Chapter 7 Types of Reactions Types of Reactions









- Slides: 19

Chapter 7 Types of Reactions

Types of Reactions �Synthesis �Decomposition �Single-replacement �Double-replacement �Combustion

Reaction Classification �Reactions are classified based on the: �Type of reactant �Number of reactants �Number of products

Synthesis Reactions � 2+ substances react to form a single substance �The reactants may be single elements or compounds �The product is always a compound �A + B → AB

Synthesis Reactions �Examples: � 2 Na + Cl 2 → 2 Na. Cl (table salt) �Sodium reacts with chlorine to form sodium chloride. � 2 H 2 + O 2 → 2 H 2 O (water) �Hydrogen reacts with oxygen to form dihydrogen monoxide or water.

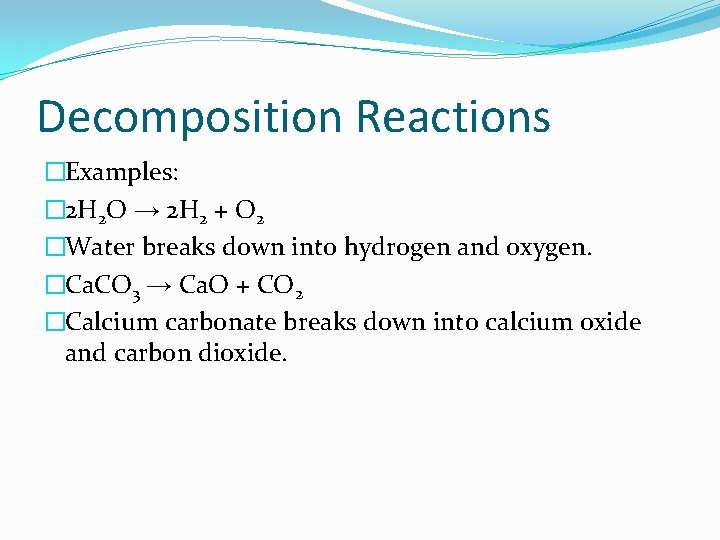
Decomposition Reactions �The opposite of synthesis reactions �A compound breaks down into 2+ simpler substances. �The reactant must be a compound. �The products may be single elements or compounds. �AB → A + B

Decomposition Reactions �Examples: � 2 H 2 O → 2 H 2 + O 2 �Water breaks down into hydrogen and oxygen. �Ca. CO 3 → Ca. O + CO 2 �Calcium carbonate breaks down into calcium oxide and carbon dioxide.

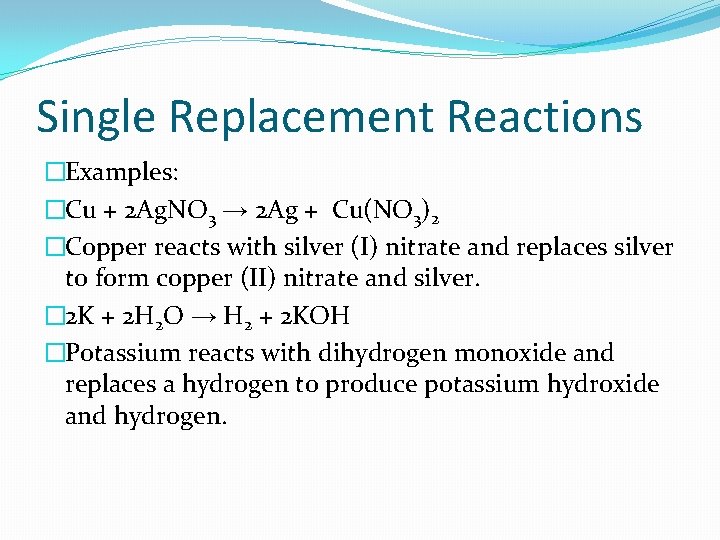
Single Replacement Reactions � 1 element takes the place of another element in a compound �A + BC → B + AC

Single Replacement Reactions �Examples: �Cu + 2 Ag. NO 3 → 2 Ag + Cu(NO 3)2 �Copper reacts with silver (I) nitrate and replaces silver to form copper (II) nitrate and silver. � 2 K + 2 H 2 O → H 2 + 2 KOH �Potassium reacts with dihydrogen monoxide and replaces a hydrogen to produce potassium hydroxide and hydrogen.

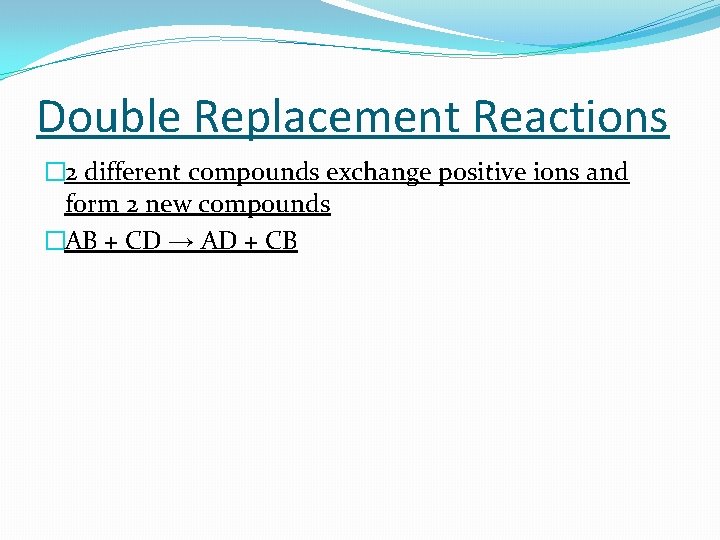
Double Replacement Reactions � 2 different compounds exchange positive ions and form 2 new compounds �AB + CD → AD + CB

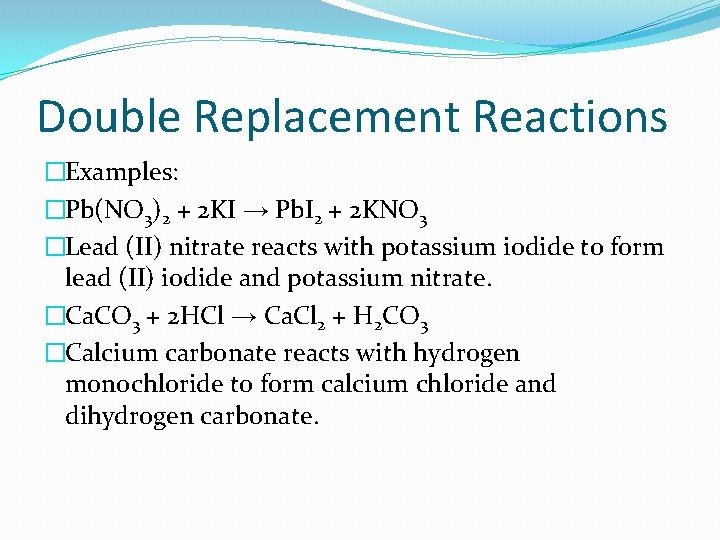
Double Replacement Reactions �Examples: �Pb(NO 3)2 + 2 KI → Pb. I 2 + 2 KNO 3 �Lead (II) nitrate reacts with potassium iodide to form lead (II) iodide and potassium nitrate. �Ca. CO 3 + 2 HCl → Ca. Cl 2 + H 2 CO 3 �Calcium carbonate reacts with hydrogen monochloride to form calcium chloride and dihydrogen carbonate.

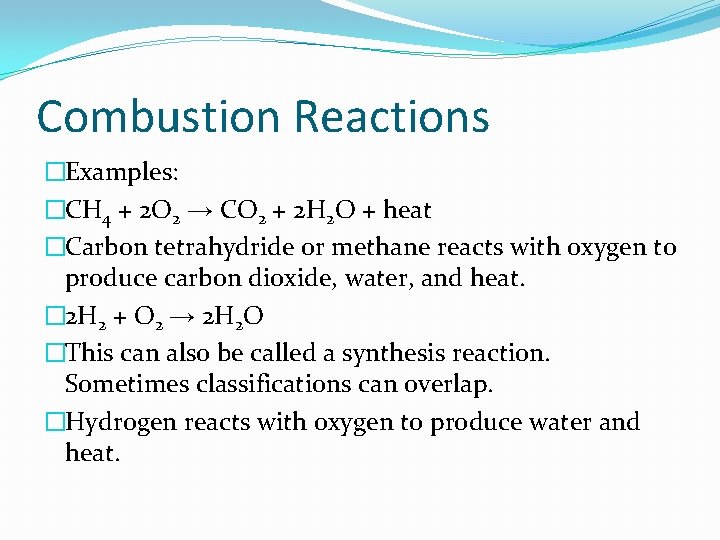
Combustion Reactions �A substance reacts rapidly with oxygen �Often heat and light are produced �This is what happens when gas is burned to power cars or fireplaces or Bunsen burners.

Combustion Reactions �Examples: �CH 4 + 2 O 2 → CO 2 + 2 H 2 O + heat �Carbon tetrahydride or methane reacts with oxygen to produce carbon dioxide, water, and heat. � 2 H 2 + O 2 → 2 H 2 O �This can also be called a synthesis reaction. Sometimes classifications can overlap. �Hydrogen reacts with oxygen to produce water and heat.

Questions! What type of reaction is it if: 1. Oxygen is a reactant? 2. 2 reactants form 1 product? 3. 1 reactant forms 2 products? 4. Both the reactants and products contain 2 compounds? 5. Both the reactants and products contain 1 compound and 1 single element?

Answers! 1. 2. 3. 4. 5. Combustion Synthesis Decomposition Double replacement Single replacement

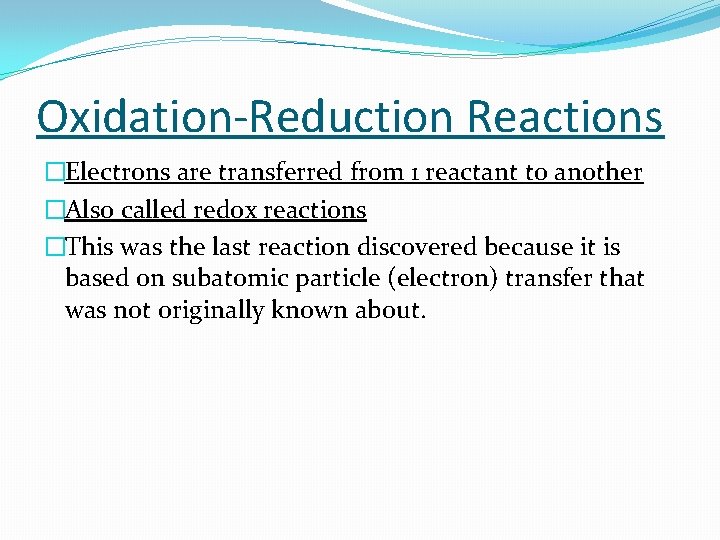
Oxidation-Reduction Reactions �Electrons are transferred from 1 reactant to another �Also called redox reactions �This was the last reaction discovered because it is based on subatomic particle (electron) transfer that was not originally known about.

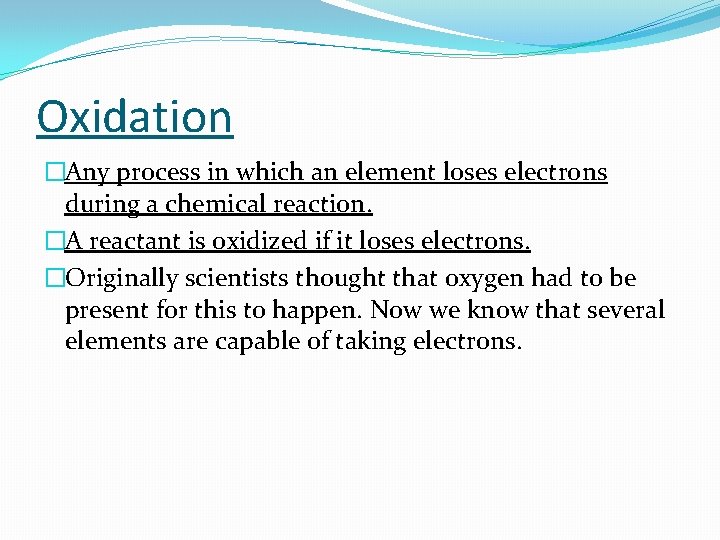
Oxidation �Any process in which an element loses electrons during a chemical reaction. �A reactant is oxidized if it loses electrons. �Originally scientists thought that oxygen had to be present for this to happen. Now we know that several elements are capable of taking electrons.

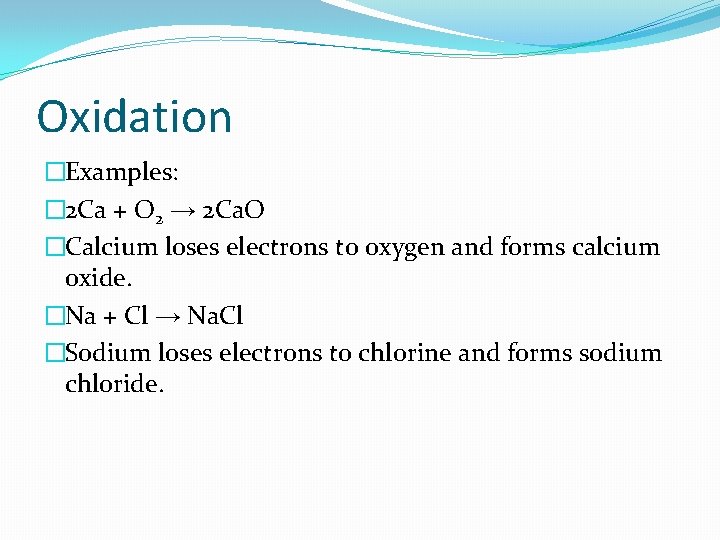
Oxidation �Examples: � 2 Ca + O 2 → 2 Ca. O �Calcium loses electrons to oxygen and forms calcium oxide. �Na + Cl → Na. Cl �Sodium loses electrons to chlorine and forms sodium chloride.

Reduction �The process in which an element gains electrons during a chemical reaction. �A reactant is said to be reduced if it gains electrons. �Oxidation and reduction ALWAYS occur together because when 1 element loses electrons the other element gains them.
 Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions How to write redox half reactions
How to write redox half reactions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Chapter 10 chemical reactions answer key
Chapter 10 chemical reactions answer key Chapter 9 chemical reactions study guide
Chapter 9 chemical reactions study guide Balancing redox reactions
Balancing redox reactions Types of reaction
Types of reaction Types of reactions
Types of reactions Single replacement reaction examples
Single replacement reaction examples Types of reactions chemistry
Types of reactions chemistry Types of chemical reactions
Types of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Nuclear fission equation
Nuclear fission equation Four types of chemical reactions
Four types of chemical reactions 5 types of reactions in chemistry
5 types of reactions in chemistry Five chemical
Five chemical 5 general types of chemical reactions
5 general types of chemical reactions Methane oxygen reaction
Methane oxygen reaction