Chemistry in day to days life Chemistry has




























- Slides: 50

Chemistry in day to days life Chemistry has established itself every where in every man’s life. But usually people are unaware that chemistry has to do something around them. Latest discoveries proved that even to think about something some chemistry has to undergo in the brain which itself is made of molecules


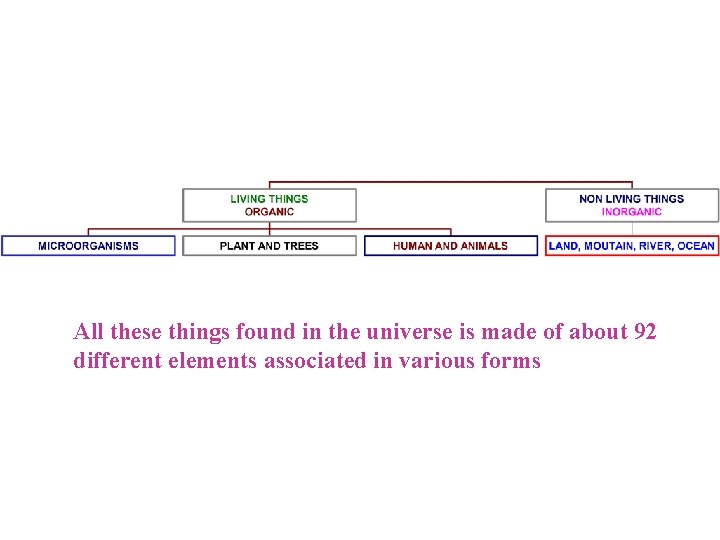
All these things found in the universe is made of about 92 different elements associated in various forms

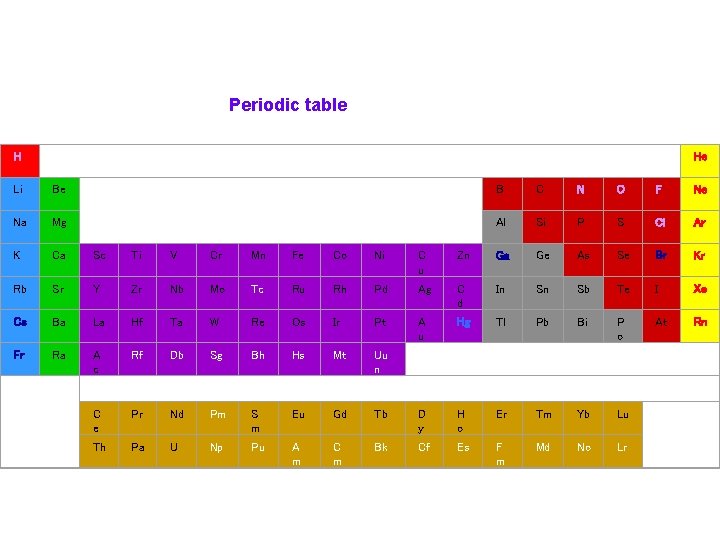
Periodic table H Li Be Na Mg K Ca Sc Ti V Cr Mn Fe Co Ni C u Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Cs Ba La Hf Ta W Re Os Ir Fr Ra A c Rf Db Sg Bh Hs C e Pr Nd Pm S m Th Pa U Np Pu He B C N O F Ne Al Si P S Cl Ar Zn Ga Ge As Se Br Kr Ag C d In Sn Sb Te I Xe Pt A u Hg Tl Pb Bi P o At Rn Mt Uu n Eu Gd Tb D y H o Er Tm Yb Lu A m C m Bk Cf Es F m Md No Lr

A molecule is the smallest unit of a compound that exhibits the properties of the compound. Chemical formula - a shorthand formula showing the number of atoms of each element present in a molecule, e. g. H 2 O, CO , O 2 , C H O Structural Formula - shows not only the number of atoms of each type but how they are fastened together. e. g. H-O-H , O=C=O , O=O

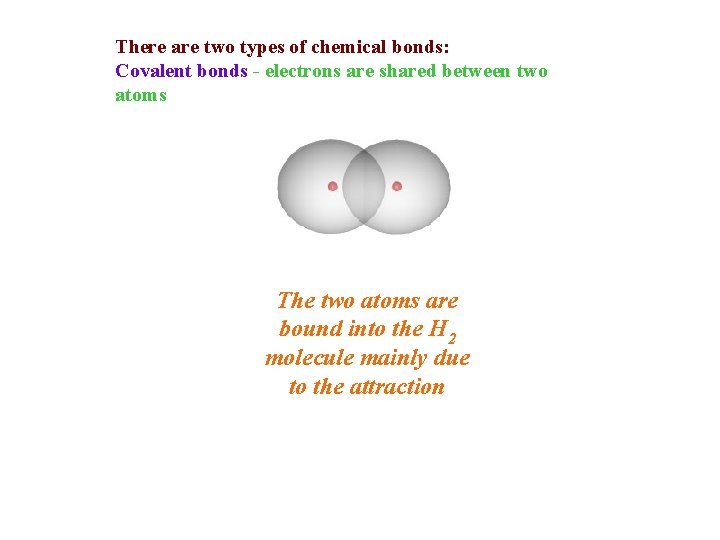
There are two types of chemical bonds: Covalent bonds - electrons are shared between two atoms The two atoms are bound into the H 2 molecule mainly due to the attraction

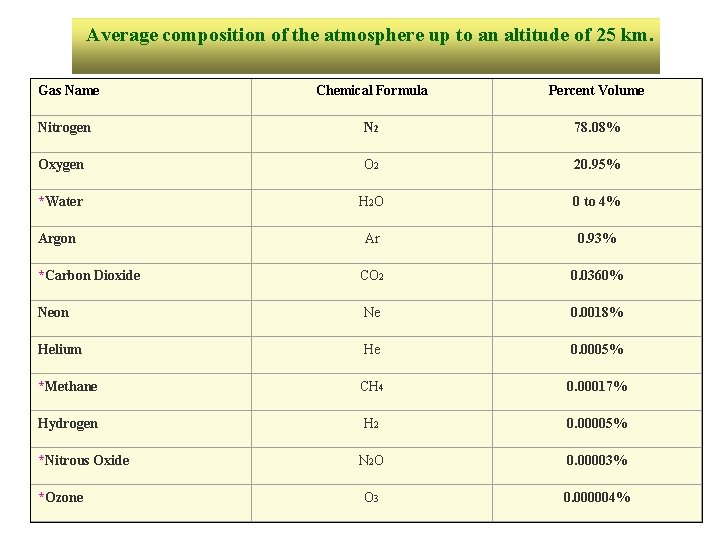
Average composition of the atmosphere up to an altitude of 25 km. Gas Name Chemical Formula Percent Volume Nitrogen N 2 78. 08% Oxygen O 2 20. 95% *Water H 2 O 0 to 4% Argon Ar 0. 93% *Carbon Dioxide CO 2 0. 0360% Neon Ne 0. 0018% Helium He 0. 0005% *Methane CH 4 0. 00017% Hydrogen H 2 0. 00005% N 2 O 0. 00003% O 3 0. 000004% *Nitrous Oxide *Ozone

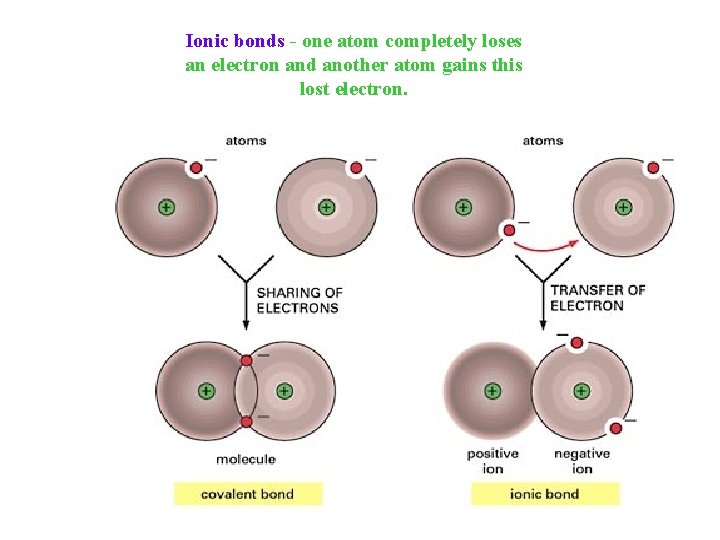
Ionic bonds - one atom completely loses an electron and another atom gains this lost electron.

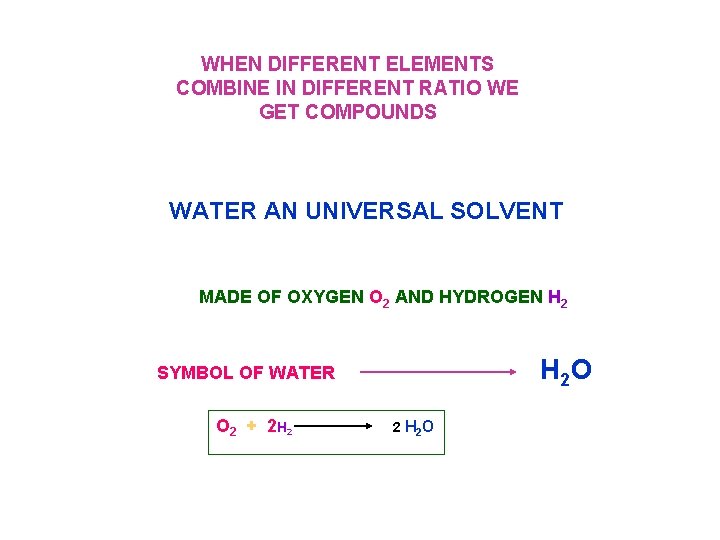
WHEN DIFFERENT ELEMENTS COMBINE IN DIFFERENT RATIO WE GET COMPOUNDS WATER AN UNIVERSAL SOLVENT MADE OF OXYGEN O 2 AND HYDROGEN H 2 SYMBOL OF WATER H 2 O O 2 + 2 H 2 2 H 2 O

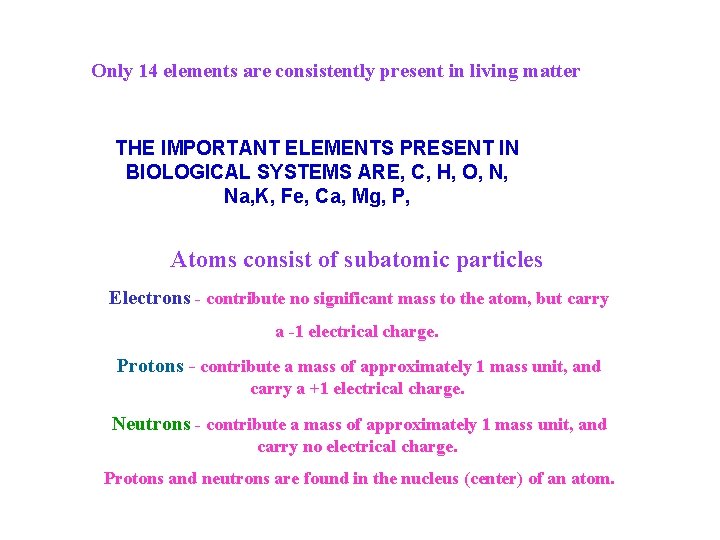
Only 14 elements are consistently present in living matter THE IMPORTANT ELEMENTS PRESENT IN BIOLOGICAL SYSTEMS ARE, C, H, O, N, Na, K, Fe, Ca, Mg, P, Atoms consist of subatomic particles Electrons - contribute no significant mass to the atom, but carry a -1 electrical charge. Protons - contribute a mass of approximately 1 mass unit, and carry a +1 electrical charge. Neutrons - contribute a mass of approximately 1 mass unit, and carry no electrical charge. Protons and neutrons are found in the nucleus (center) of an atom.

Let us take the example of human 1. He/she breadths Oxygen. Without oxygen no life. 2. As soon as he/she gets up he uses toothpaste which is made of chemicals The exact composition of a particular toothpaste varies with each manufacturer, but a typical formulation is abrasive 1040%, humectant 20 -70%, water 5 -30%, binder 1 -2%, detergent 1 -3%, flavour 1 -2%, preservative 0. 05 -0. 5% and therapeutic agent 0. 1 -0. 5%. 3. The brush that he/she uses to clean the teeth is made of chemical known as polymers e. g. polyurethanes

After brushing he drinks tea or coffee composition 1. Milk is composed of water, carbohydrate (lactose), fat, protein, minerals and vitamins. 2. Tea powder Apart from polyphenols, tea also contains caffeine, theophylline and theobromine in significant quantities. Caffeine is a mild stimulant which affects the central nervous system, the respiratory and metabolic processes of the cells. It is caffeine which provides the relaxing and uplifting effect of tea. 3. Sugar Carbohydrates

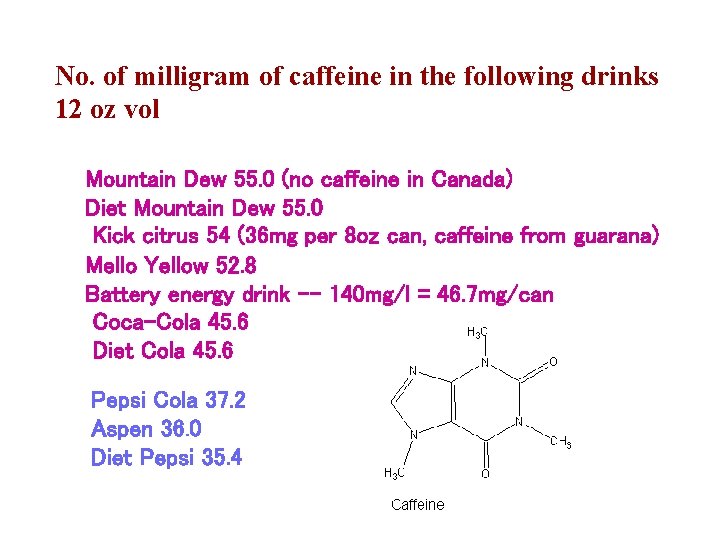
No. of milligram of caffeine in the following drinks 12 oz vol Mountain Dew 55. 0 (no caffeine in Canada) Diet Mountain Dew 55. 0 Kick citrus 54 (36 mg per 8 oz can, caffeine from guarana) Mello Yellow 52. 8 Battery energy drink -- 140 mg/l = 46. 7 mg/can Coca-Cola 45. 6 Diet Cola 45. 6 Pepsi Cola 37. 2 Aspen 36. 0 Diet Pepsi 35. 4

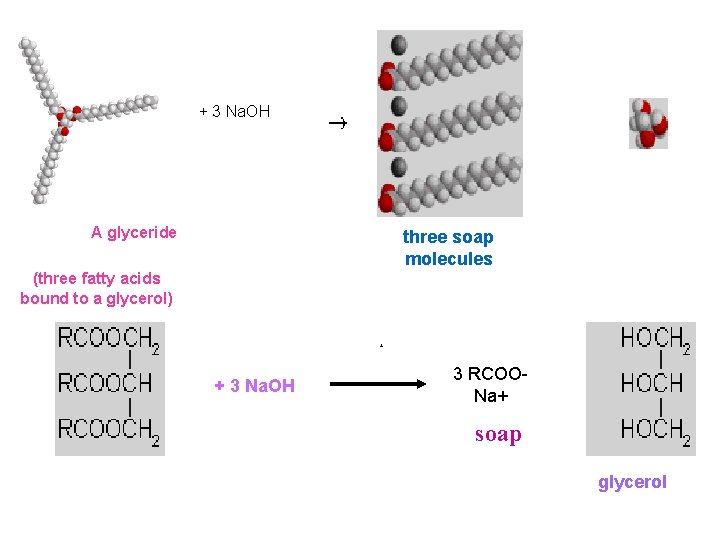
3. Taking bath a. Soap sodium salt of higher fatty acids What is soap made of? Soaps are made of molecules that are both fat and water soluble. The molecule has a long hydrocarbon tail that allows it to dissolve grease, and a polar head that is water soluble. A soap molecule. The grey, red, and white balls represent carbon, oxygen, and hydrogen atoms, respectively. The sodium ion near the negatively charged oxygen atoms is not shown. The head is the sodium or potassium salt of an organic acid. How do you make a molecule like this? Animal fat contains them, chemically bound to a small three-armed molecule called glycerol. The links between each of the three acid molecules and the glycerol are easily broken in a hot, alkaline solution:

A soap molecule. The grey, red, and white balls represent carbon, oxygen, and hydrogen atoms, respectively. The sodium ion near the negatively charged oxygen atoms is not shown. The head is the sodium or potassium salt of an organic acid. How do you make a molecule like this? Animal fat contains them, chemically bound to a small threearmed molecule called glycerol. The links between each of the three acid molecules and the glycerol are easily broken in a hot, alkaline solution

+ 3 Na. OH A glyceride + three soap molecules (three fatty acids bound to a glycerol) + 3 Na. OH 3 RCOO- Na+ soap glycerol

4. Shampoo 5. Bath towel halogenated organic compounds, formaldehyde, musk fragrances Cotton, Pure cellulose To wipeout water from our body we use cotton cloth not the synthetic cloth

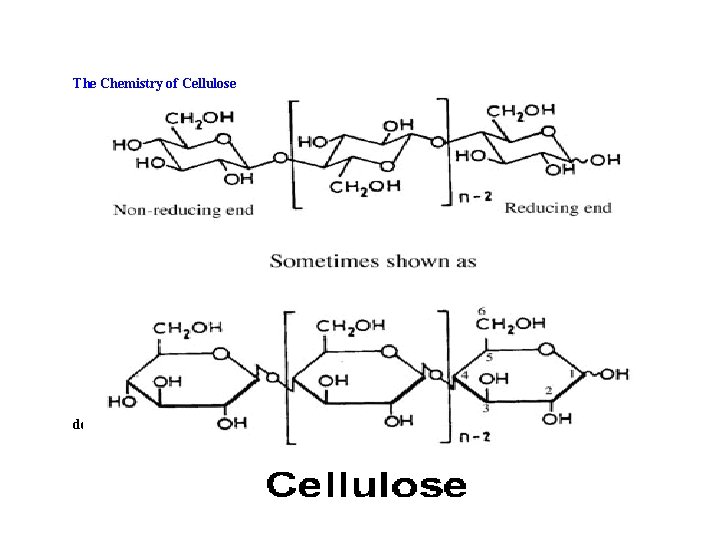
The Chemistry of Cellulose Although it took many decades after the identification of cellulose by Payen,

Cellulose is the major component of most plant cell walls. Cellulose is a major constituent of cotton, wood, and paper. cellulose, like starch, is also a polymer made from glucose units linked to each other. Unlike starch, animals do not make enzymes for breaking down cellulose. Cellulose does serve as a source of fiber in animal diets, however the only organisms that make enzymes that can break down cellulose are fungi and bacteria. Animals that live on materials rich in cellulose, e. g. cattle and sheep, contain bacteria in their stomach that are able to break down cellulose for use by the animal.

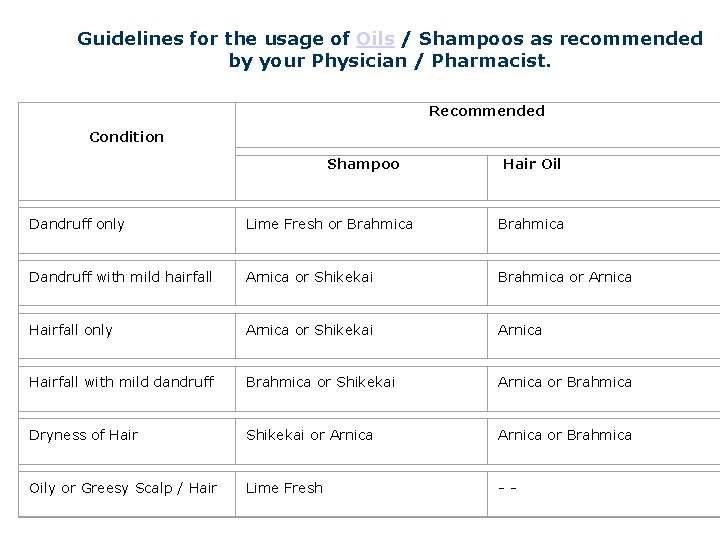
Guidelines for the usage of Oils / Shampoos as recommended by your Physician / Pharmacist. Recommended Condition Shampoo Hair Oil Dandruff only Lime Fresh or Brahmica Dandruff with mild hairfall Arnica or Shikekai Brahmica or Arnica Hairfall only Arnica or Shikekai Arnica Hairfall with mild dandruff Brahmica or Shikekai Arnica or Brahmica Dryness of Hair Shikekai or Arnica or Brahmica Oily or Greesy Scalp / Hair Lime Fresh - -

6. Hair oil Glyceryl esters Essential oils Oil and fat

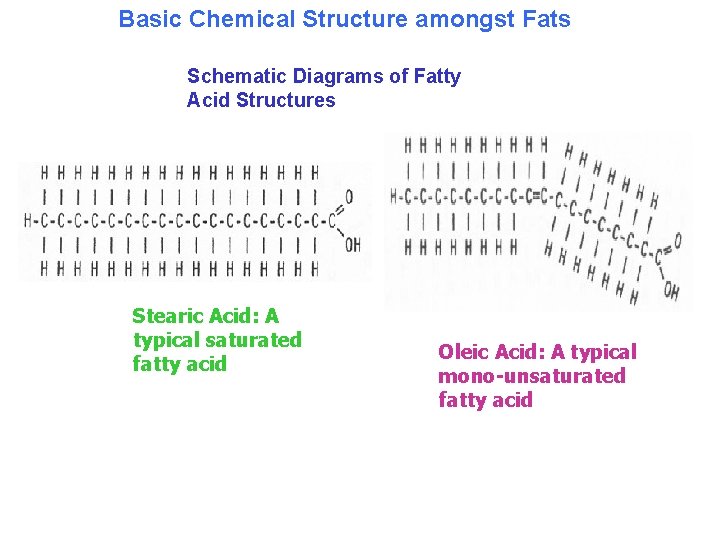
Lipids are fats or fat-like substances. Lipids are a heterogeneous group of compounds composed of carbon and hydrogen mostly and some oxygen, but some lipids also contain phosphorus. They are oily substances that are typically not very water soluble (nonpolar), although some lipids contain both polar and nonpolar regions.

Basic Chemical Structure amongst Fats Schematic Diagrams of Fatty Acid Structures Stearic Acid: A typical saturated fatty acid Oleic Acid: A typical mono-unsaturated fatty acid





6. Reading news paper Paper is made of cellulose 7. break fast Idle wade sambar Idle is made of exclusively carbohydrates-starch Wade is made of proteins and oil(majority) Sambar is really a big mixture of organic compounds

8. Wearing shirt and pant All these wears are made of cotton, polystyrene, silk, woolen Ofcourse you wont were only white, but many prefer coloured. COLOUR

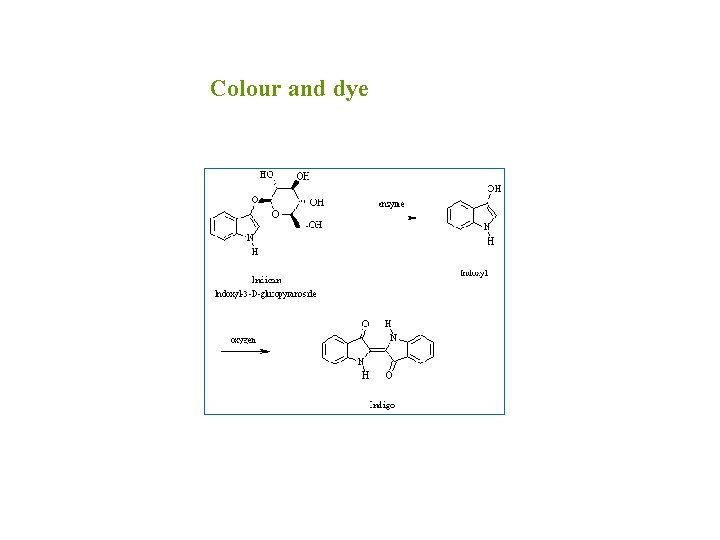
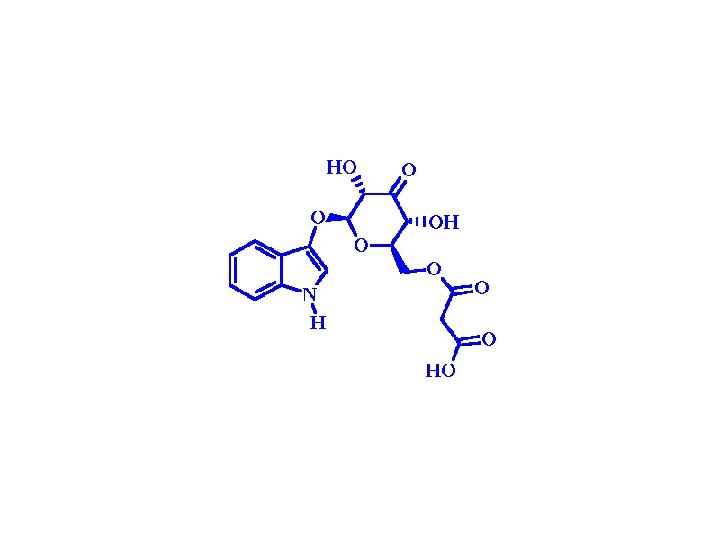
Colour and dye


Face powder Talc is a hydrated magnesium sheet silicate with the chemical formula Mg 3 Si 4 O 10(OH)2. The elementary sheet is composed of a layer of magnesium-oxygen/hydroxyl, sandwiched between two layers of silicon oxygen. The main or basal surfaces of this elementary sheet do not contain hydroxyl groups or active ions, which explains talc’s hydrophobicity and inertness

Question: What is make-up made of? Mostly solid and liquid foundations are made by a vanishing cream usually an oilin-water emulsion containing some fatty acid (as steric acid) and some coloring product. Face powder is more complicated and made by a mixture of products: talc (to help to spread), chalk or kaolin (to give moisture absorbing qualities), magnesium stearate (gives adherence), zinc oxide and titanium oxide (to help cover the skin thorougly) and pigments (for colour). Mascara for the eyes is made with vanishing cream and pigments. These have a fatty base together with pigments. Of course these are the general ingredients and the different commercial products have different formulas sometimes with fancy names. Many people are allergic to cosmetics and must be careful with the products they use, mostly with the ones that go near sensitive areas (lips and eyes). So, young ladies, if you want to be always pretty, use make up but always with care!

Perfumes It is one of the important factor in the modern life. The quality of perfume some time judge the prestige of the person. But chemistry wise there is no difference. All of them are made of simple elements.

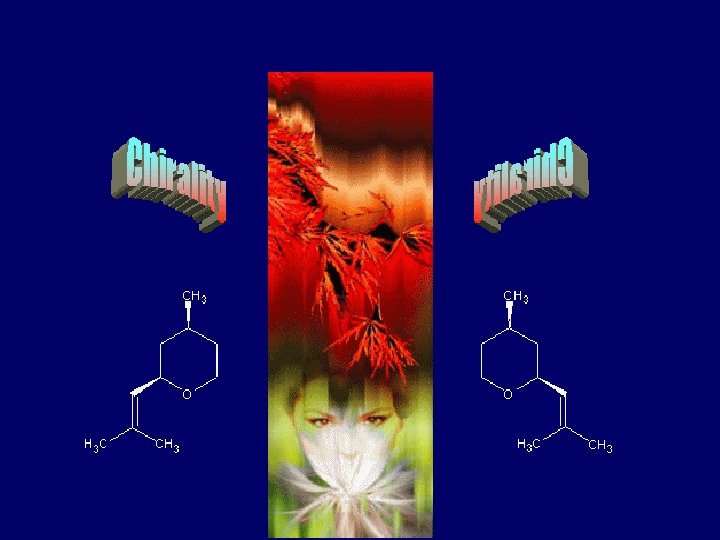
Chirality & Odour Perception

Vaseline------Petroleum Polycyclic aromatic hydrocarbons Petroleum wax---- Component of chewing gum Antioxidant ---- t-butylhydroquinone Preservative, bleaching agent---- SO 2


Petroleum consists of three main hydrocarbon groups: Paraffins These consist of straight or branched carbon rings saturated with hydrogen atoms, the simplest of which is methane (CH 4) the main ingredient of natural gas. Others in this group include ethane (C 2 H 6), and propane (C 3 H 8).

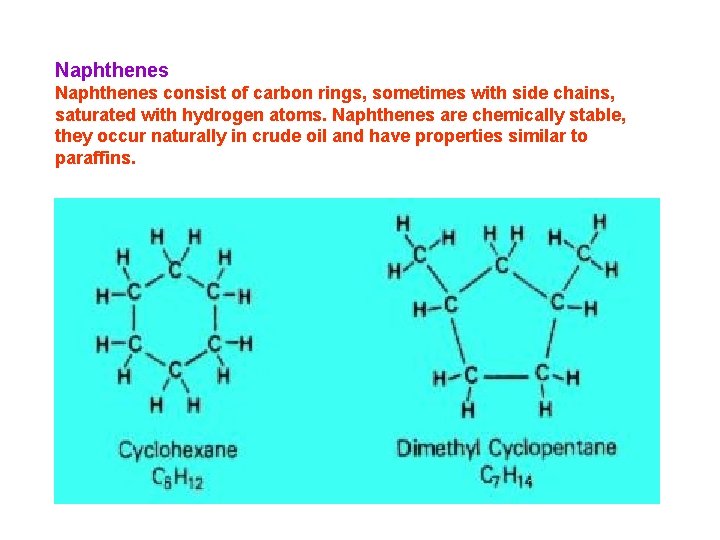
Naphthenes consist of carbon rings, sometimes with side chains, saturated with hydrogen atoms. Naphthenes are chemically stable, they occur naturally in crude oil and have properties similar to paraffins.

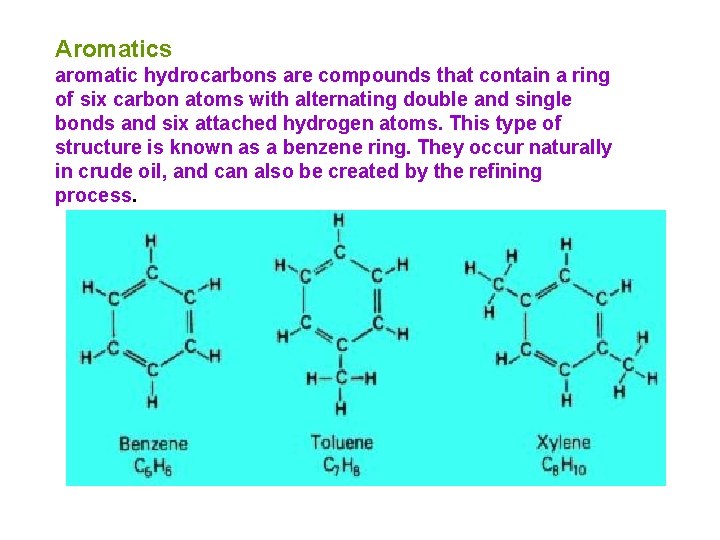
Aromatics aromatic hydrocarbons are compounds that contain a ring of six carbon atoms with alternating double and single bonds and six attached hydrogen atoms. This type of structure is known as a benzene ring. They occur naturally in crude oil, and can also be created by the refining process.

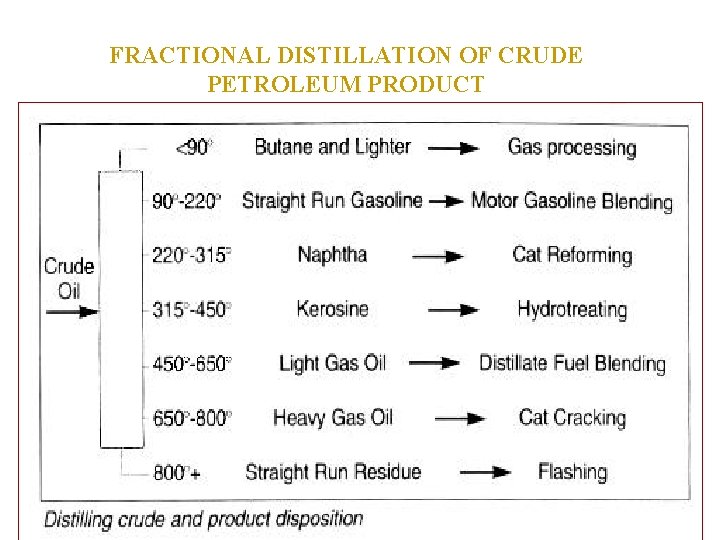
FRACTIONAL DISTILLATION OF CRUDE PETROLEUM PRODUCT

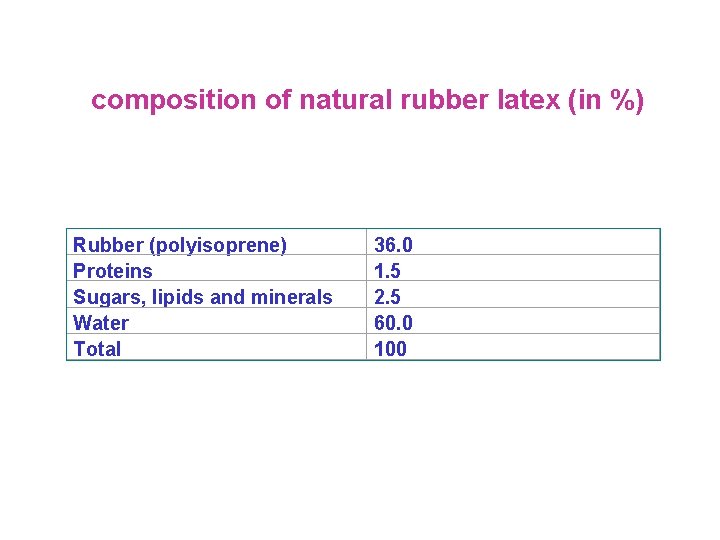
composition of natural rubber latex (in %) Rubber (polyisoprene) Proteins Sugars, lipids and minerals Water Total 36. 0 1. 5 2. 5 60. 0 100




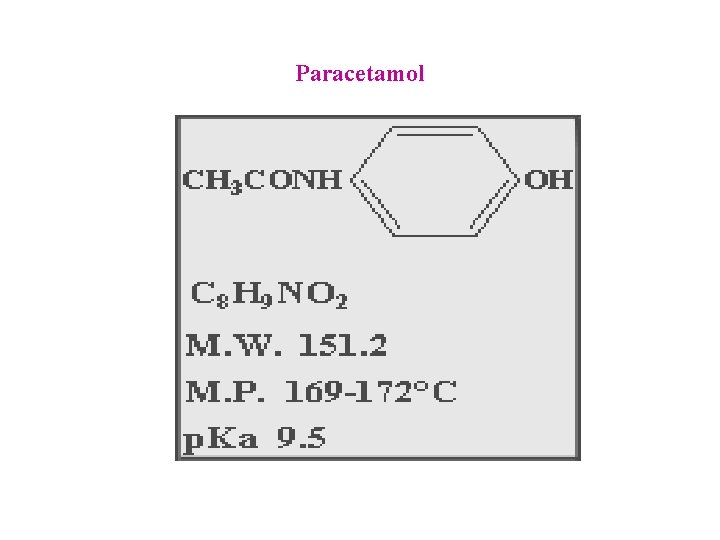
Paracetamol




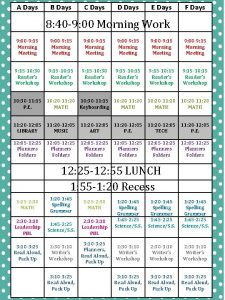
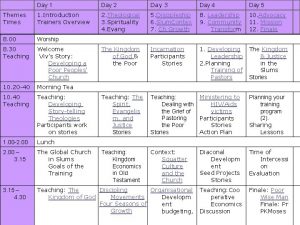
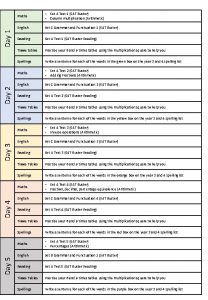
 Day 1 day 2 day 3 day 4
Day 1 day 2 day 3 day 4 Day 1 day 2 day 817
Day 1 day 2 day 817 Day of pentecost 50 days after
Day of pentecost 50 days after 30 days has september april june and november
30 days has september april june and november Last days of life toolkit
Last days of life toolkit Cec last days of life toolkit
Cec last days of life toolkit Pgcps calendar a day b day
Pgcps calendar a day b day Ocean the part day after day
Ocean the part day after day Day to day maintenance
Day to day maintenance As your room gets messier day by day, entropy is
As your room gets messier day by day, entropy is I don't know tomorrow
I don't know tomorrow Beautiful sunday evening
Beautiful sunday evening Growing day by day
Growing day by day Seed germination inhibitors examples
Seed germination inhibitors examples Germination conclusion
Germination conclusion Seeds vs spores
Seeds vs spores I live for jesus day after day
I live for jesus day after day Oh glorious day
Oh glorious day Day one day one noodle ss2
Day one day one noodle ss2 Day one day one noodles ss2
Day one day one noodles ss2 Whats a simple sentence
Whats a simple sentence The weather has been nice but it may snow again any day
The weather has been nice but it may snow again any day The weather has been nice but it may snow again any day
The weather has been nice but it may snow again any day Roots valentines
Roots valentines 24 hour time
24 hour time Easter legends and symbols
Easter legends and symbols Great britain has day ever easter
Great britain has day ever easter Functional groups ib chemistry
Functional groups ib chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry A day in the life of a scrum master
A day in the life of a scrum master One day can change your whole life
One day can change your whole life A day in the life of a compliance officer
A day in the life of a compliance officer One day your life will flash
One day your life will flash Frank has an eraser
Frank has an eraser A problem has been
A problem has been A problem has been detected and windows has been
A problem has been detected and windows has been Spring has sprung, the grass is riz
Spring has sprung, the grass is riz Every picture has a story and every story has a moment
Every picture has a story and every story has a moment He who has ears to hear let him hear
He who has ears to hear let him hear You light up my life chemistry lab answer key
You light up my life chemistry lab answer key Half life kinetics
Half life kinetics Chemistry
Chemistry Chapter 2 the chemistry of life section 2-3 answer key
Chapter 2 the chemistry of life section 2-3 answer key Concept 2 chemistry of life
Concept 2 chemistry of life Concept 2 chemistry of life
Concept 2 chemistry of life Deficiency disease of protein
Deficiency disease of protein Chemistry of life
Chemistry of life Chemistry for life
Chemistry for life Wonderful plan
Wonderful plan City life and country life paragraph
City life and country life paragraph Farm life vs city life
Farm life vs city life