 166/1011 170/1021 0. 92 (0. 74 -1. 13) 0. 87 (0. 76 -0. 99), P=0. 038 Siontis et al. EHJ 2016, in press

TI NO I II ON ER High TN HR 1 A ER 1 B Extreme PA R ER lve Va re Co TN Va lve ER STS score (%) 12 PA R re Co TN PA R RCT in TAVI Intermediate Low 10 8 6 4 2 0

TI NO I II ON ER 10 100 8 80 6 60 4 40 2 20 0 0 Age (years) High TN HR 1 A ER 1 B Extreme PA R ER lve Va re Co TN Va lve ER STS score (%) 12 PA R re Co TN PA R RCT in TAVI – same age! Intermediate Low

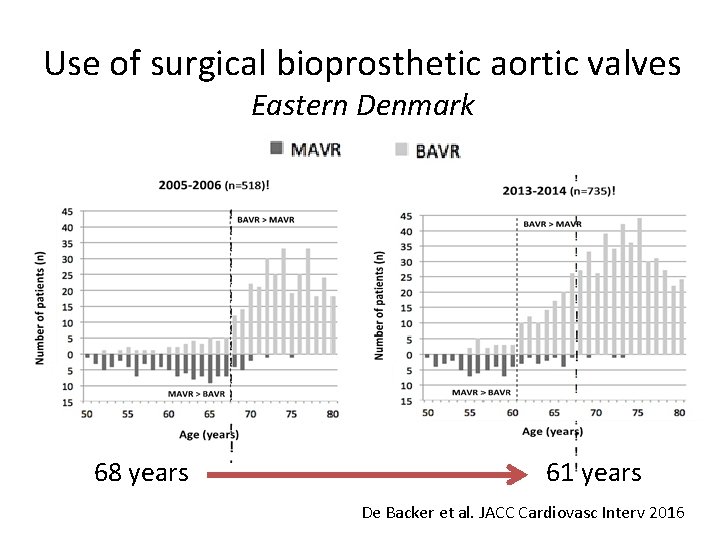
Use of surgical bioprosthetic aortic valves Eastern Denmark 68 years 61 years De Backer et al. JACC Cardiovasc Interv 2016

NOTION I – Mortality at 2 Years

NOTION II Study design Inclusion criteria • Severe symptomatic aortic stenosis • Age ≤ 75 years & STS score ≤ 4% • Anticipated usage of aortic bioprosthesis Primary end-point • Composite rate of all-cause mortality, stroke & MI at 1 year Design • RCT, 1: 1, TF TAVI vs. SAVR, superior, N=992 • Bicuspid valves & revascularization (CABG or PCI) allowed • Any aortic bioprosthesis allowed