6 2 Types of Reactions Types of Reactions









- Slides: 15

(6 -2) Types of Reactions

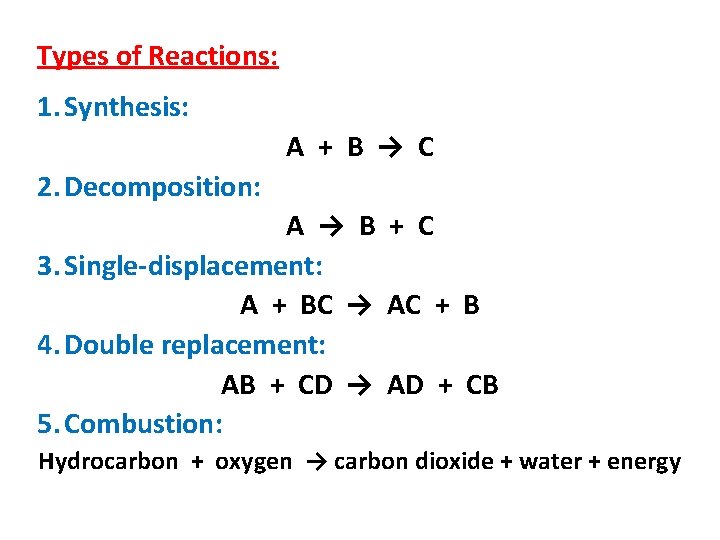
Types of Reactions: 1. Synthesis: A + B → C 2. Decomposition: A → B + C 3. Single-displacement: A + BC → AC + B 4. Double replacement: AB + CD → AD + CB 5. Combustion: Hydrocarbon + oxygen → carbon dioxide + water + energy

Synthesis When two substances combine to form a compound. – Rusting of iron: (producing iron (III) oxide) 4 Fe + 3 O 2 → 2 Fe 2 O 3 – Acid rain: (producing carbonic acid) H 2 O + CO 2 → H 2 CO 3 – Formation of iron sulfide: Fe + S → Fe. S


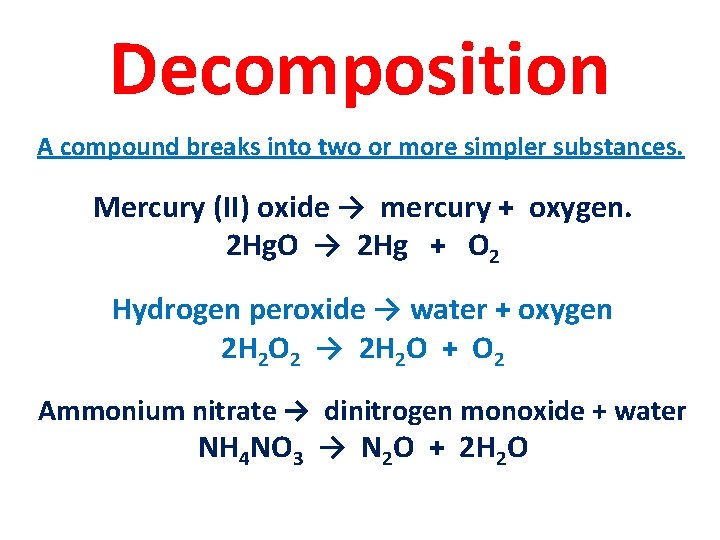
Decomposition A compound breaks into two or more simpler substances. Mercury (II) oxide → mercury + oxygen. 2 Hg. O → 2 Hg + O 2 Hydrogen peroxide → water + oxygen 2 H 2 O 2 → 2 H 2 O + O 2 Ammonium nitrate → dinitrogen monoxide + water NH 4 NO 3 → N 2 O + 2 H 2 O


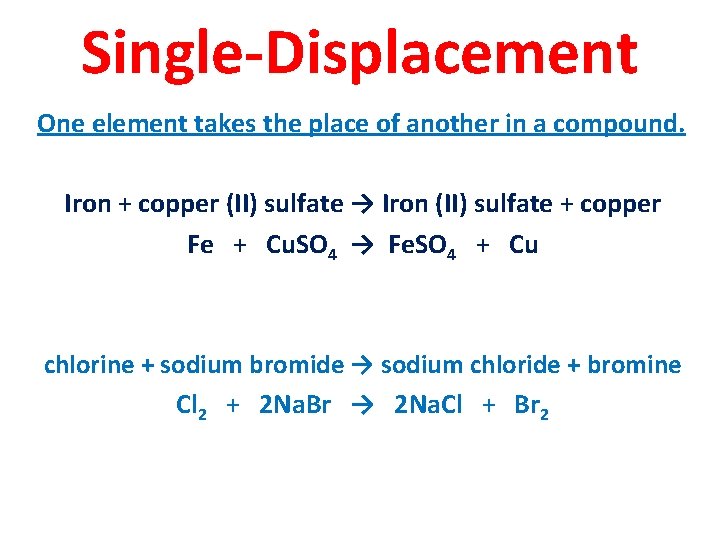
Single-Displacement One element takes the place of another in a compound. Iron + copper (II) sulfate → Iron (II) sulfate + copper Fe + Cu. SO 4 → Fe. SO 4 + Cu chlorine + sodium bromide → sodium chloride + bromine Cl 2 + 2 Na. Br → 2 Na. Cl + Br 2


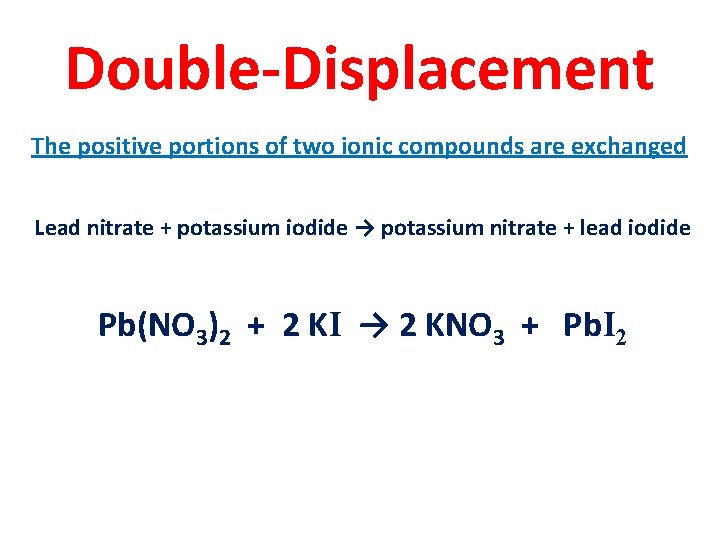
Double-Displacement The positive portions of two ionic compounds are exchanged Lead nitrate + potassium iodide → potassium nitrate + lead iodide Pb(NO 3)2 + 2 KI → 2 KNO 3 + Pb. I 2


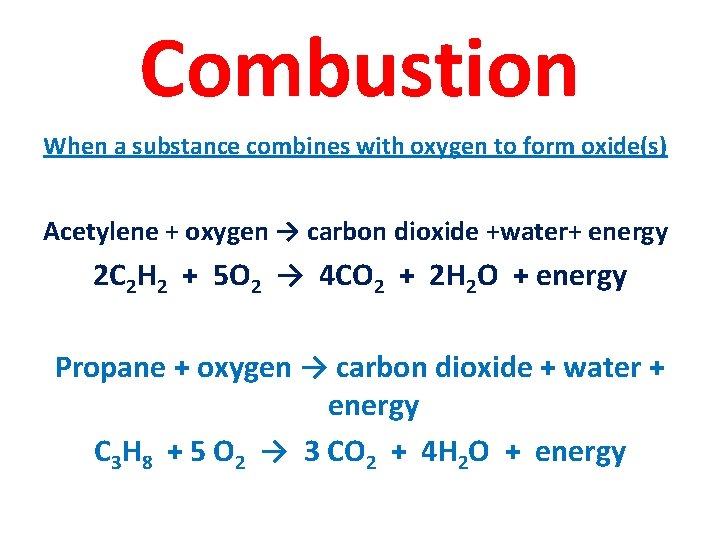
Combustion When a substance combines with oxygen to form oxide(s) Acetylene + oxygen → carbon dioxide +water+ energy 2 C 2 H 2 + 5 O 2 → 4 CO 2 + 2 H 2 O + energy Propane + oxygen → carbon dioxide + water + energy C 3 H 8 + 5 O 2 → 3 CO 2 + 4 H 2 O + energy



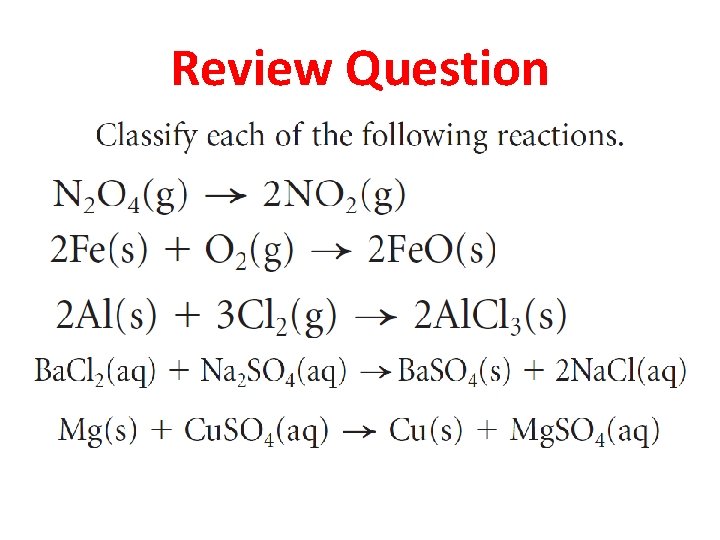
Review Question

Answer • Decomposition • Synthesis • Double displacement • Single displacement
 Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Balance redox half reactions
Balance redox half reactions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Types of reactions
Types of reactions Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Balancing redox reactions
Balancing redox reactions Identify types of reactions
Identify types of reactions Types of reactions
Types of reactions Single replacement reaction examples
Single replacement reaction examples 4 types of chemical reactions
4 types of chemical reactions 5 types of chemical reactions
5 types of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Co 60
Co 60 Four types of chemical reactions
Four types of chemical reactions 5 types of reactions chemistry
5 types of reactions chemistry Five chemical
Five chemical