CARCINOMA DELLA PROSTATA PROSTATE CANCER Prostate Anatomy Prostate










- Slides: 62

CARCINOMA DELLA PROSTATA

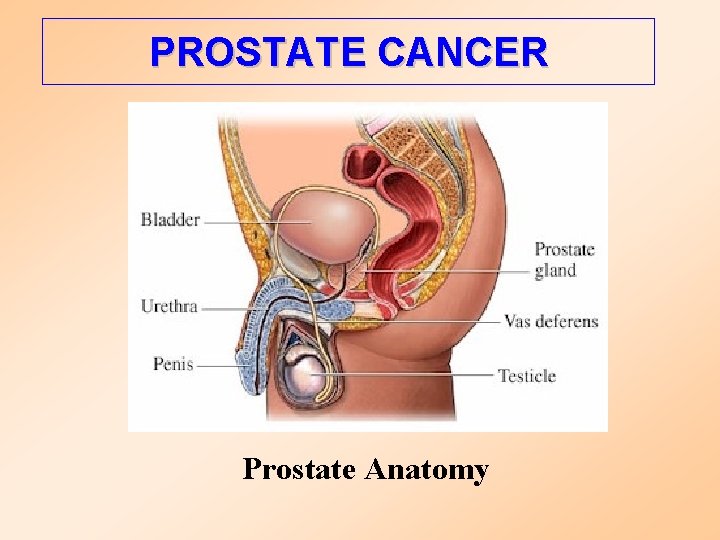
PROSTATE CANCER Prostate Anatomy

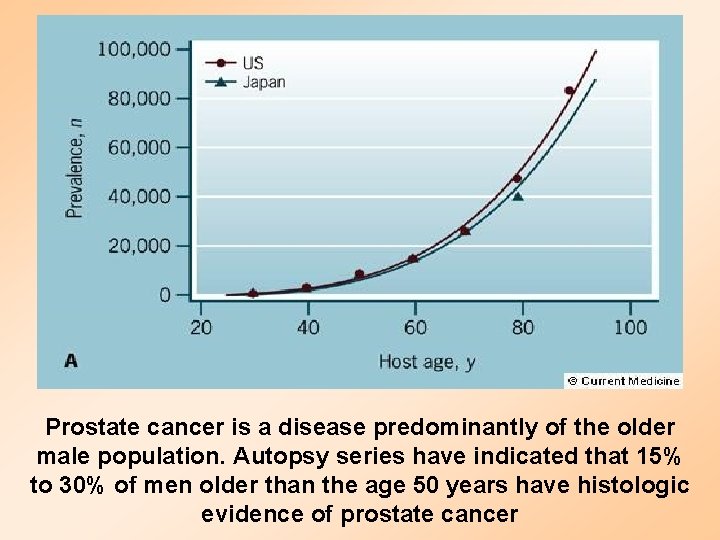
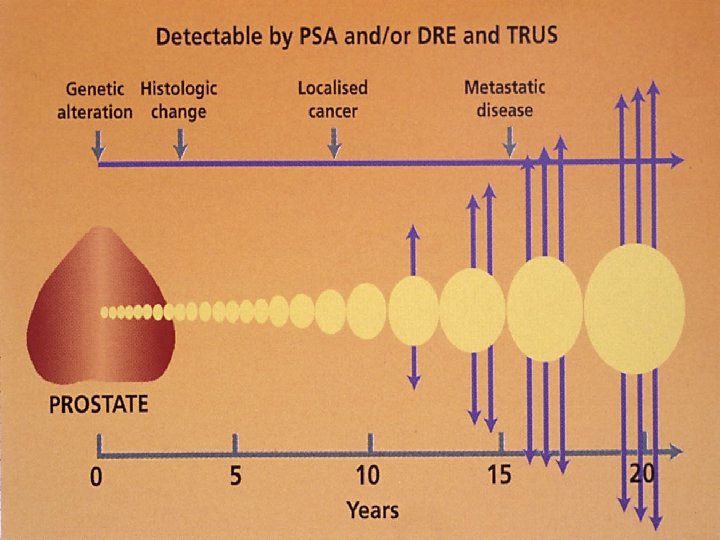
Prostate cancer is a disease predominantly of the older male population. Autopsy series have indicated that 15% to 30% of men older than the age 50 years have histologic evidence of prostate cancer

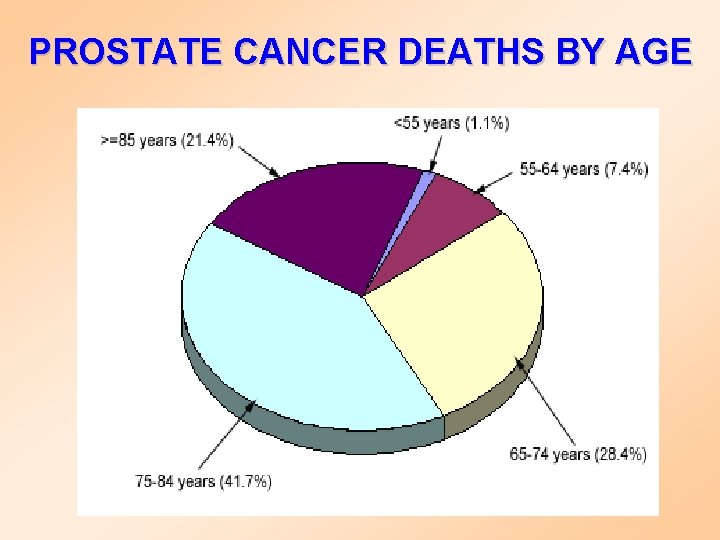
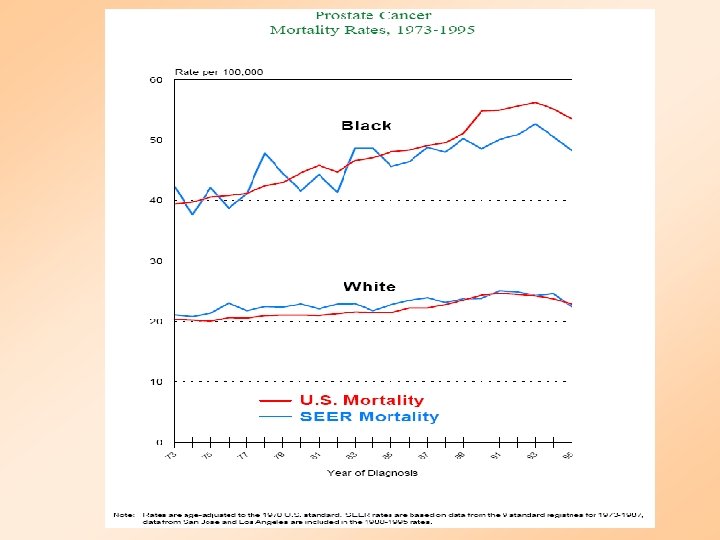
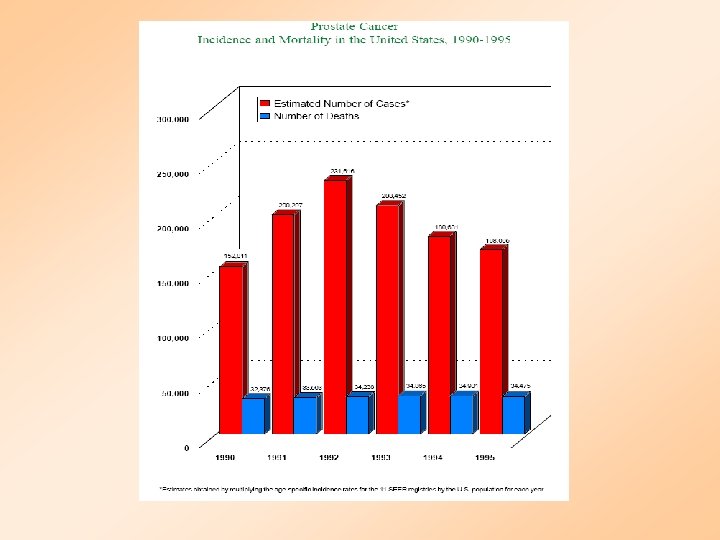
PROSTATE CANCER DEATHS BY AGE





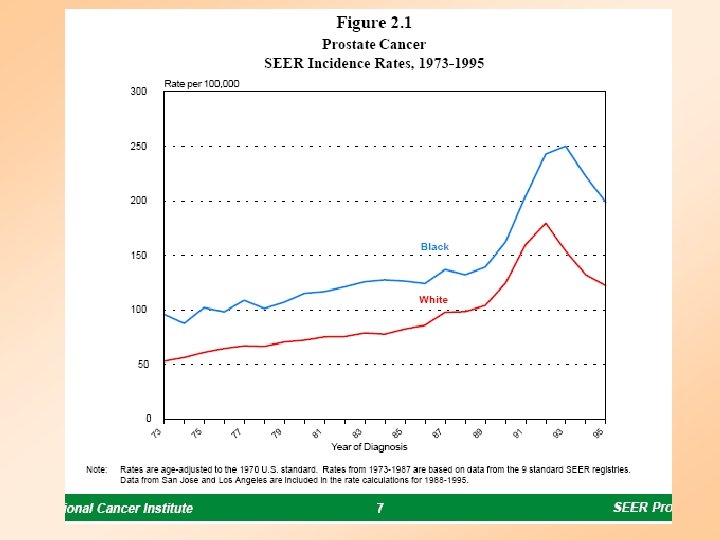
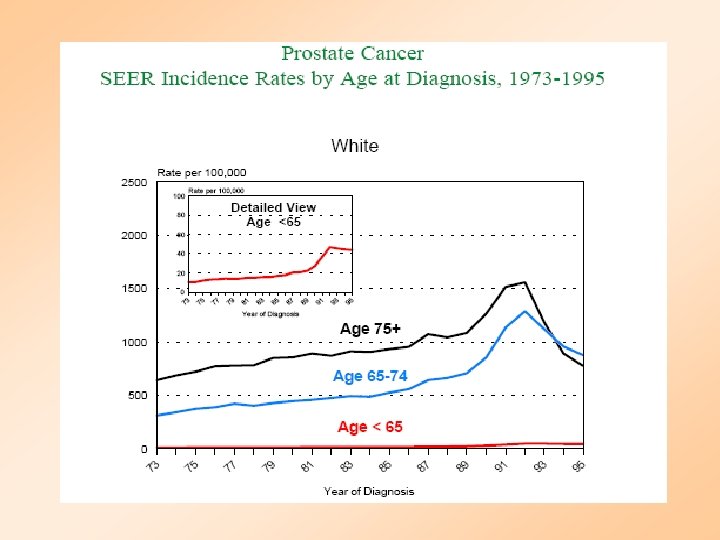
Risk Factors for Prostate Cancer • Age – Found mainly in men over age 55. Average of diagnosis is 70 • Family History – Men’s risk is higher if father or brother is diagnosed before the age of 60 • Race – Prostate cancer is found more often in African American men then White men. It is less common in Asian and American Indian men • Dietary factors – Evidence suggests that a diet high in fat may increase the risk of prostate cancer and diets high in fruits and vegetables decrease the risk

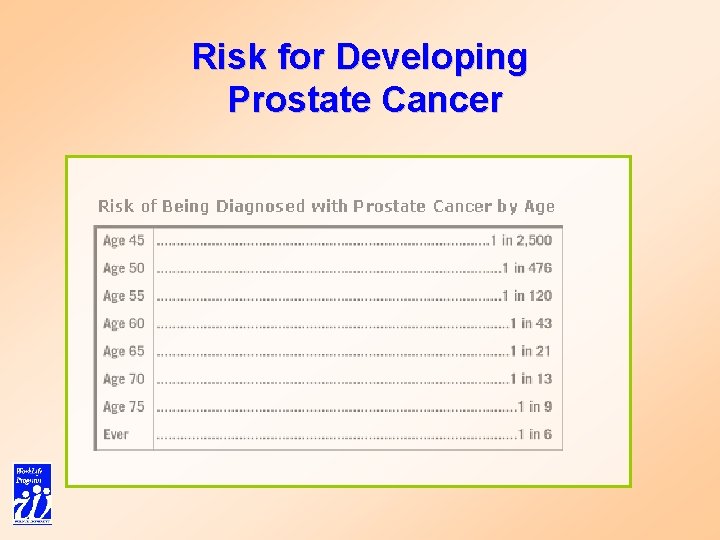
Risk for Developing Prostate Cancer

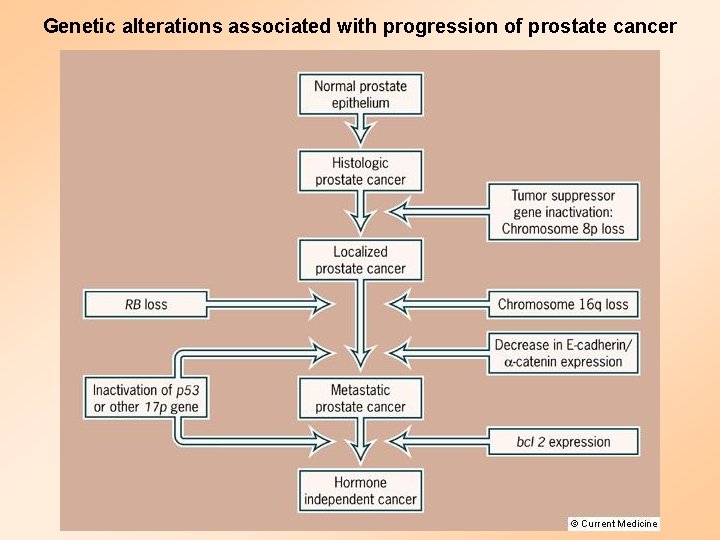
Genetic alterations associated with progression of prostate cancer

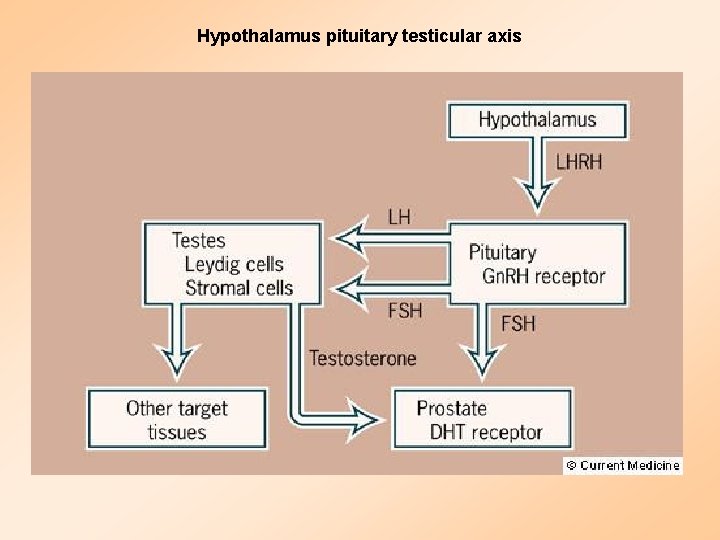
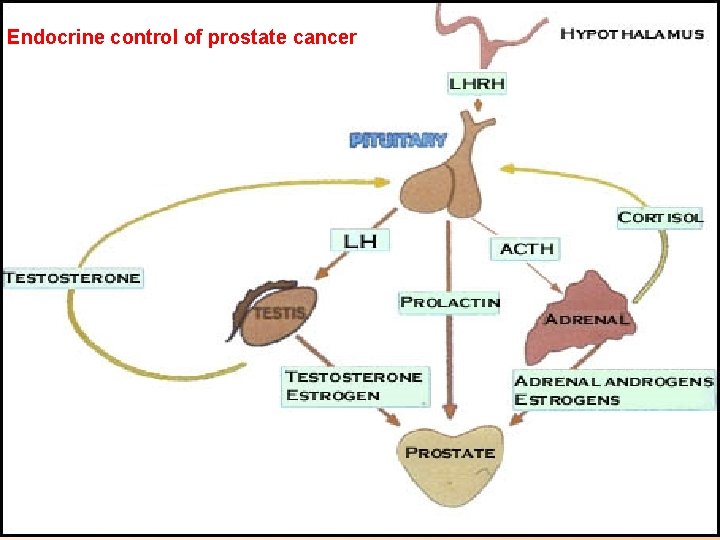
Hypothalamus pituitary testicular axis

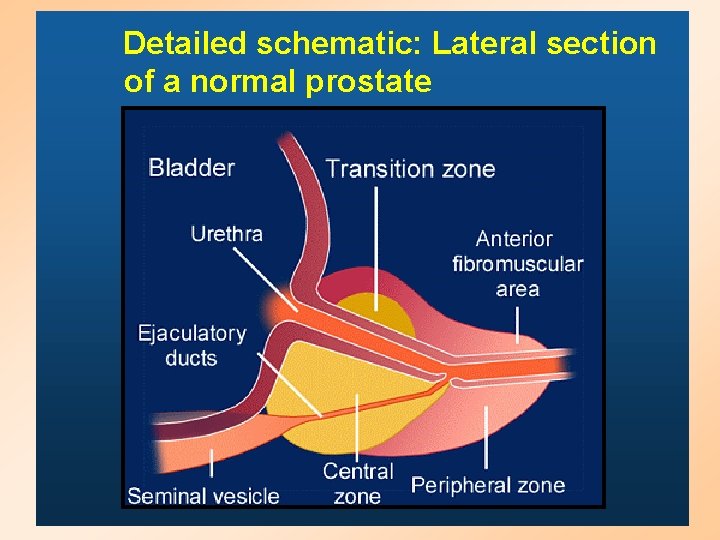
Detailed schematic: Lateral section of a normal prostate

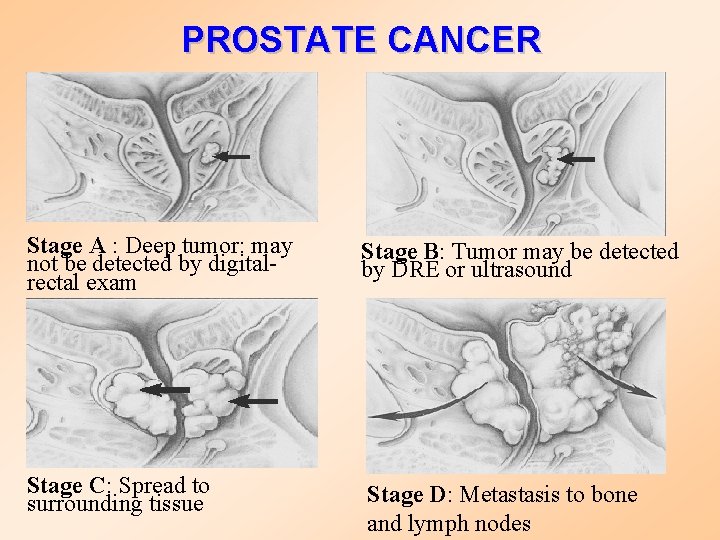
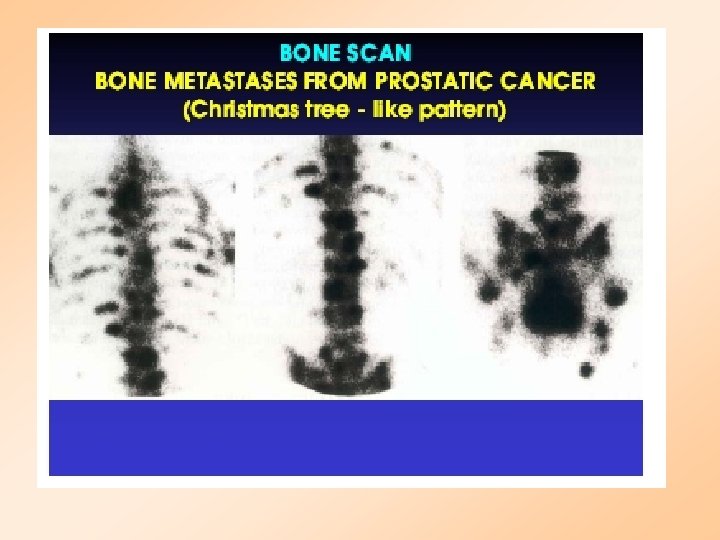
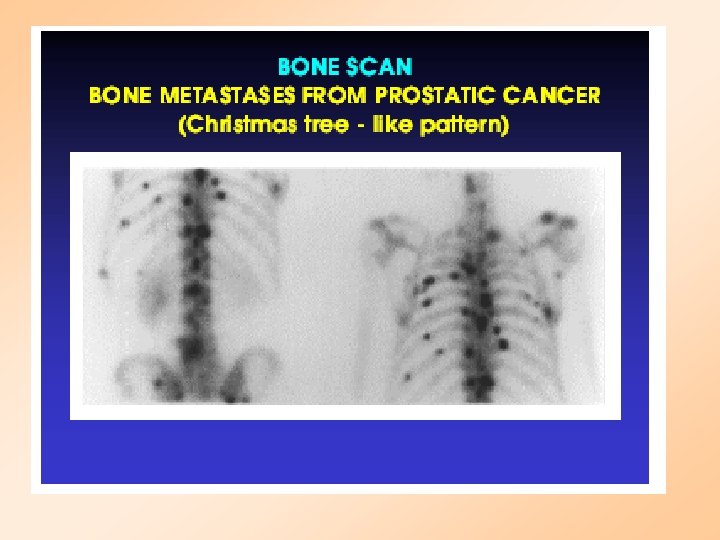
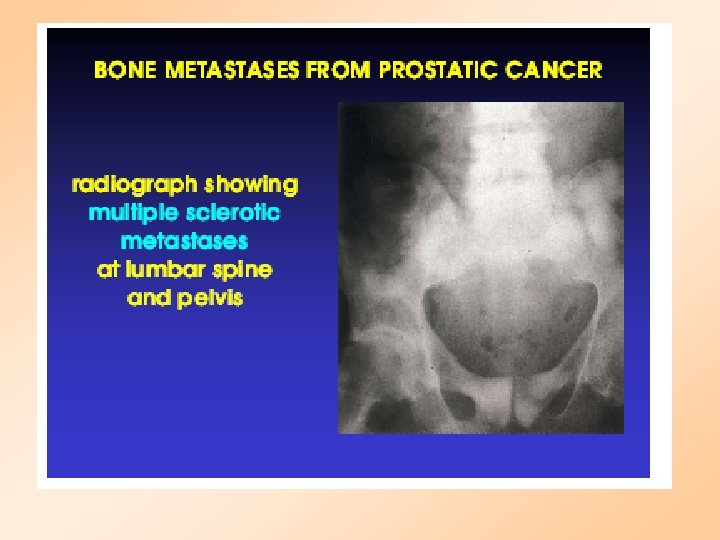
PROSTATE CANCER Stage A : Deep tumor: may not be detected by digitalrectal exam Stage B: Tumor may be detected by DRE or ultrasound Stage C: Spread to surrounding tissue Stage D: Metastasis to bone and lymph nodes


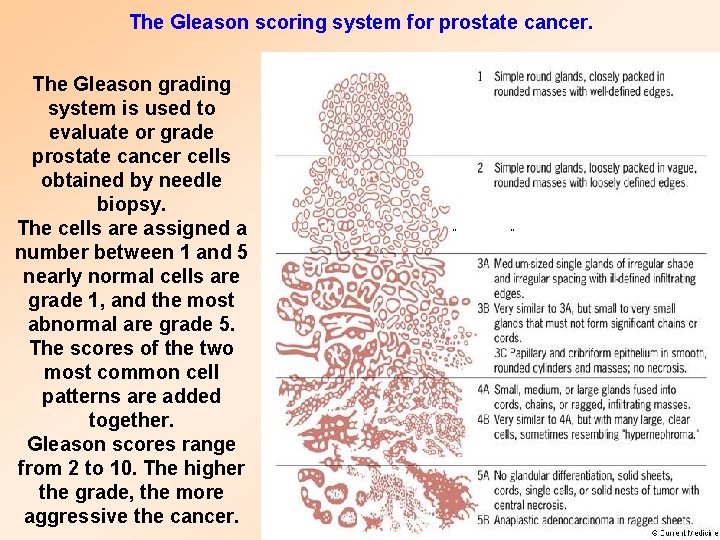
The Gleason scoring system for prostate cancer. The Gleason grading system is used to evaluate or grade prostate cancer cells obtained by needle biopsy. The cells are assigned a number between 1 and 5 nearly normal cells are grade 1, and the most abnormal are grade 5. The scores of the two most common cell patterns are added together. Gleason scores range from 2 to 10. The higher the grade, the more aggressive the cancer.


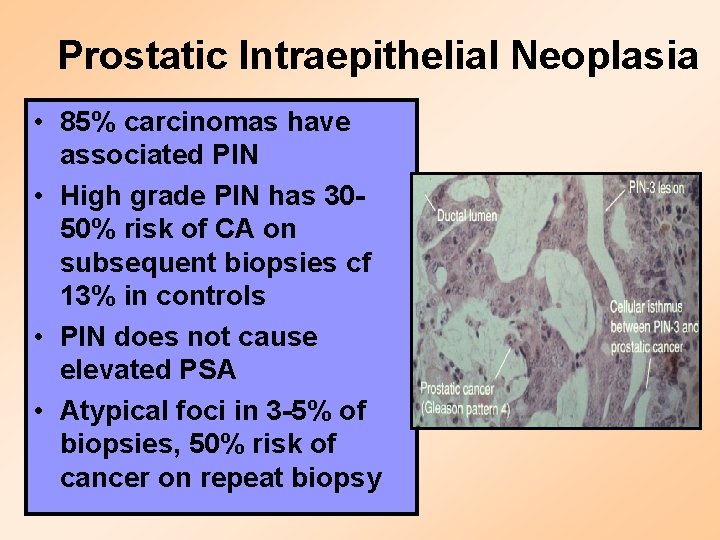
Prostatic Intraepithelial Neoplasia • 85% carcinomas have associated PIN • High grade PIN has 3050% risk of CA on subsequent biopsies cf 13% in controls • PIN does not cause elevated PSA • Atypical foci in 3 -5% of biopsies, 50% risk of cancer on repeat biopsy

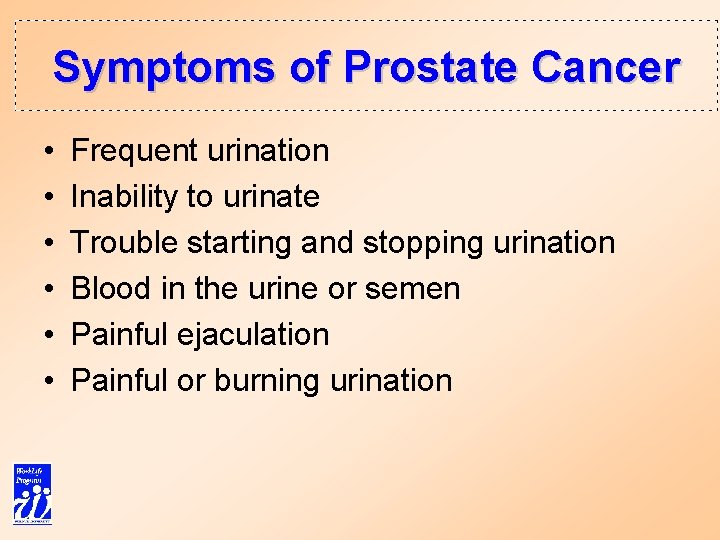
Symptoms of Prostate Cancer • • • Frequent urination Inability to urinate Trouble starting and stopping urination Blood in the urine or semen Painful ejaculation Painful or burning urination

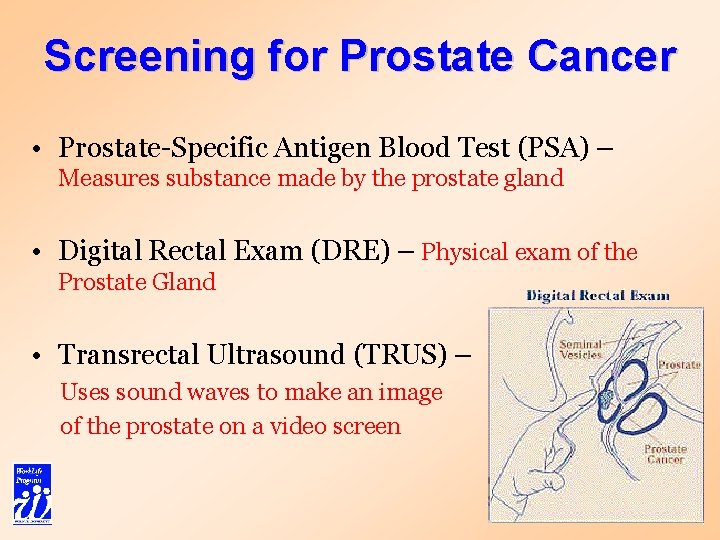
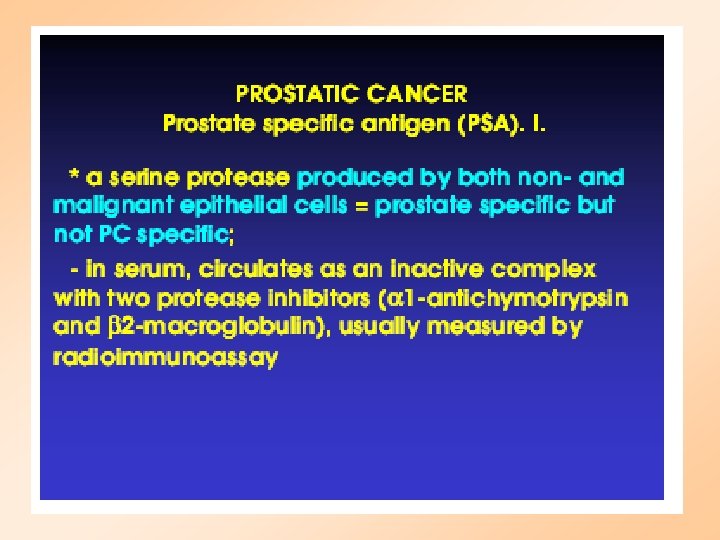
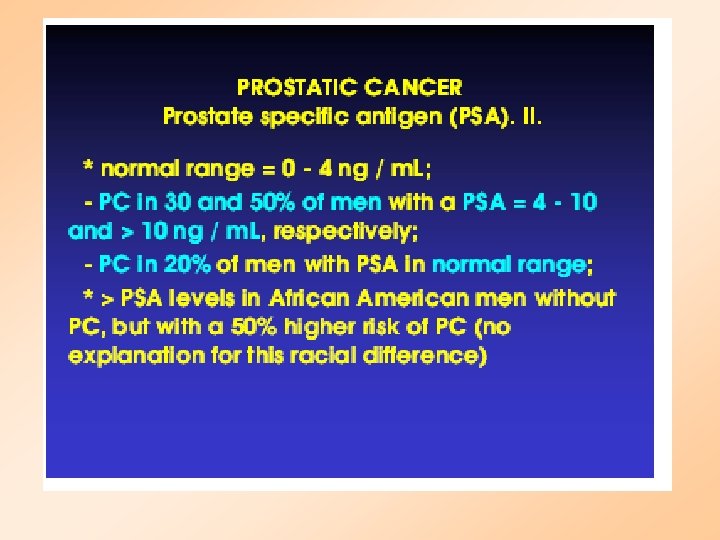
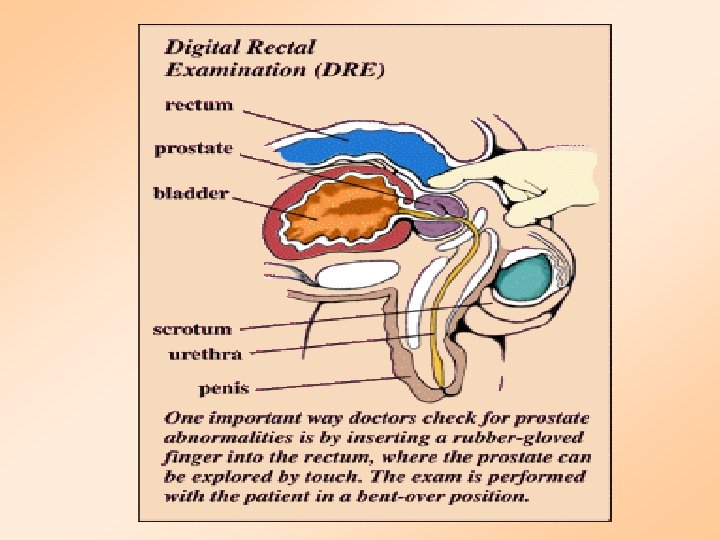
Screening for Prostate Cancer • Prostate-Specific Antigen Blood Test (PSA) – Measures substance made by the prostate gland • Digital Rectal Exam (DRE) – Physical exam of the Prostate Gland • Transrectal Ultrasound (TRUS) – Uses sound waves to make an image of the prostate on a video screen

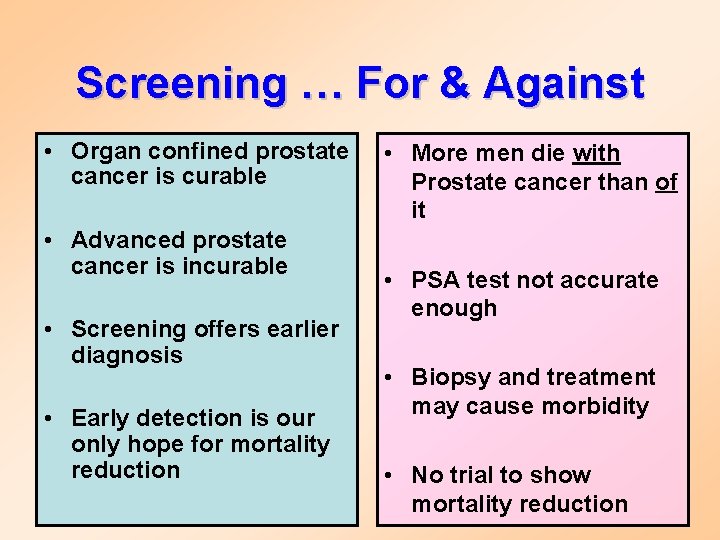
Screening … For & Against • Organ confined prostate cancer is curable • Advanced prostate cancer is incurable • Screening offers earlier diagnosis • Early detection is our only hope for mortality reduction • More men die with Prostate cancer than of it • PSA test not accurate enough • Biopsy and treatment may cause morbidity • No trial to show mortality reduction



Factors Increasing PSA • • Cycling Prostate massage Cystoscopy Ejaculation Prostate biopsy Transrectal Ultrasound Prostate disease

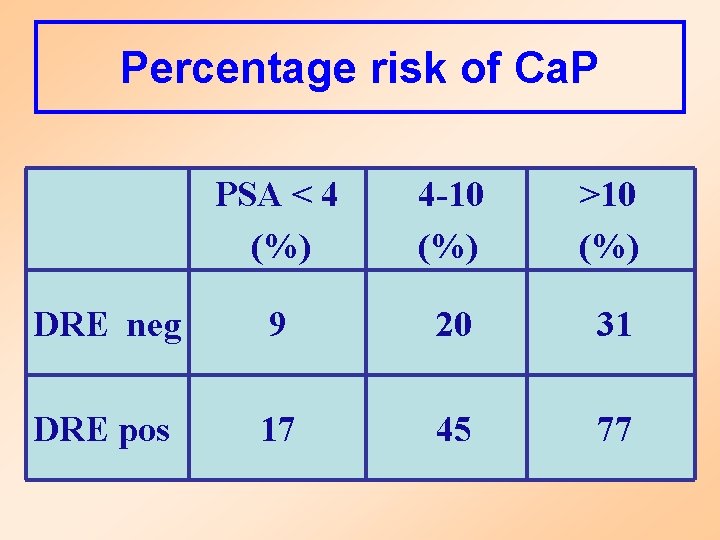
Percentage risk of Ca. P PSA < 4 (%) 4 -10 (%) >10 (%) DRE neg 9 20 31 DRE pos 17 45 77

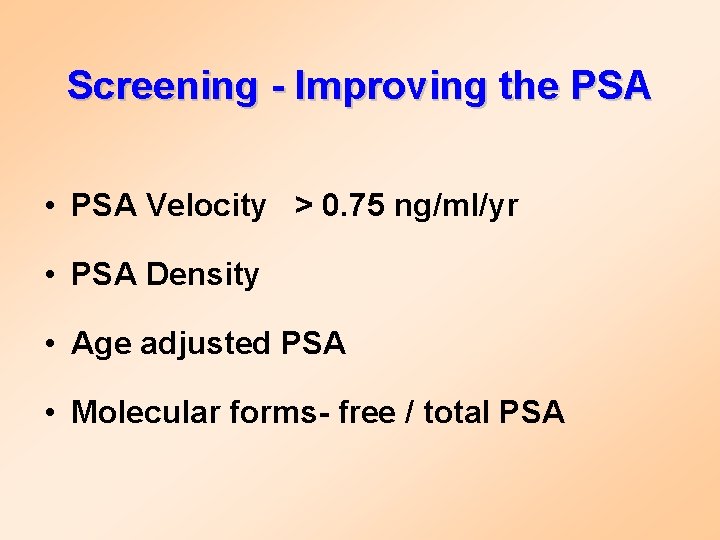
Screening - Improving the PSA • PSA Velocity > 0. 75 ng/ml/yr • PSA Density • Age adjusted PSA • Molecular forms- free / total PSA

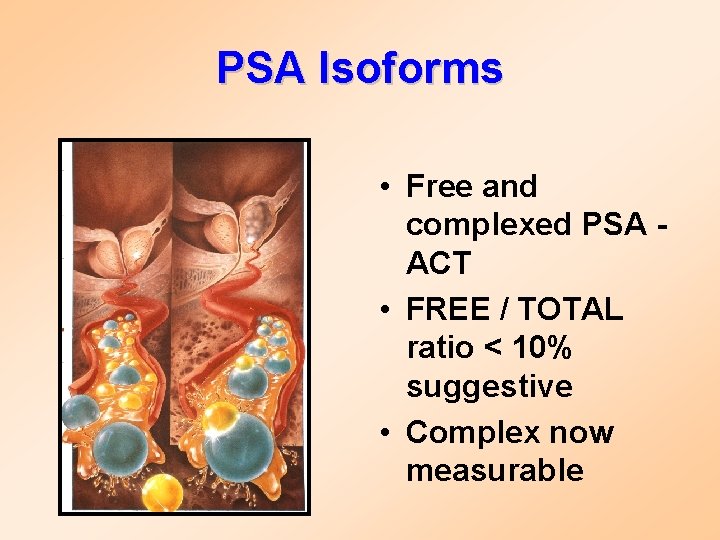
PSA Isoforms • Free and complexed PSA - ACT • FREE / TOTAL ratio < 10% suggestive • Complex now measurable

Digital Rectal Exam for Prostate Tumors

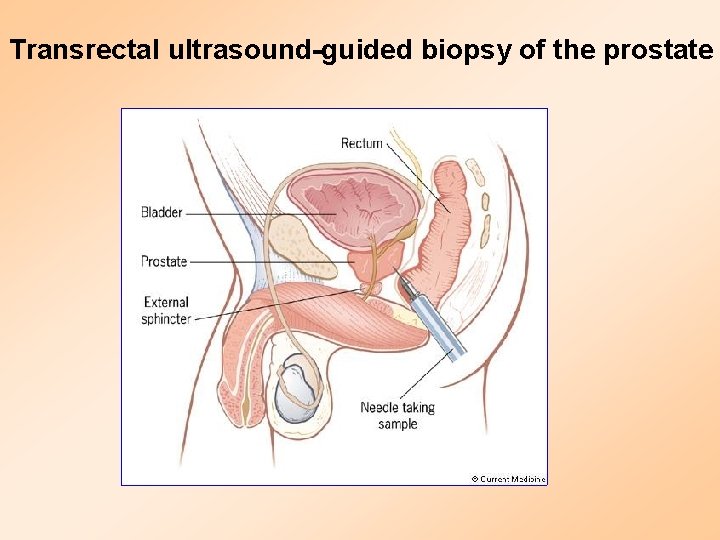
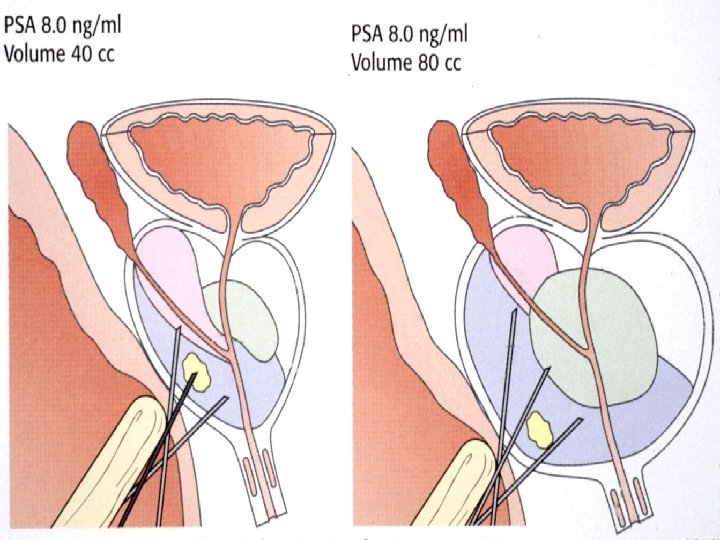
Transrectal ultrasound-guided biopsy of the prostate


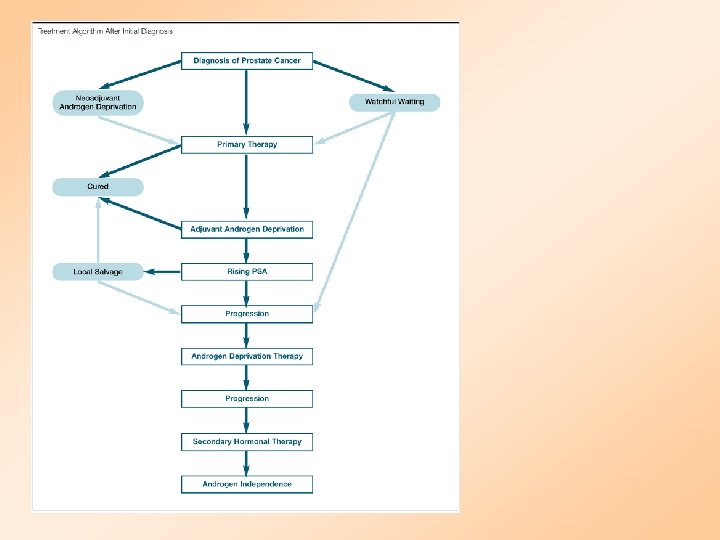
Management Alternatives • Expectant -- Watchful Waiting • Radical Prostatectomy • Radiation Therapy -- EBRT, 3 D - CRT, Brachytherapy: HDR, Seed • Hormonal -- Mono Rx, MAB • Combination

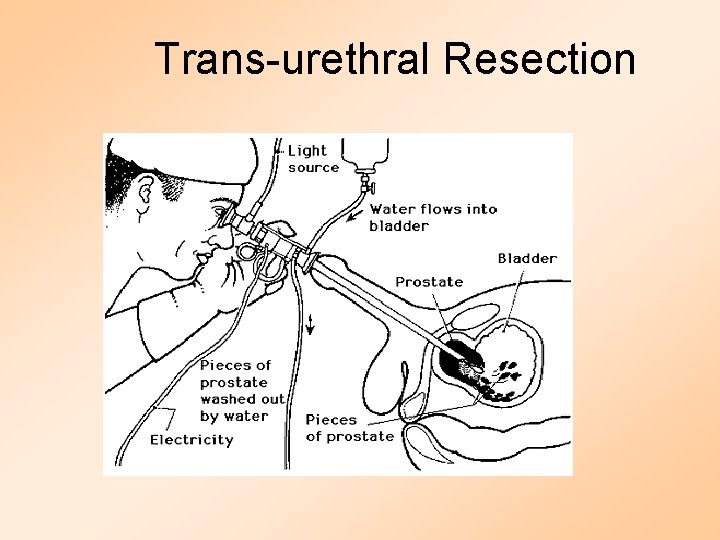
Trans-urethral Resection


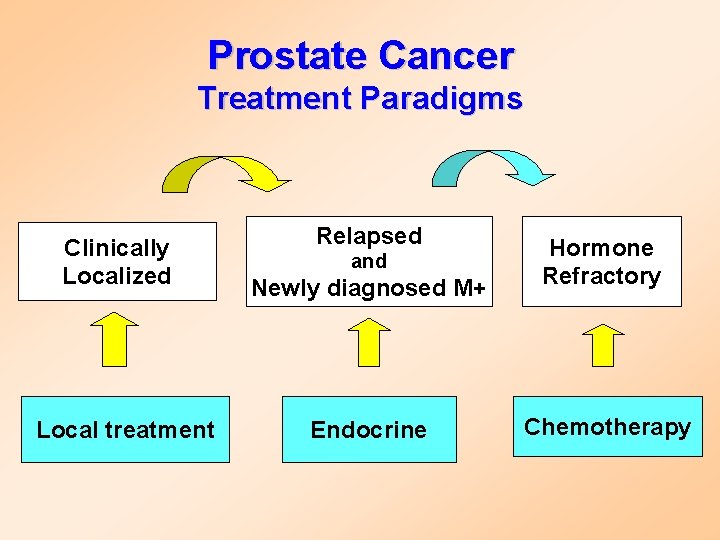
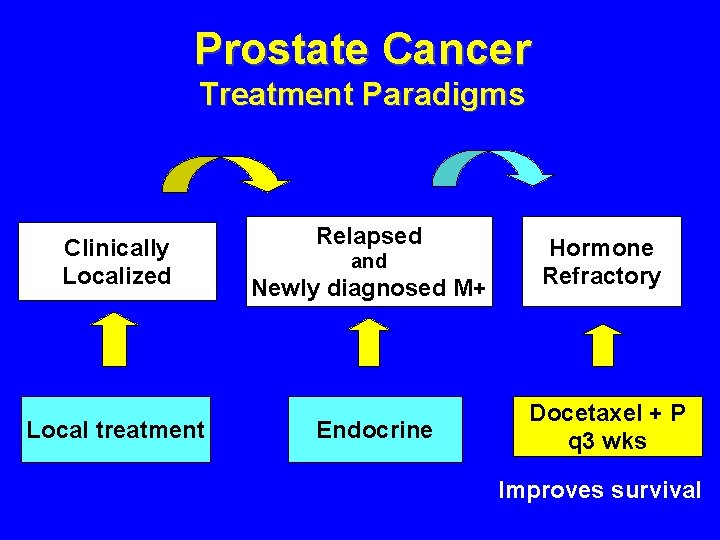
Prostate Cancer Treatment Paradigms Clinically Localized Local treatment Relapsed Newly diagnosed M+ Hormone Refractory Endocrine Chemotherapy and

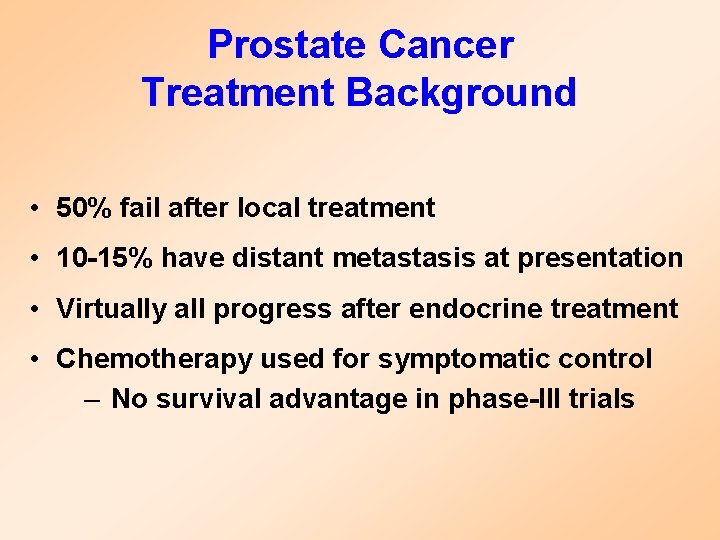
Prostate Cancer Treatment Background • 50% fail after local treatment • 10 -15% have distant metastasis at presentation • Virtually all progress after endocrine treatment • Chemotherapy used for symptomatic control – No survival advantage in phase-III trials

Endocrine control of prostate cancer

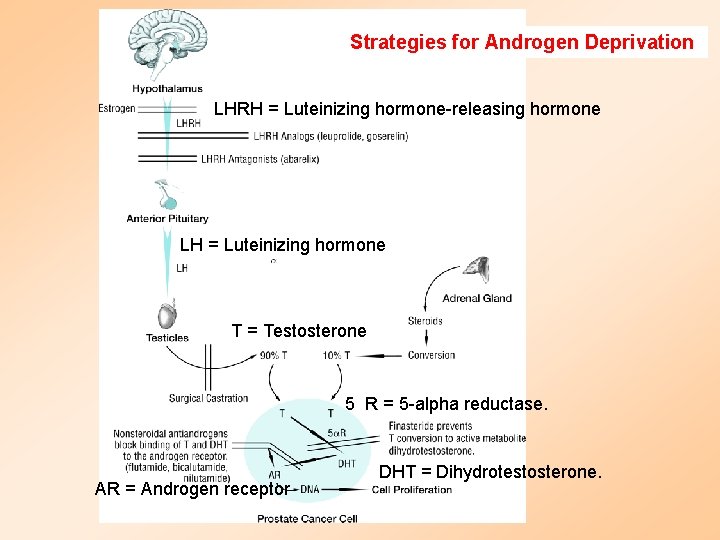
Strategies for Androgen Deprivation LHRH = Luteinizing hormone-releasing hormone LH = Luteinizing hormone T = Testosterone 5 R = 5 -alpha reductase. AR = Androgen receptor DHT = Dihydrotestosterone.

Types of Androgen Deprivation Monotherapy Bilateral orchiectomy Medical castration Estrogen LHRH agonist: leuprolide, goserelin Steroidal antiandrogens Megesterol acetate Cyproterone acetate Nonsteroidal antiandrogens Flutamide Bicalutamide Nilutamide Primary gonadal suppression + antiandrogen

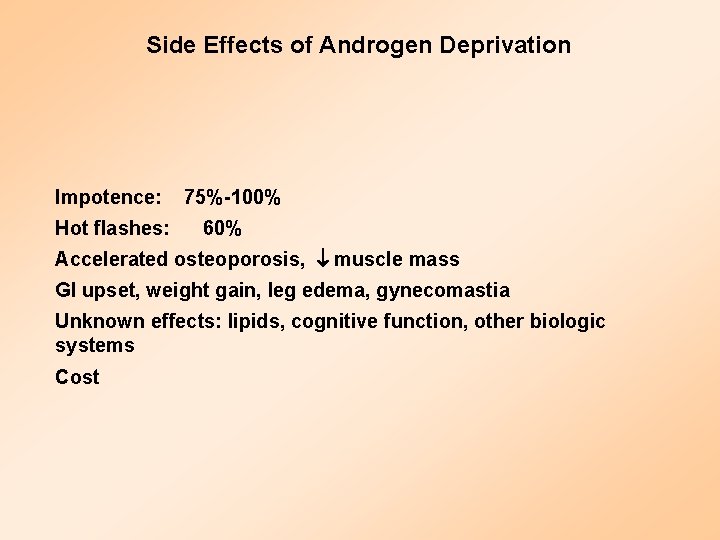
Side Effects of Androgen Deprivation Impotence: 75%-100% Hot flashes: 60% Accelerated osteoporosis, muscle mass GI upset, weight gain, leg edema, gynecomastia Unknown effects: lipids, cognitive function, other biologic systems Cost

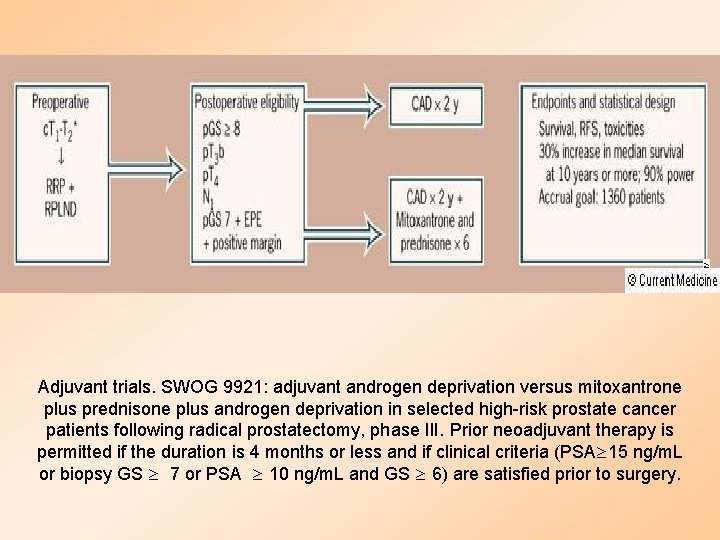
Adjuvant trials. SWOG 9921: adjuvant androgen deprivation versus mitoxantrone plus prednisone plus androgen deprivation in selected high-risk prostate cancer patients following radical prostatectomy, phase III. Prior neoadjuvant therapy is permitted if the duration is 4 months or less and if clinical criteria (PSA 15 ng/m. L or biopsy GS 7 or PSA 10 ng/m. L and GS 6) are satisfied prior to surgery.

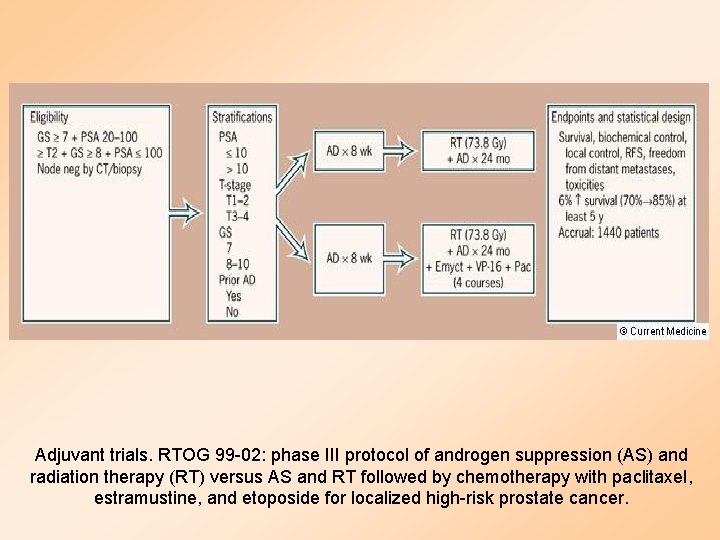
Adjuvant trials. RTOG 99 -02: phase III protocol of androgen suppression (AS) and radiation therapy (RT) versus AS and RT followed by chemotherapy with paclitaxel, estramustine, and etoposide for localized high-risk prostate cancer.

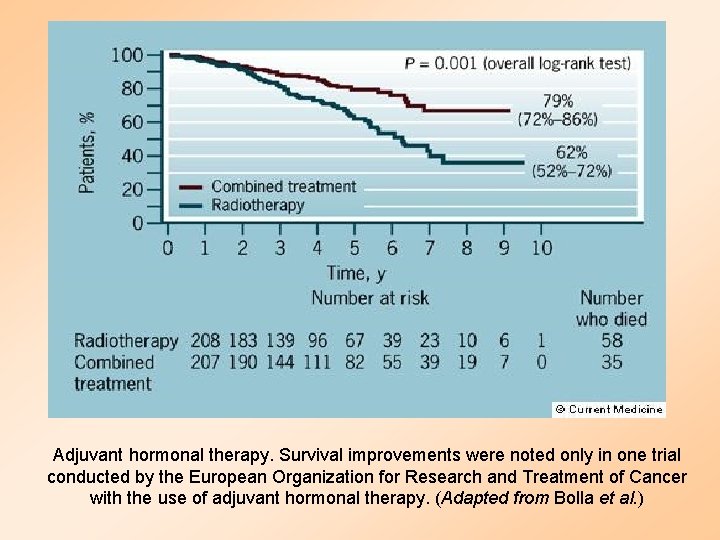
Adjuvant hormonal therapy. Survival improvements were noted only in one trial conducted by the European Organization for Research and Treatment of Cancer with the use of adjuvant hormonal therapy. (Adapted from Bolla et al. )

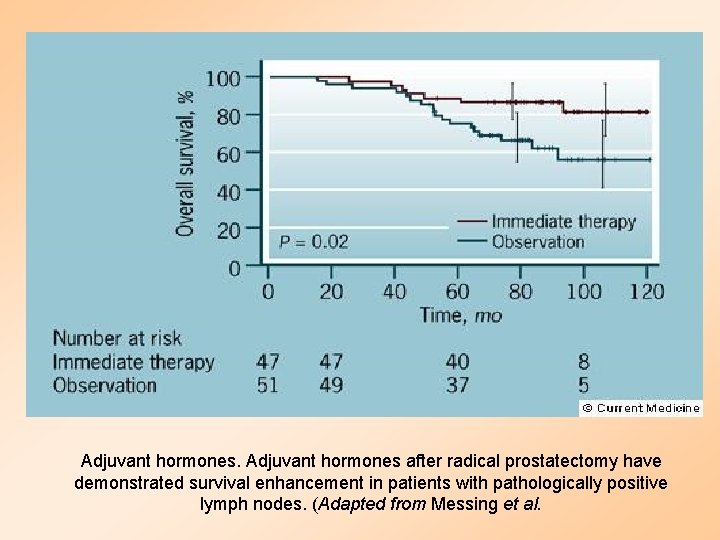
Adjuvant hormones after radical prostatectomy have demonstrated survival enhancement in patients with pathologically positive lymph nodes. (Adapted from Messing et al.

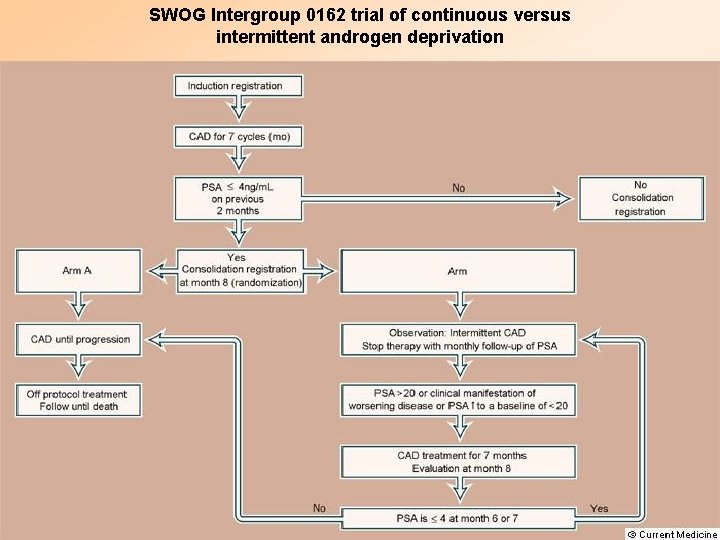
SWOG Intergroup 0162 trial of continuous versus intermittent androgen deprivation

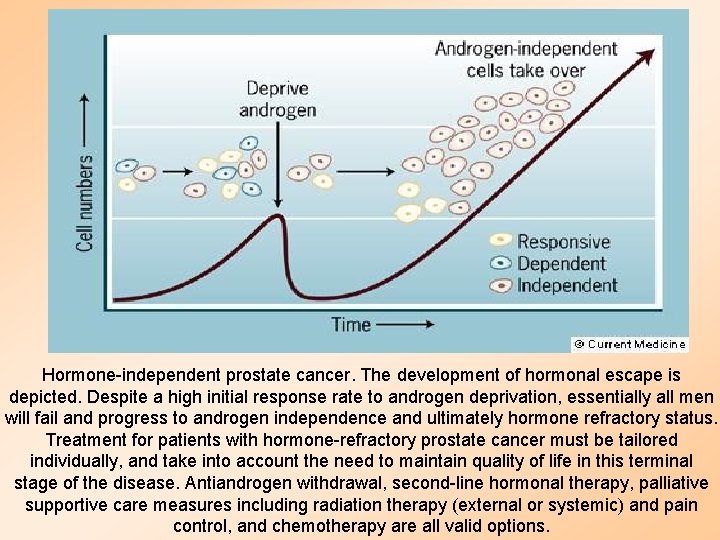
Hormone-independent prostate cancer. The development of hormonal escape is depicted. Despite a high initial response rate to androgen deprivation, essentially all men will fail and progress to androgen independence and ultimately hormone refractory status. Treatment for patients with hormone-refractory prostate cancer must be tailored individually, and take into account the need to maintain quality of life in this terminal stage of the disease. Antiandrogen withdrawal, second-line hormonal therapy, palliative supportive care measures including radiation therapy (external or systemic) and pain control, and chemotherapy are all valid options.

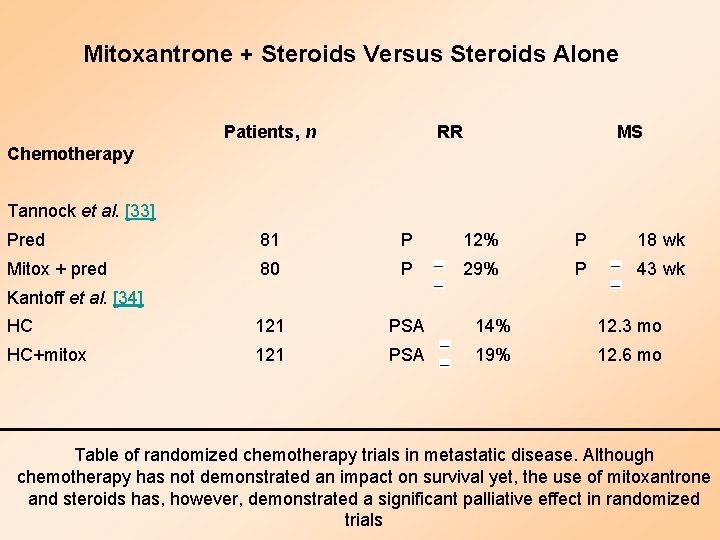
Mitoxantrone + Steroids Versus Steroids Alone Patients, n RR MS Pred 81 P 12% P 18 wk Mitox + pred 80 P 29% P 43 wk HC 121 PSA 14% 12. 3 mo HC+mitox 121 PSA 19% 12. 6 mo Chemotherapy Tannock et al. [33] Kantoff et al. [34] Table of randomized chemotherapy trials in metastatic disease. Although chemotherapy has not demonstrated an impact on survival yet, the use of mitoxantrone and steroids has, however, demonstrated a significant palliative effect in randomized trials

SWOG trial of chemotherapy in metastatic disease. SWOG Intergroup 9916 randomized phase III study of docetaxel + estramustine versus mitoxantrone + prednisone in patients with hormone-refractory prostate cancer; 620 patients must be entered to detect a 33% survival difference. Future directions include exploring biologic therapies such as epithelial growth factor receptor inhibitors and antiangiogenesis strategies.

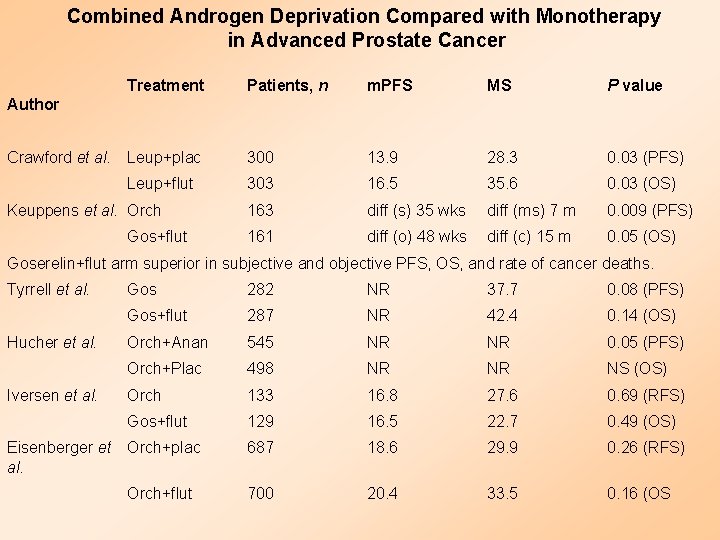
Combined Androgen Deprivation Compared with Monotherapy in Advanced Prostate Cancer Treatment Patients, n m. PFS MS P value 300 13. 9 28. 3 0. 03 (PFS) 303 16. 5 35. 6 0. 03 (OS) 163 diff (s) 35 wks diff (ms) 7 m 0. 009 (PFS) 161 diff (o) 48 wks diff (c) 15 m 0. 05 (OS) Author Crawford et al. Leup+plac Leup+flut Keuppens et al. Orch Gos+flut Goserelin+flut arm superior in subjective and objective PFS, OS, and rate of cancer deaths. Tyrrell et al. Hucher et al. Iversen et al. Gos 282 NR 37. 7 0. 08 (PFS) Gos+flut 287 NR 42. 4 0. 14 (OS) Orch+Anan 545 NR NR 0. 05 (PFS) Orch+Plac 498 NR NR NS (OS) Orch 133 16. 8 27. 6 0. 69 (RFS) Gos+flut 129 16. 5 22. 7 0. 49 (OS) 687 18. 6 29. 9 0. 26 (RFS) 700 20. 4 33. 5 0. 16 (OS Eisenberger et Orch+plac al. Orch+flut

Docetaxel in HRPC • • Multiple phase II studies Responses in 45 -82% (similar 95% CI duration) Estramustine based RR higher but more toxic Single agent data (weekly and every 3 wks) consistently safe and effective • Superior to mitoxantrone + prednisone?

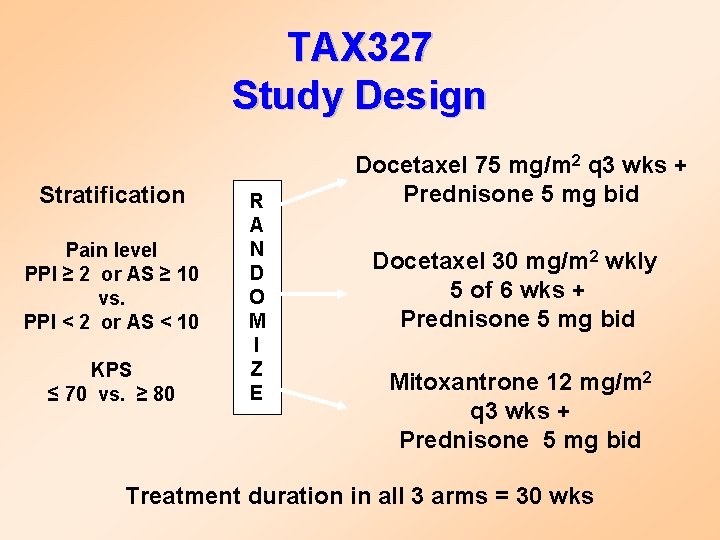
TAX 327 Study Design Stratification Pain level PPI ≥ 2 or AS ≥ 10 vs. PPI < 2 or AS < 10 KPS ≤ 70 vs. ≥ 80 R A N D O M I Z E Docetaxel 75 mg/m 2 q 3 wks + Prednisone 5 mg bid Docetaxel 30 mg/m 2 wkly 5 of 6 wks + Prednisone 5 mg bid Mitoxantrone 12 mg/m 2 q 3 wks + Prednisone 5 mg bid Treatment duration in all 3 arms = 30 wks

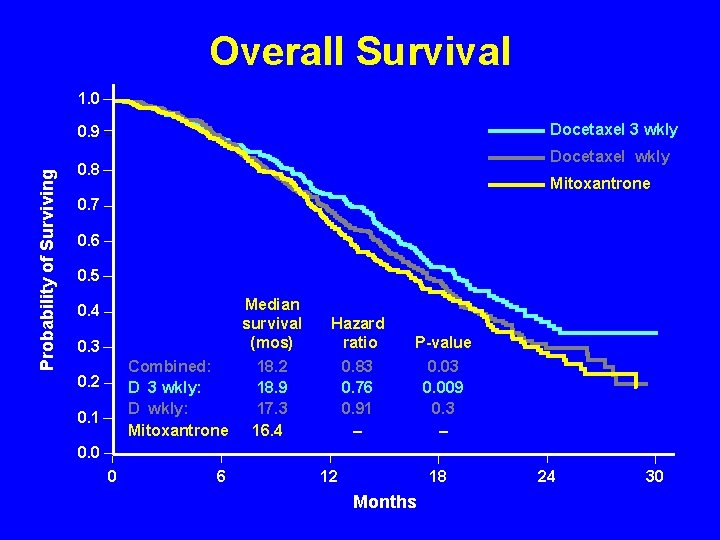
Overall Survival 1. 0 Docetaxel 3 wkly Probability of Surviving 0. 9 Docetaxel wkly 0. 8 Mitoxantrone 0. 7 0. 6 0. 5 0. 4 0. 3 Combined: D 3 wkly: D wkly: Mitoxantrone 0. 2 0. 1 Median survival (mos) Hazard ratio P-value 18. 2 18. 9 17. 3 16. 4 0. 83 0. 76 0. 91 – 0. 03 0. 009 0. 3 – 0. 0 0 6 12 18 Months 24 30

TAX 327 Docetaxel 3 Weekly • Safe • Significantly improves: – Survival (18. 9 vs 16. 5 months) 24% reduction in the risk of death (95% CI 0. 62 -0. 94, p=. 009) – PSA decline - 45% vs. 32%, p<. 0005 – Pain response - 35% vs. 22%, p=. 01 – Quality of life

Prostate Cancer Treatment Paradigms Clinically Localized Local treatment Relapsed Newly diagnosed M+ Hormone Refractory Endocrine Docetaxel + P q 3 wks and Improves survival

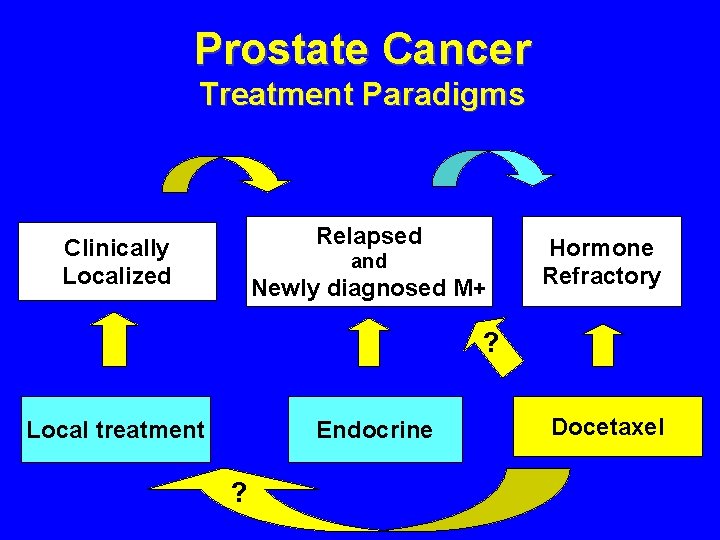
Prostate Cancer Treatment Paradigms Relapsed Clinically Localized and Newly diagnosed M+ Hormone Refractory ? Local treatment Endocrine ? Docetaxel

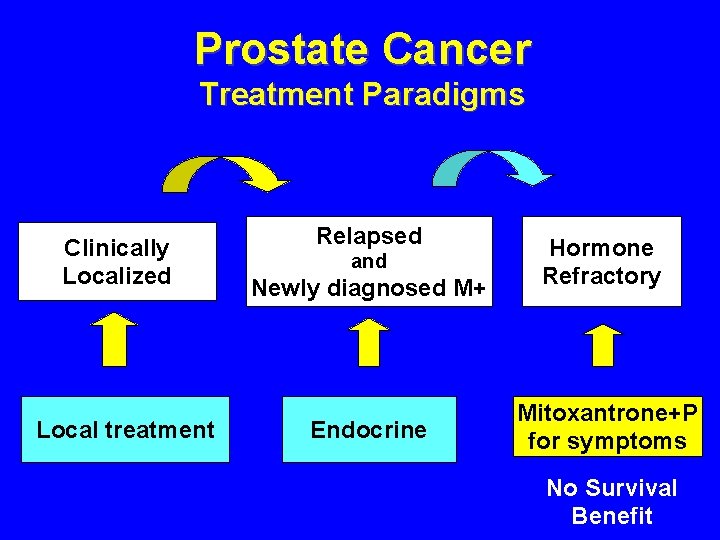
Prostate Cancer Treatment Paradigms Clinically Localized Local treatment Relapsed Newly diagnosed M+ Hormone Refractory Endocrine Mitoxantrone+P for symptoms and No Survival Benefit







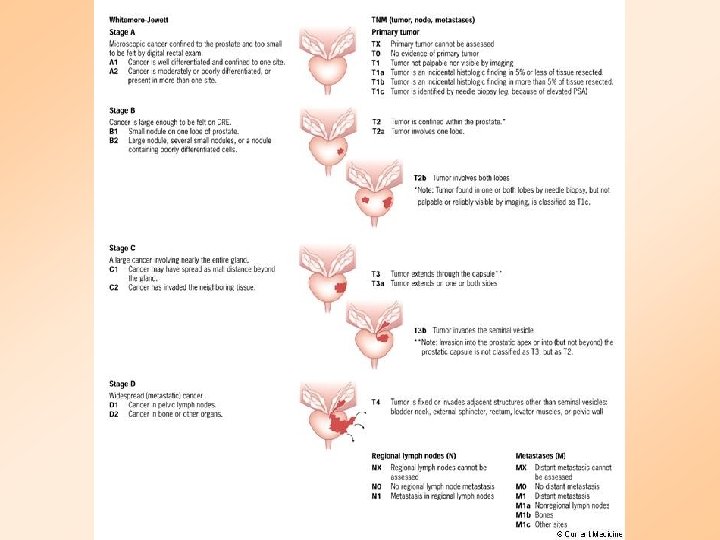
 Prostate cancer staging
Prostate cancer staging Mdv3100 prostate cancer
Mdv3100 prostate cancer Prostate
Prostate Laprp
Laprp Prostate cancer tnm classification
Prostate cancer tnm classification Quel taux de psa pour un cancer de la prostate ?
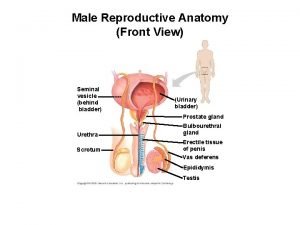
Quel taux de psa pour un cancer de la prostate ? Function of prostate gland
Function of prostate gland What does the seminal vesicle do
What does the seminal vesicle do Prostata biopsie nachbluten
Prostata biopsie nachbluten Adkp
Adkp Pdta prostata
Pdta prostata Pielonefrite xantogranulomatosa
Pielonefrite xantogranulomatosa Adk prostata
Adk prostata Mittfåra prostata
Mittfåra prostata Valores flujometria
Valores flujometria Farmacologia
Farmacologia Karcinom prostaty
Karcinom prostaty Prostata
Prostata Basis prostatae
Basis prostatae Ipertrofia
Ipertrofia Adenocarcinoma acinar de prostata
Adenocarcinoma acinar de prostata Adenom de prostata complicatii
Adenom de prostata complicatii Breast cancer anatomy and early warning signs
Breast cancer anatomy and early warning signs Cervix carcinoma
Cervix carcinoma Naphthylamine
Naphthylamine Neuro derm
Neuro derm Md frcpc definition
Md frcpc definition Invasive ductal carcinoma with medullary features
Invasive ductal carcinoma with medullary features Breast lump differential diagnosis
Breast lump differential diagnosis Carcinoma de mama
Carcinoma de mama Carcinoma in situ
Carcinoma in situ Carcinoma in situ
Carcinoma in situ Icd 10 ca nasofaring
Icd 10 ca nasofaring Wikipedia commons
Wikipedia commons Squamous cell carcinoma louisiana
Squamous cell carcinoma louisiana Follicular carcinoma of thyroid
Follicular carcinoma of thyroid Breast papillary carcinoma
Breast papillary carcinoma Hormones
Hormones Arteria axilar
Arteria axilar Epithelial component
Epithelial component Carcinoma of stomach
Carcinoma of stomach Cancer de pulmon
Cancer de pulmon Haggit kriterleri
Haggit kriterleri Nodular melanoma
Nodular melanoma Mucoepidermoid carcinoma pathology outlines
Mucoepidermoid carcinoma pathology outlines Carcinoma
Carcinoma Esophagus
Esophagus Carcinoma comedonico
Carcinoma comedonico Carcinoma
Carcinoma Squamous cell carcinoma
Squamous cell carcinoma Carcinoma on scalp
Carcinoma on scalp Carcinoma renal de células claras fuhrman
Carcinoma renal de células claras fuhrman Lichen sclerosus vulvare
Lichen sclerosus vulvare Tnm cáncer de pulmón 2021
Tnm cáncer de pulmón 2021 Carcinoma epidermoide microinfiltrante
Carcinoma epidermoide microinfiltrante Lichen sclerosus vulvare
Lichen sclerosus vulvare Bcc pathology
Bcc pathology Carcinoma epidermoide
Carcinoma epidermoide Warthin's tumor
Warthin's tumor Carcinoma squamoso polmone
Carcinoma squamoso polmone Carcinoma squamoso polmone
Carcinoma squamoso polmone Dr. aldo azael garza galindo
Dr. aldo azael garza galindo Follicular adenoma
Follicular adenoma