Clash Of The Titans Convergence Of Tau And





- Slides: 34

Clash Of The Titans! Convergence Of Tau And Alpha-Synuclein Amyloid In Neurodegenerative Diseases John Q. Trojanowski, M. D. , Ph. D. Institute on Aging Center for Neurodegenerative Disease Research Department of Pathology and Laboratory Medicine University of Pennsylvania Philadelphia, PA

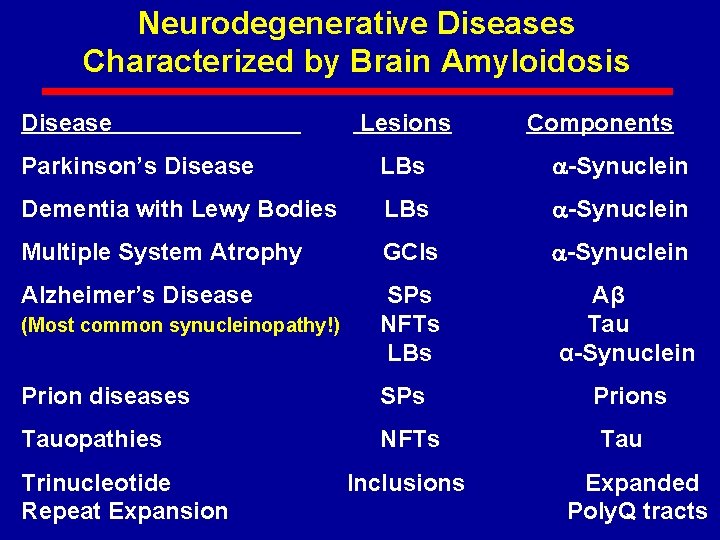
Neurodegenerative Diseases Characterized by Brain Amyloidosis Disease Lesions Components Parkinson’s Disease LBs -Synuclein Dementia with Lewy Bodies LBs -Synuclein Multiple System Atrophy GCIs -Synuclein Alzheimer’s Disease (Most common synucleinopathy!) SPs NFTs LBs Aβ Tau α-Synuclein Prion diseases SPs Prions Tauopathies NFTs Trinucleotide Repeat Expansion Inclusions Tau Expanded Poly. Q tracts

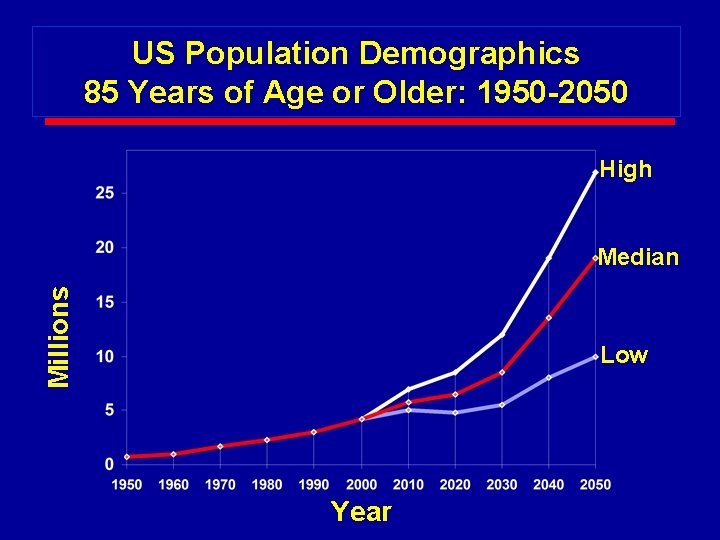
US Population Demographics 85 Years of Age or Older: 1950 -2050 High Millions Median Low Year

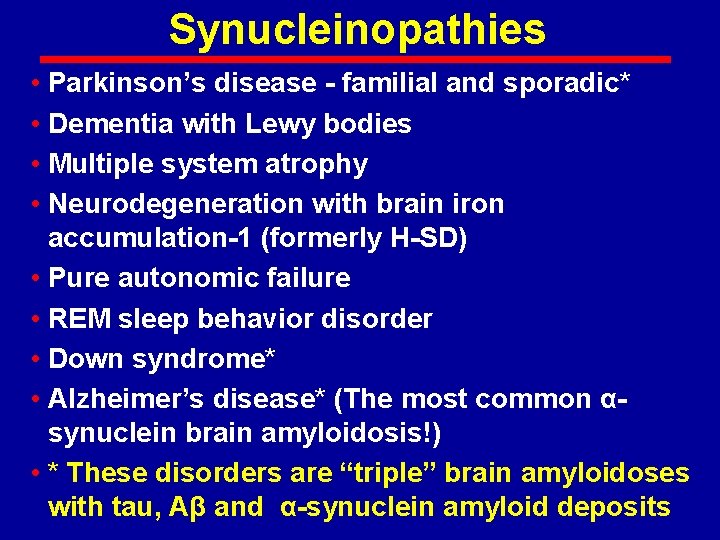
Synucleinopathies • Parkinson’s disease - familial and sporadic* • Dementia with Lewy bodies • Multiple system atrophy • Neurodegeneration with brain iron accumulation-1 (formerly H-SD) • Pure autonomic failure • REM sleep behavior disorder • Down syndrome* • Alzheimer’s disease* (The most common αsynuclein brain amyloidosis!) • * These disorders are “triple” brain amyloidoses with tau, Aβ and α-synuclein amyloid deposits

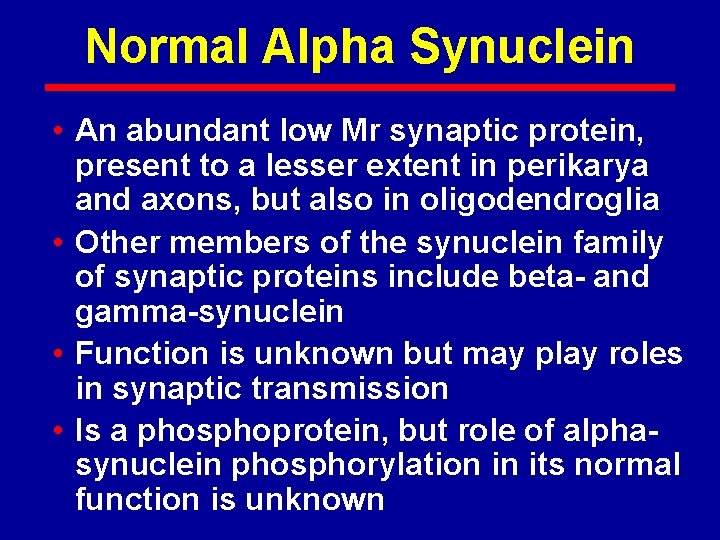
Normal Alpha Synuclein • An abundant low Mr synaptic protein, present to a lesser extent in perikarya and axons, but also in oligodendroglia • Other members of the synuclein family of synaptic proteins include beta- and gamma-synuclein • Function is unknown but may play roles in synaptic transmission • Is a phosphoprotein, but role of alphasynuclein phosphorylation in its normal function is unknown

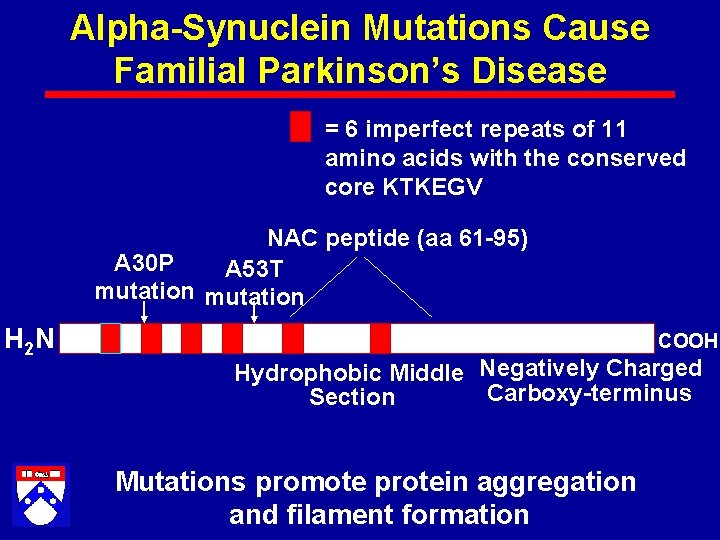
Alpha-Synuclein Mutations Cause Familial Parkinson’s Disease = 6 imperfect repeats of 11 amino acids with the conserved core KTKEGV NAC peptide (aa 61 -95) A 30 P A 53 T mutation H 2 N COOH Hydrophobic Middle Negatively Charged Carboxy-terminus Section Mutations promote protein aggregation and filament formation

Pathological Alpha-Synuclein • Forms insoluble filamentous aggregates with the properties of amyloid • Amino acids 71 -82 in the NAC domain are the minimal, essential sequences required for fibrilization • Filamentous alpha-synuclein inclusions form in neuronal perikarya, processes and in glia. I cells • Is abnormally phosphorylated, nitrated and ubiquitinated

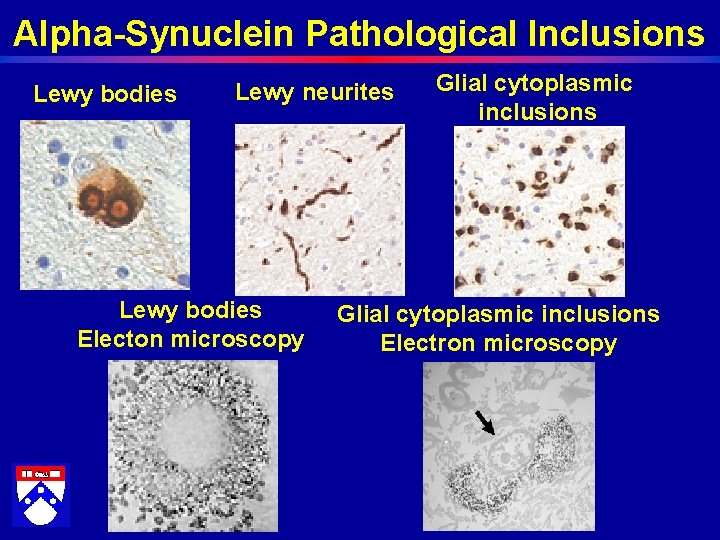
Alpha-Synuclein Pathological Inclusions Lewy bodies Lewy neurites Lewy bodies Electon microscopy Glial cytoplasmic inclusions Electron microscopy


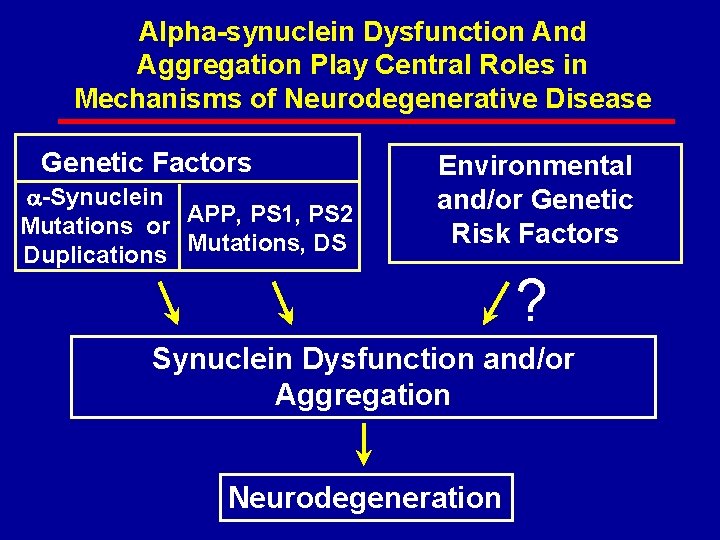
Alpha-synuclein Dysfunction And Aggregation Play Central Roles in Mechanisms of Neurodegenerative Disease Genetic Factors -Synuclein Mutations or APP, PS 1, PS 2 Duplications Mutations, DS Environmental and/or Genetic Risk Factors ? Synuclein Dysfunction and/or Aggregation Neurodegeneration

But hold on! Tau and Alpha-Synuclein Pathology Commonly Co-occurr in Neurodegenerative Diseases – So what does this mean? • Diseases with tau, α-synuclein and abundant A deposits – Lewy body variant of Alzheimer’s disease – Familial Alzheimer’s disease – Down’s syndrome • Diseases with tau, α-synuclein and scant/no A deposits – Parkinson’s disease/Dementia with Lewy bodies – Multiple system atrophy – Guam ALS/PDC – Neurodegeneration with brain iron accumulation type 1 – Some cases of PSP, CBD, and Pick’s disease

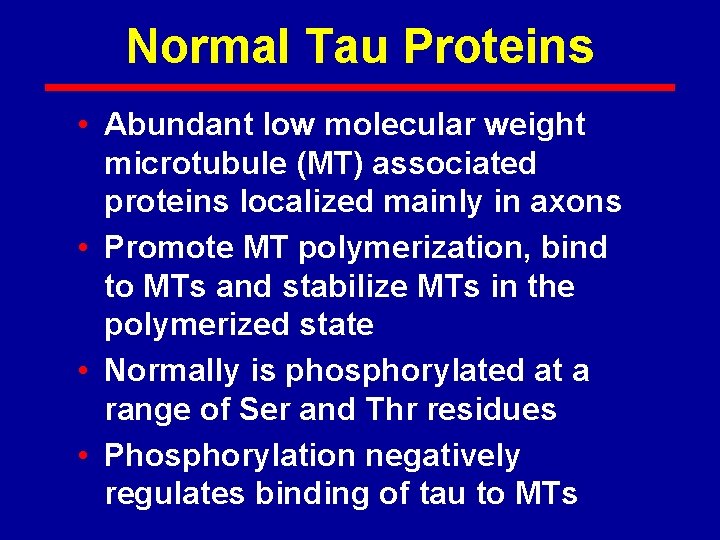
Normal Tau Proteins • Abundant low molecular weight microtubule (MT) associated proteins localized mainly in axons • Promote MT polymerization, bind to MTs and stabilize MTs in the polymerized state • Normally is phosphorylated at a range of Ser and Thr residues • Phosphorylation negatively regulates binding of tau to MTs

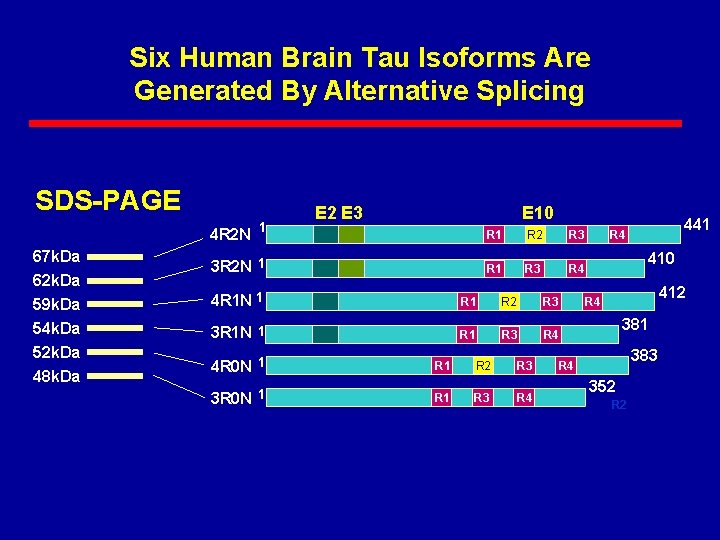
Six Human Brain Tau Isoforms Are Generated By Alternative Splicing SDS-PAGE 4 R 2 N 67 k. Da 62 k. Da 59 k. Da 54 k. Da 52 k. Da 48 k. Da 1 E 2 E 3 E 10 3 R 2 N 1 R 2 R 3 R 1 R 3 R 4 4 R 1 N 1 R 2 R 3 3 R 1 N 1 R 3 R 4 4 R 0 N 1 R 2 R 3 3 R 0 N 1 R 3 R 4 441 R 4 410 412 R 4 381 383 R 4 352 R 2

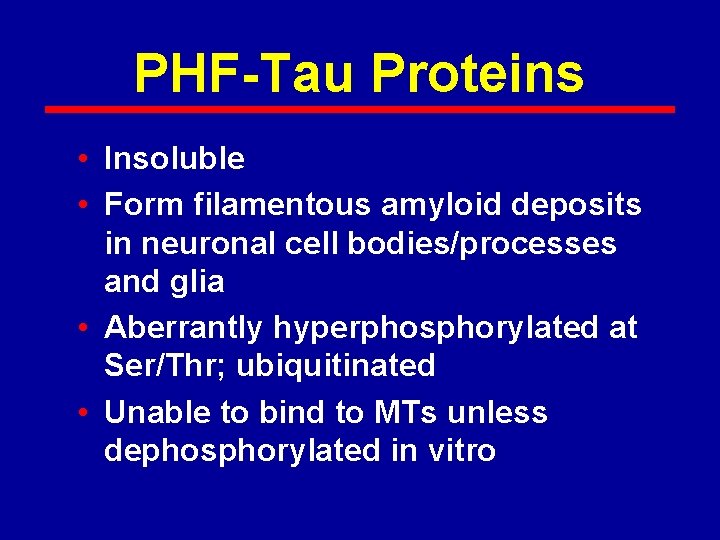
PHF-Tau Proteins • Insoluble • Form filamentous amyloid deposits in neuronal cell bodies/processes and glia • Aberrantly hyperphosphorylated at Ser/Thr; ubiquitinated • Unable to bind to MTs unless dephosphorylated in vitro

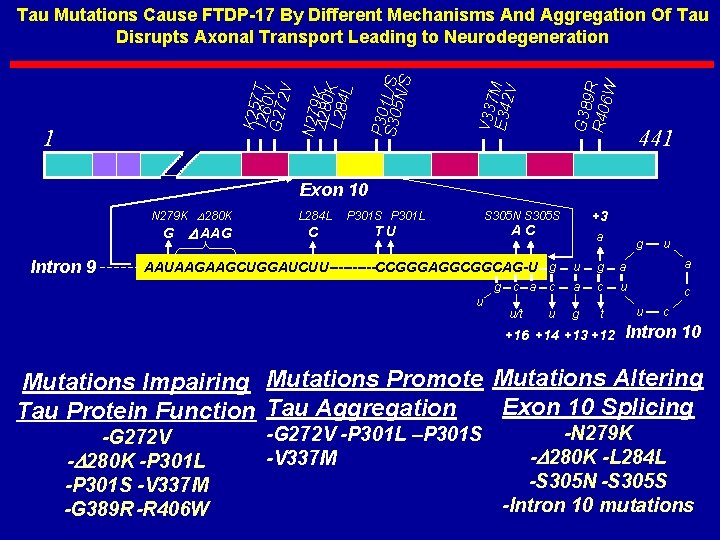
G 389 R 406 R W V 337 E 342 M V 1 P 3 S 30051 L/S N/S K 257 I 260 T G 272 V V N 279 D 280 K L 284 K L Tau Mutations Cause FTDP-17 By Different Mechanisms And Aggregation Of Tau Disrupts Axonal Transport Leading to Neurodegeneration 441 Exon 10 N 279 K D 280 K G Intron 9 D AAG L 284 L P 301 S P 301 L S 305 N S 305 S C TU AC a u c a u/t g u u g a a c u c u g t AAUAAGAAGCUGGAUCUU-----CCGGGAGGCGGCAG-U g g +3 +16 +14 +13 +12 u c Intron 10 Mutations Impairing Mutations Promote Mutations Altering Exon 10 Splicing Tau Protein Function Tau Aggregation -G 272 V -D 280 K -P 301 L -P 301 S -V 337 M -G 389 R-R 406 W -G 272 V -P 301 L –P 301 S -V 337 M -N 279 K -D 280 K -L 284 L -S 305 N -S 305 S -Intron 10 mutations

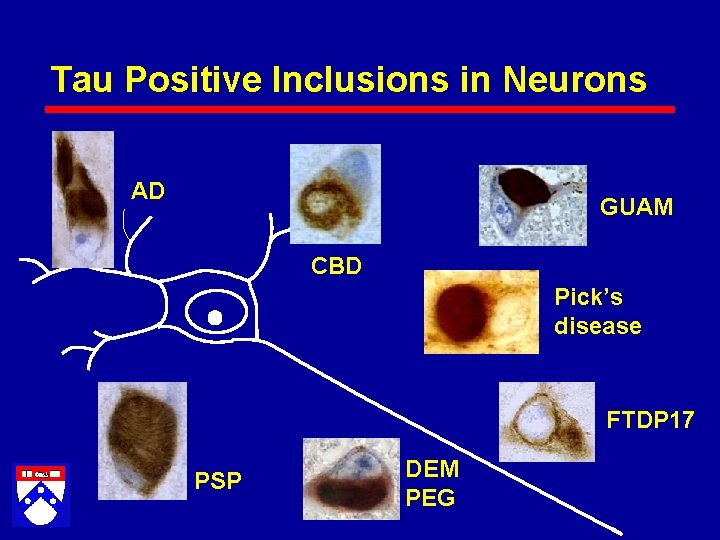
Tau Positive Inclusions in Neurons AD GUAM CBD Pick’s disease FTDP 17 PSP DEM PEG

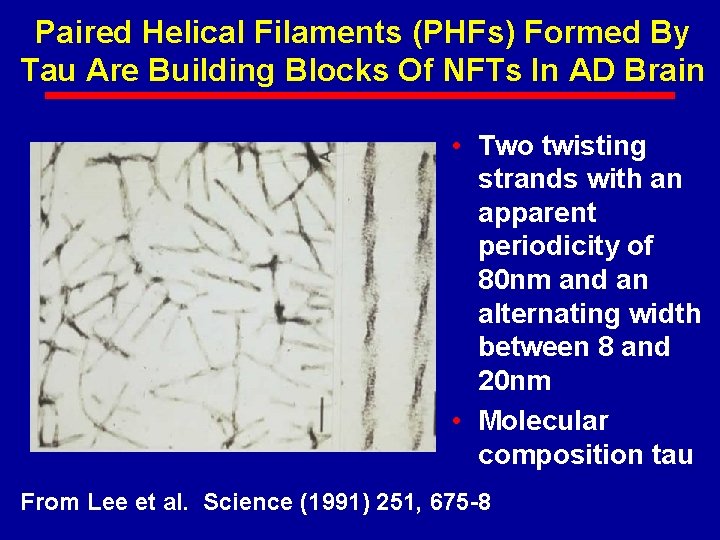
Paired Helical Filaments (PHFs) Formed By Tau Are Building Blocks Of NFTs In AD Brain • Two twisting strands with an apparent periodicity of 80 nm and an alternating width between 8 and 20 nm • Molecular composition tau From Lee et al. Science (1991) 251, 675 -8

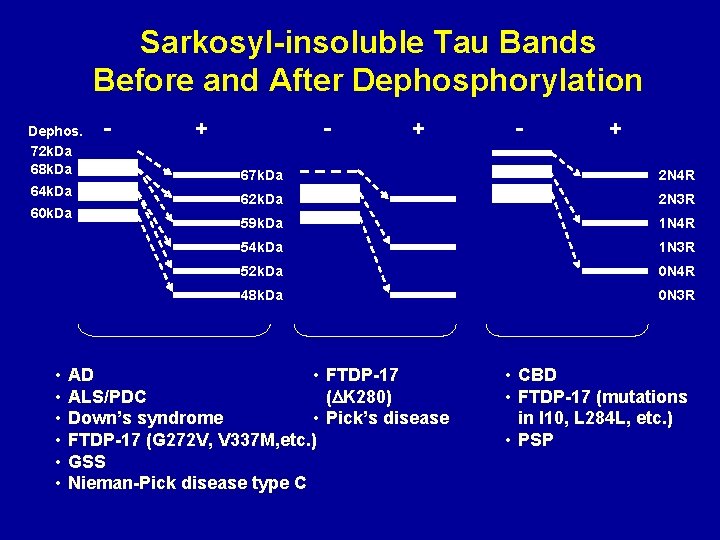
Sarkosyl-insoluble Tau Bands Before and After Dephosphorylation Dephos. 72 k. Da 68 k. Da 64 k. Da 60 k. Da • • • - + - + 67 k. Da 2 N 4 R 62 k. Da 2 N 3 R 59 k. Da 1 N 4 R 54 k. Da 1 N 3 R 52 k. Da 0 N 4 R 48 k. Da 0 N 3 R AD • FTDP-17 ALS/PDC (DK 280) Down’s syndrome • Pick’s disease FTDP-17 (G 272 V, V 337 M, etc. ) GSS Nieman-Pick disease type C • CBD • FTDP-17 (mutations in I 10, L 284 L, etc. ) • PSP

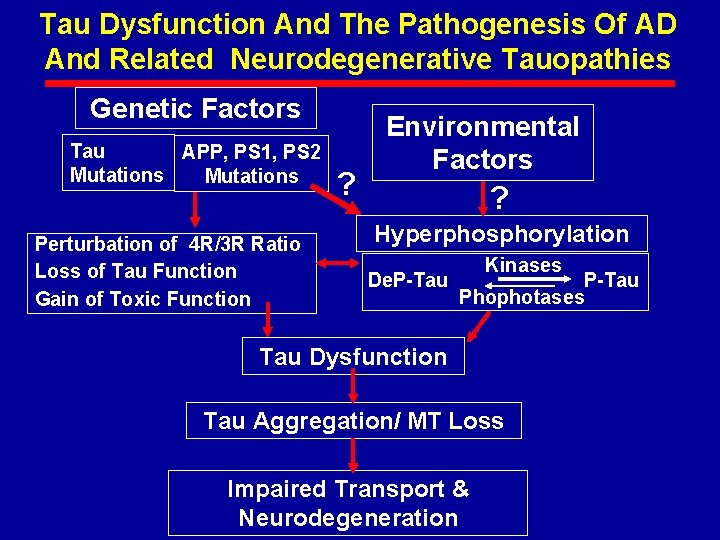
Tau Dysfunction And The Pathogenesis Of AD And Related Neurodegenerative Tauopathies Genetic Factors Tau APP, PS 1, PS 2 Mutations Perturbation of 4 R/3 R Ratio Loss of Tau Function Gain of Toxic Function ? Environmental Factors ? Hyperphosphorylation De. P-Tau Kinases P-Tau Phophotases Tau Dysfunction Tau Aggregation/ MT Loss Impaired Transport & Neurodegeneration

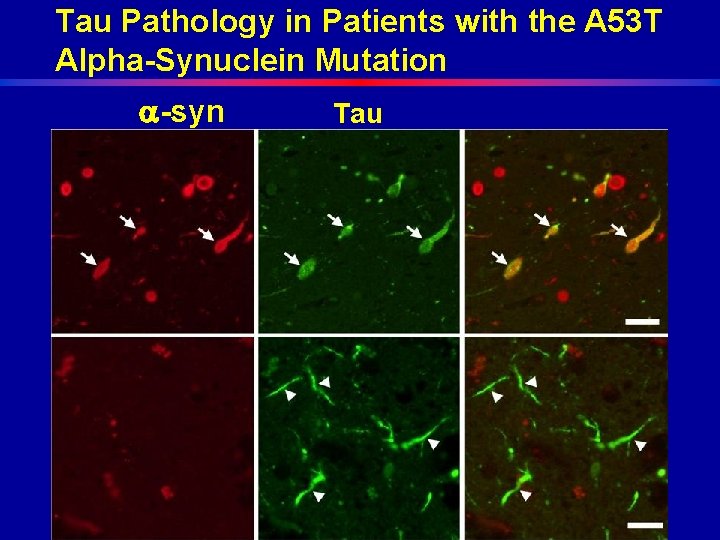
Tau Pathology in Patients with the A 53 T Alpha-Synuclein Mutation -syn Tau

Summary of -Synuclein Assembly Studies • -Synuclein readily assembles into 10 nm diameter filaments • A 53 T mutation facilitates -synuclein assembly • -Synuclein is incapable of fibrillogenesis • Residue 71 -82 is required for synuclein filament assembly

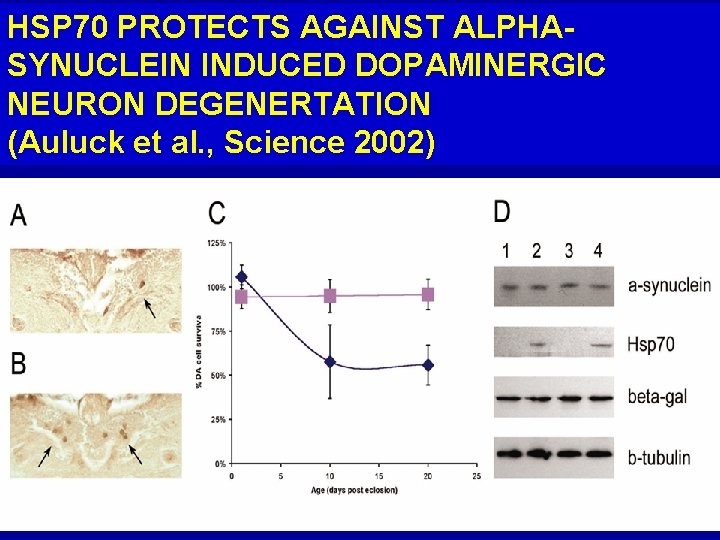
HSP 70 PROTECTS AGAINST ALPHASYNUCLEIN INDUCED DOPAMINERGIC NEURON DEGENERTATION (Auluck et al. , Science 2002)

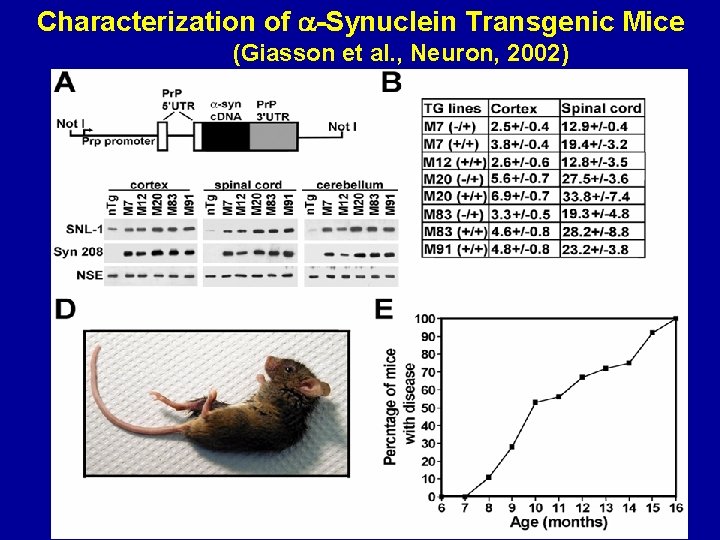
Characterization of -Synuclein Transgenic Mice (Giasson et al. , Neuron, 2002)

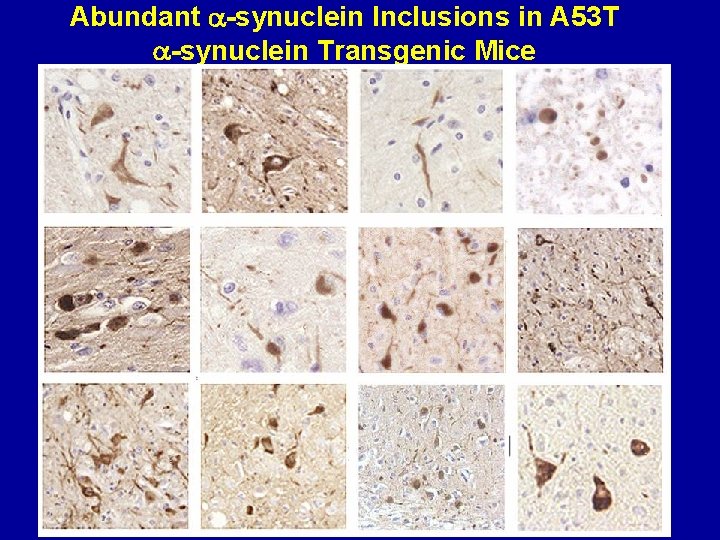
Abundant -synuclein Inclusions in A 53 T -synuclein Transgenic Mice SNL-4 Spinal cord Syn 303 Raphe Syn 505 pons Syn 506 Syn 303 pons SNL-4 Spinal cord Syn 505 pons Syn 505 locus ceruleus SNL-4 Spinal cord Syn 303 midbrain Syn 505 cerebellum

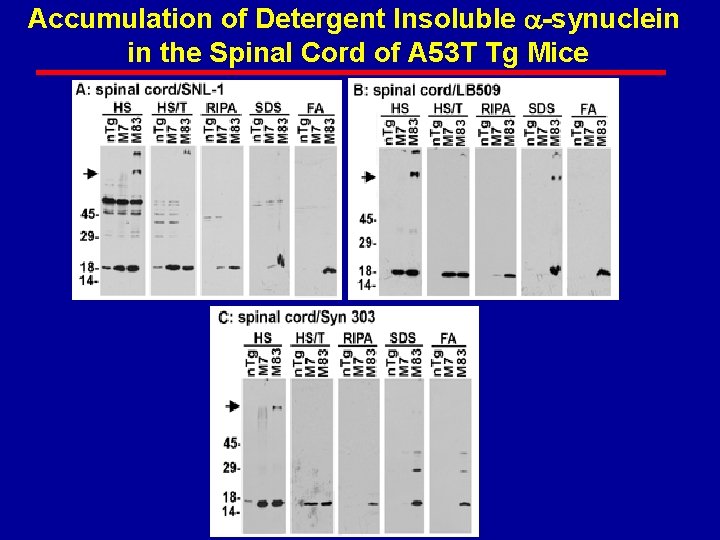
Accumulation of Detergent Insoluble -synuclein in the Spinal Cord of A 53 T Tg Mice

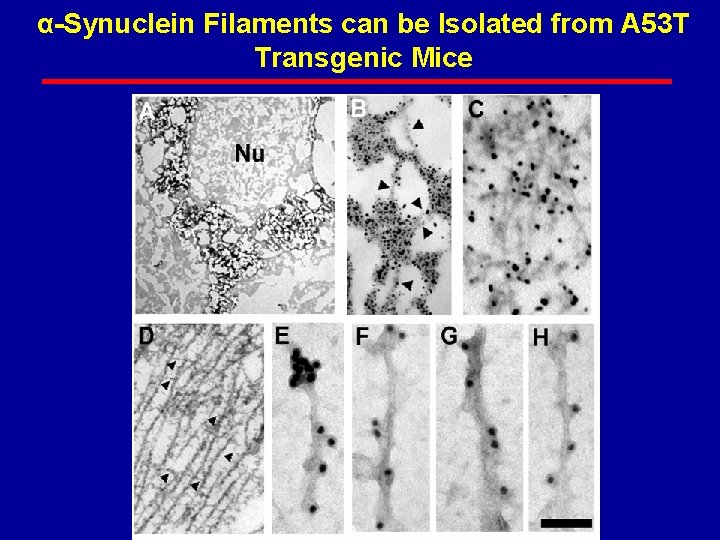
α-Synuclein Filaments can be Isolated from A 53 T Transgenic Mice

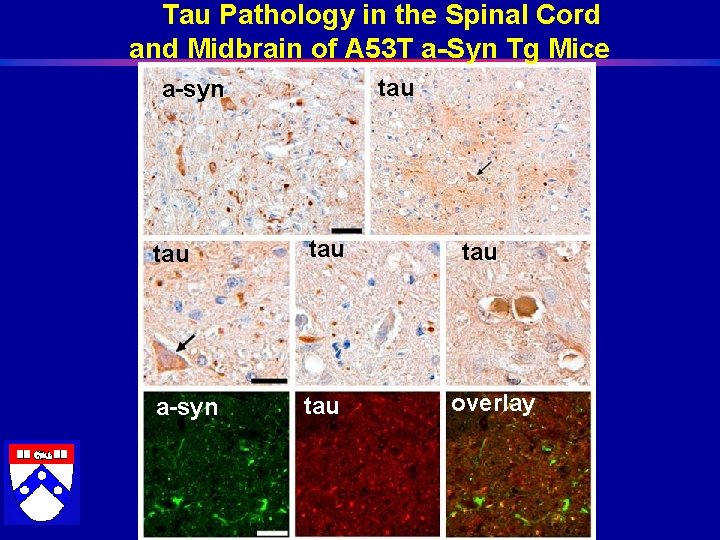
Tau Pathology in the Spinal Cord and Midbrain of A 53 T a-Syn Tg Mice tau a-syn tau tau overlay

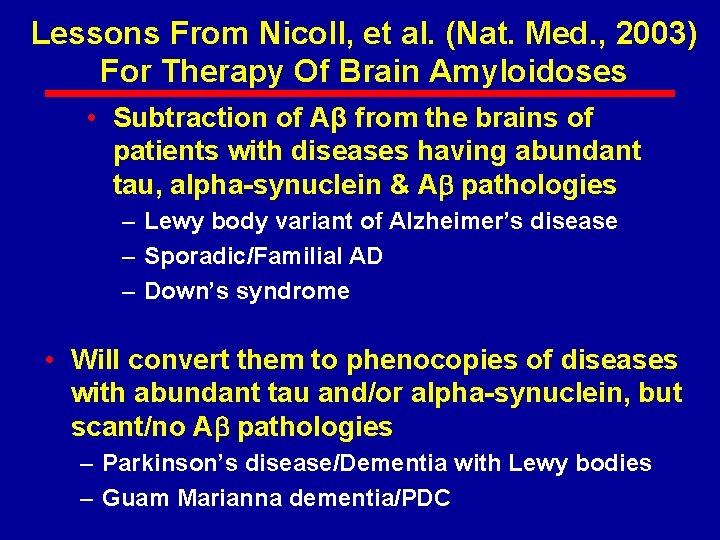
Lessons From Nicoll, et al. (Nat. Med. , 2003) For Therapy Of Brain Amyloidoses • Subtraction of Aβ from the brains of patients with diseases having abundant tau, alpha-synuclein & A pathologies – Lewy body variant of Alzheimer’s disease – Sporadic/Familial AD – Down’s syndrome • Will convert them to phenocopies of diseases with abundant tau and/or alpha-synuclein, but scant/no A pathologies – Parkinson’s disease/Dementia with Lewy bodies – Guam Marianna dementia/PDC

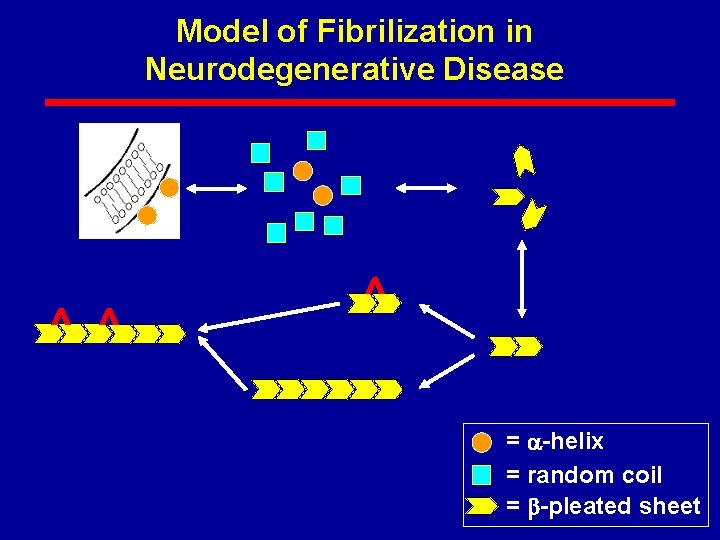
Model of Fibrilization in Neurodegenerative Disease ^ ^ ^ = -helix = random coil = -pleated sheet

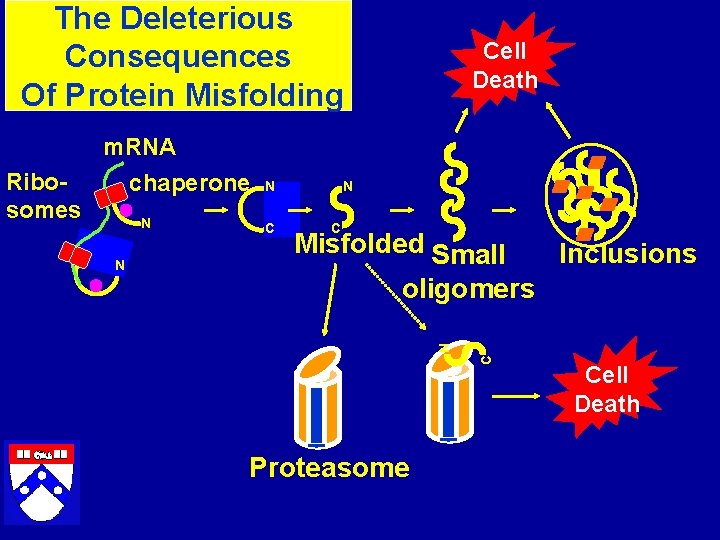
The Deleterious Consequences Of Protein Misfolding Cell Death m. RNA Ribosomes chaperone C Misfolded Small Inclusions oligomers C N N N N Proteasome Cell Death

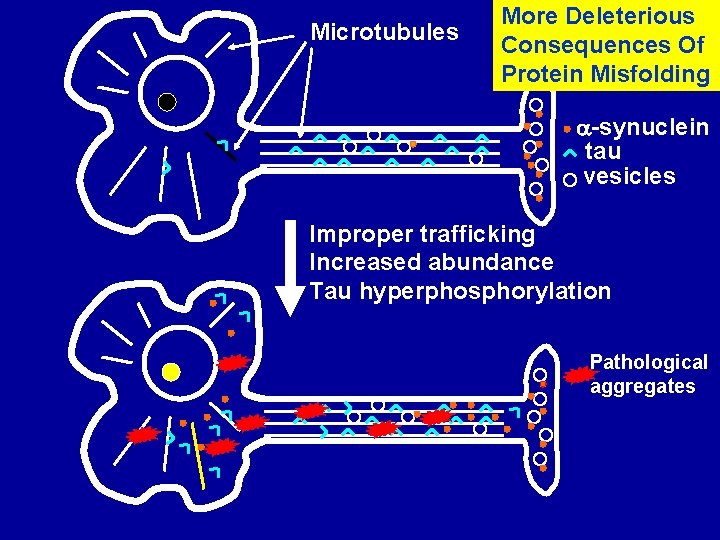
Microtubules More Deleterious Consequences Of Protein Misfolding -synuclein tau vesicles Improper trafficking Increased abundance Tau hyperphosphorylation Pathological aggregates

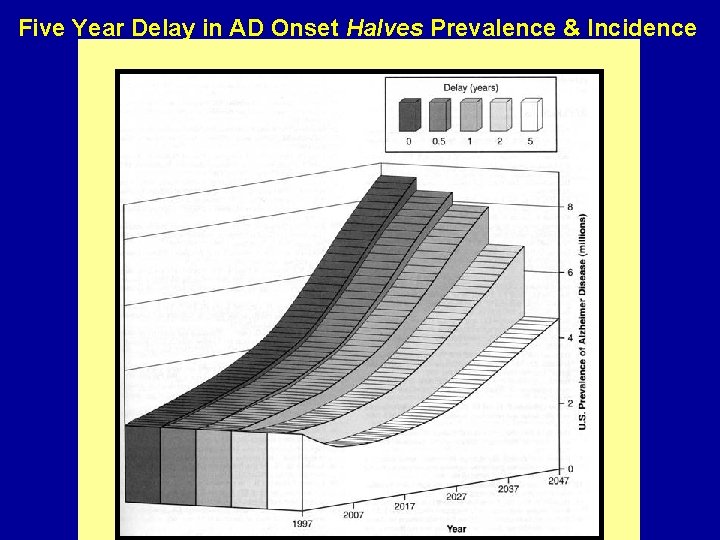
Five Year Delay in AD Onset Halves Prevalence & Incidence

It Takes Great A Team! • • Mark Forman Benoit Giasson Makoto Higuchi Hiro Uryu Bin Zhang CNDR Members Virginia Lee • • N. Bonini, J. Duda J. Galvin, D. Galasko L. Golbe, M. Grossman H. Hurtig, T. Iwatsubo C. Lippa, B. Miller D. Murphy, M. Stern Nat’l AD Coordinating Center • NIA Alzheimer Disease Centers • Penn Head Injury Center Supported by Grants from the NIA, the Alzheimer’s Association, Michael J. Fox Foundation and the Families of our Patients

DISCLAIMER If you attended to the content of this lecture, you may have participated in effortful mental activity, and this may reduce your risk for dementia. * However, this lecture is not intended as a therapeutic intervention and there is no guarantee that this lecture has therapeutic benefit. * Wilson et al. JAMA 287: 742 -748, 2003; Verghese et al. NEJM, 34: 2508 -2516, 2003
 Higgs to tau tau
Higgs to tau tau Tau vs titans
Tau vs titans Example of conditional convergence
Example of conditional convergence Onomatopoeia poem definition
Onomatopoeia poem definition Chapter 29 the clash between traditionalism and modernism
Chapter 29 the clash between traditionalism and modernism Why did johnson and congress clash over reconstruction
Why did johnson and congress clash over reconstruction Tau beta sigma flag
Tau beta sigma flag Why do ethnicities clash
Why do ethnicities clash What is the struggle between opposing forces
What is the struggle between opposing forces Clash royale clan manager
Clash royale clan manager Superordinate and hyponym
Superordinate and hyponym Hapsburg triumphs
Hapsburg triumphs Clash of lights 10
Clash of lights 10 Col to come together with great force to clash
Col to come together with great force to clash Lesson 3 a clash of values
Lesson 3 a clash of values What clash does the scopes trial represent?
What clash does the scopes trial represent? Chapter 21 section 3 central european monarchs clash
Chapter 21 section 3 central european monarchs clash X bogen clash of clans
X bogen clash of clans Chapter 5 a clash of cultures
Chapter 5 a clash of cultures Clash of civilizations huntington
Clash of civilizations huntington Clash of civilizations huntington
Clash of civilizations huntington Lesson 1 planning reconstruction
Lesson 1 planning reconstruction Jeopardy league of legends
Jeopardy league of legends Hku course disapprove
Hku course disapprove Chapter 13 section 1 cultures clash on the prairie
Chapter 13 section 1 cultures clash on the prairie Mc formers
Mc formers A conflict is a struggle between forces in a story
A conflict is a struggle between forces in a story Clash of armies
Clash of armies Central european monarchs clash
Central european monarchs clash Balanceamento clash
Balanceamento clash 12 titan gods
12 titan gods Poseidon's kids
Poseidon's kids Opening karakia timatanga
Opening karakia timatanga Kia tau kia tatou katoa karakia
Kia tau kia tatou katoa karakia Elastisk tau biltema
Elastisk tau biltema