Chemotherapy in prostate cancer Dr Mina Tajvidi Radiation




















- Slides: 25

Chemotherapy in prostate cancer Dr. Mina Tajvidi Radiation oncologist

Chemotherapy in prostate cancer ØNeoadjuvant chemotherapy Ø chemotherapy in hormone refractory prostate cancer

Neoadjuvant chemotherapy (Radical prostatectomy) ØNeoadjuvant ADT may decrease tumor volume and improve the rate of complete resection in men with c. T 3 prostate cancer , However, randomized trials have not demonstrated an improvement in long-term outcome despite the nearly universal decline in serum PSA in such men ØNewer approaches have added neoadjuvant or induction chemotherapy to ADT (chemohormonal therapy) in RP Ø In the past, chemotherapy was considered to be relatively ineffective in prostate cancer. However, docetaxel chemotherapy has led to higher rates of both objective and biochemical response, and prolonged survival in some cases ØThe ultimate goal is to establish a neoadjuvant regimen that improves local control and survival by eradicating clinically inapparent micrometastatic disease

Neoadjuvant chemotherapy (Radical prostatectomy) ØThere is no evidence that neoadjuvant therapy improves resectability or longterm outcomes from prostatectomy, and these approaches should not be considered standard of care.

Neoadjuvant chemotherapy(RT) ØThe combination of chemotherapy, ADT, and 3 D conformal RT (3 D-CRT) has been explored in men with locally advanced prostate cancer in at least two studies ØIn the only trial with long-enough follow-up to estimate the impact on disease control, neoadjuvant ADT plus chemotherapy (two 21 -day cycles of estramustine plus oral etoposide, followed by oral estramustine concurrent with 3 D-CRT) was administered to 18 men with locally advanced or high-risk localized disease (T 3 -4 or T 1 c-2 c with Gleason score 7 and serum PSA >15 ng/m. L) ØAlthough these results are encouraging, adjuvant chemotherapy for locally advanced disease is an investigational approach and it should be used only in the context of clinical trials ØThe role of using chemotherapy with ADT in men undergoing EBRT for prostate cancer should become clearer when the results of the recently completed RTOG trial 99 -02 become available.

adjuvant chemotherapy ØThe activity of docetaxel and other agents has led to the evaluation of adjuvant chemotherapy in men with resected highrisk prostate cancer ØMultiple large randomized trials are in progress to determine whether this approach can improve long-term outcomes ØThe potential importance of late toxicity was illustrated by a report from the Southwest Oncology Group trial 9921 in which patients with high-risk prostate cancer who had undergone radical prostatectomy were randomly assigned to two years of combined androgen deprivation therapy (goserelin plus bicalutamide), with or without six cycles of mitoxantrone plus prednisone. The trial was terminated after 983 of the planned 1360 patients were enrolled, when acute myeloid leukemia was observed in three patients who had received mitoxantrone. The enrolled patients continue to be observed, but efficacy data are not yet available

adjuvant chemotherapy Øpatients with advanced prostate cancer should be encouraged to participate in clinical trials and referred early to a oncologist(NCCN) Ø based upon phase III data , every 3 -week docetaxel and prednisone is the preferred first -line chemotherapy treatment Øalternative regimens include every 3 -weekly docetaxel and estramustin , weekly docetaxel and prednisone , and every 3 -weekly mitoxantron and prednisone

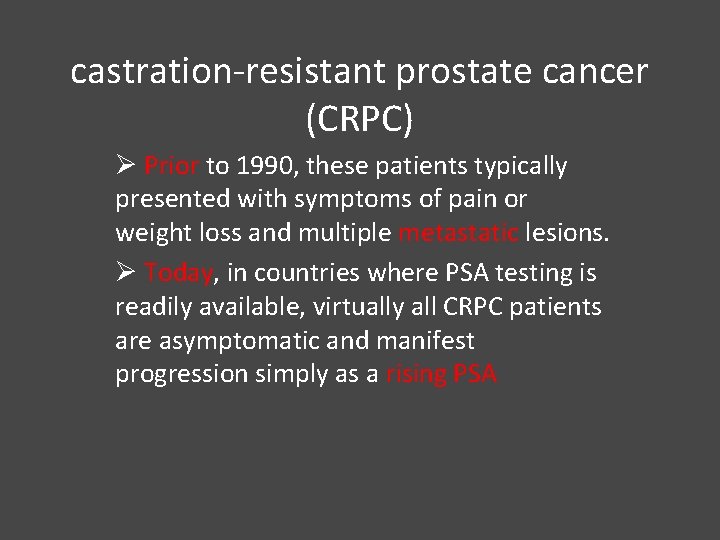
hormone refractory prostate cancer or castration-resistant prostate cancer (CRPC) Øit is generally agreed that patients with progressive prostate cancer despite a castrate testosterone level (50 ng/d. L or less) have a distinct prognosis and set of therapeutic options

castration-resistant prostate cancer (CRPC) Ø Prior to 1990, these patients typically presented with symptoms of pain or weight loss and multiple metastatic lesions. Ø Today, in countries where PSA testing is readily available, virtually all CRPC patients are asymptomatic and manifest progression simply as a rising PSA

castration-resistant prostate cancer (CRPC) ØAttempts to prospectively assess the effects of secondary hormonal treatments as compared with earlier use of chemotherapy have failed consequent to poor accrual. Ø Most secondary hormonal manipulations are reasonably well tolerated, particularly as compared with chemotherapy

Mitoxantrone Ø Mitoxantrone is an anthraquinone that is structurally related to the anthracyclines ØMitoxantrone has palliative activity as a single agent and was the first chemotherapy shown to confer clinical benefit in randomized trials for patients with CRPC Øthe combination of mitoxantrone(12 to 14 mg/m 2 intravenously every 3 weeks) plus prednisone was approved by the FDA in 1996 for the treatment of CRPC patients

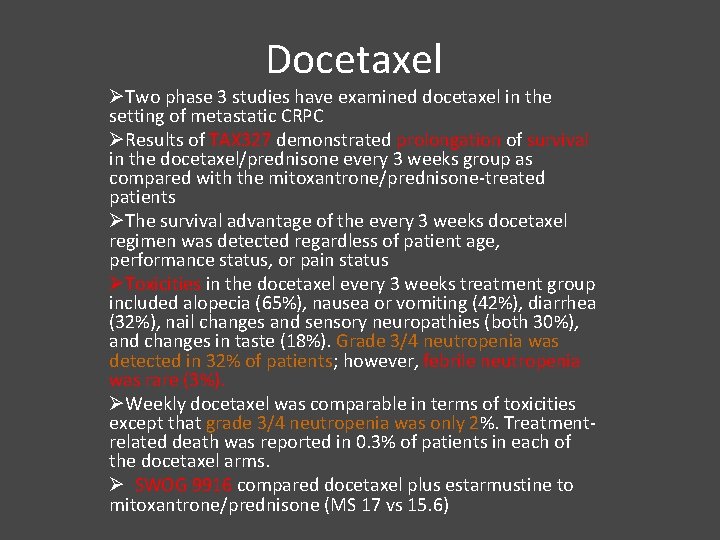
Docetaxel ØTwo phase 3 studies have examined docetaxel in the setting of metastatic CRPC ØResults of TAX 327 demonstrated prolongation of survival in the docetaxel/prednisone every 3 weeks group as compared with the mitoxantrone/prednisone-treated patients ØThe survival advantage of the every 3 weeks docetaxel regimen was detected regardless of patient age, performance status, or pain status ØToxicities in the docetaxel every 3 weeks treatment group included alopecia (65%), nausea or vomiting (42%), diarrhea (32%), nail changes and sensory neuropathies (both 30%), and changes in taste (18%). Grade 3/4 neutropenia was detected in 32% of patients; however, febrile neutropenia was rare (3%). ØWeekly docetaxel was comparable in terms of toxicities except that grade 3/4 neutropenia was only 2%. Treatmentrelated death was reported in 0. 3% of patients in each of the docetaxel arms. Ø SWOG 9916 compared docetaxel plus estarmustine to mitoxantrone/prednisone (MS 17 vs 15. 6)

Other docetaxel combinations Ø none of these has been demonstrated to be superior to docetaxel plus prednisone in randomized, phase III trials ØDocetaxel plus estramustine ØDocetaxel plus calcitriol ØDocetaxel plus vinorelbine: ØDocetaxel plus capecitabine ØDocetaxel plus epirubicin ØDocetaxel plus carboplatin

Paclitaxel ØThe taxane paclitaxel has been less extensively evaluated than docetaxel in men with hormone refractory prostate cancer Ø efficacy of weekly therapy was illustrated in a study of 43 men with hormone refractory prostate cancer who received paclitaxel (80 mg/m 2 over one hour) weekly for 6 of every 8 weeks ØPSA response was noted in 36 percent

Paclitaxel ØPaclitaxel has been combined with estramustine in several phase II studies. ØAttempts to augment the activity of the paclitaxel plus estramustine combination have included the addition of a third agent such as etoposide and carboplatin

ESTRAMUSTINE Øthe use of estramustine was complicated by an increased risk of both arterial and venous thromboembolic events. Although daily aspirin (325 mg daily) and low dose warfarin (2 mg daily) have been proposed as prophylaxis

newer agents Among newer agents, the epothilone, ixabepilone, and the platinum derivative, satraplatin have been more extensively studied

Ixabepilone Ø Ixabepilone: a new class of non-taxane tubulin polymerizing agents ØIxabepilone( 35 -40 mg/m 2 over three hours every three weeks) ØEarly clinical studies suggest that ixabepilone has significant activity in men with hormone refractory prostate cancer both in chemotherapynaive and previously treated patients ØFurther follow-up of these studies and additional randomized trials are needed to clarify the role of ixabepilone in men with hormone refractory prostate cancer

Satraplatin ØSatraplatin : is an orally active platinum compound that has significant activity in cisplatin-resistant tumor models (80 mg/m 2 for five days every five weeks) ØActivity in prostate cancer was suggested in early clinical studies ØSatraplatin was evaluated more extensively in a phase III trial, in which 950 men who had progressed after first-line chemotherapy for hormone refractory prostate cancer ØFinal results of this trial were presented at the American Society of Clinical Oncology (ASCO) meetings in 2008 ØProgression-free survival (PFS) was significantly increased in patients assigned to satraplatin compared to placebo ØThere was no difference in overall survival (61 weeks on both treatment arms, HR 0. 95, 95% CI 0. 84 -1. 15)

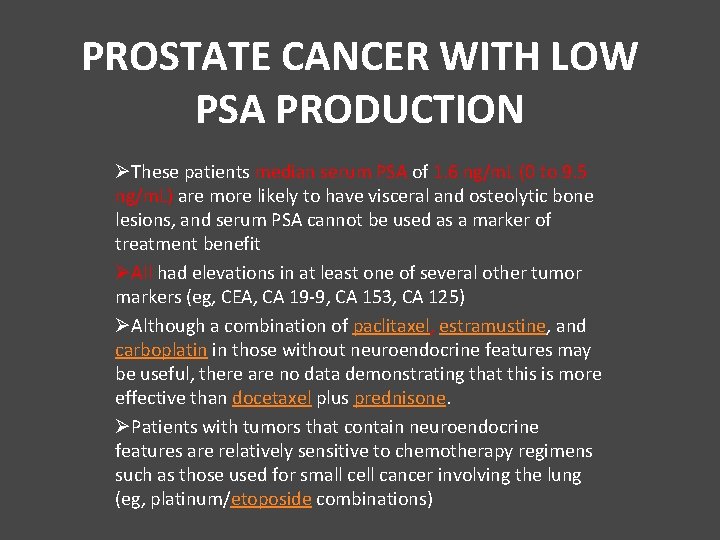
PROSTATE CANCER WITH LOW PSA PRODUCTION ØThese patients median serum PSA of 1. 6 ng/m. L (0 to 9. 5 ng/m. L) are more likely to have visceral and osteolytic bone lesions, and serum PSA cannot be used as a marker of treatment benefit ØAll had elevations in at least one of several other tumor markers (eg, CEA, CA 19 -9, CA 153, CA 125) ØAlthough a combination of paclitaxel, estramustine, and carboplatin in those without neuroendocrine features may be useful, there are no data demonstrating that this is more effective than docetaxel plus prednisone. ØPatients with tumors that contain neuroendocrine features are relatively sensitive to chemotherapy regimens such as those used for small cell cancer involving the lung (eg, platinum/etoposide combinations)

SUMMARY AND RECOMMENDATIONS Øpatients with advanced prostate cancer should be encouraged to participate in clinical trials and referred early to a oncologist(NCCN) Ø based upon phase III data , every 3 -week docetaxel and prednisone is the preferred first line chemotherapy treatment Øalternative regimens include every 3 -weekly docetaxel and estramustin , weekly docetaxel and prednisone , and every 3 -weekly mitoxantron and prednisone

SUMMARY AND RECOMMENDATIONS ØFor chemotherapy-naive men with hormone refractory prostate cancer without neuroendocrine features, we recommend chemotherapy with docetaxel (75 mg/m 2 every three weeks) plus oral prednisone (5 mg twice a day) ØWe suggest that gonadal androgen suppression (but not antiandrogens) be continued during chemotherapy ØThe best treatment for men who fail docetaxel-based therapy is unclear. In this setting, both ixabepilone and the combination of mitoxantrone plus prednisone appear to have activity in some men. Patients should be encouraged to participate in clinical trials whenever possible

SUMMARY AND RECOMMENDATIONS ØPatients with hormone refractory prostate cancer and a low serum PSA: For patients with poorly differentiated adenocarcinoma without neuroendocrine features, we suggest a combination of docetaxel plus prednisone ØA combination of paclitaxel, estramustine, and carboplatin may be an alternative ØThose patients whose tumors have a substantial component with neuroendocrine features may benefit from treatment with a chemotherapy regimen similar to that used for patients with small cell lung cancer


tanks
 Mina tajvidi
Mina tajvidi Mina tajvidi
Mina tajvidi Mina tajvidi
Mina tajvidi Mdv3100 prostate cancer
Mdv3100 prostate cancer Hormonothérapie cancer prostate
Hormonothérapie cancer prostate Stephen ko md
Stephen ko md Laprp
Laprp Prostate cancer staging
Prostate cancer staging Prostate cancer tnm classification
Prostate cancer tnm classification Principles of chemotherapy
Principles of chemotherapy 4ac 4t chemotherapy
4ac 4t chemotherapy General principles of chemotherapy
General principles of chemotherapy Bsa calculation formula for chemotherapy
Bsa calculation formula for chemotherapy Chemotherapy
Chemotherapy Icd 9 code for aphthous ulcer
Icd 9 code for aphthous ulcer Caf abbreviation
Caf abbreviation Debra forman
Debra forman Normal weight of prostate
Normal weight of prostate Prostate
Prostate Prostate adenocarcinoma perineural invasion
Prostate adenocarcinoma perineural invasion Istilah berarti: absennya satu atau kedua buah pelir adalah
Istilah berarti: absennya satu atau kedua buah pelir adalah Prostate pathology
Prostate pathology Tuip prostate
Tuip prostate Prostate
Prostate Lobes of prostate
Lobes of prostate Anatomie zonale de mac neal
Anatomie zonale de mac neal