Chemical Reactions Types of Reactions 5 Types of



- Slides: 12

Chemical Reactions Types of Reactions

5 Types of Reactions ►Synthesis or Combination ►Decompostion ►Single Replacement ►Double Replacement ►Combustion

Synthesis or Combination Reaction ►Two or more substances combine to form another substance § Example: A + B AB ► 2 H 2 (g) + O 2 (g) 2 H 2 O (g)

Decomposition Reaction ►One substance breaks down or decomposes into two or more simpler substances § Example: AB A + B ► 2 H 2 O 2 H 2 + O 2 § Just the opposite of a synthesis reaction

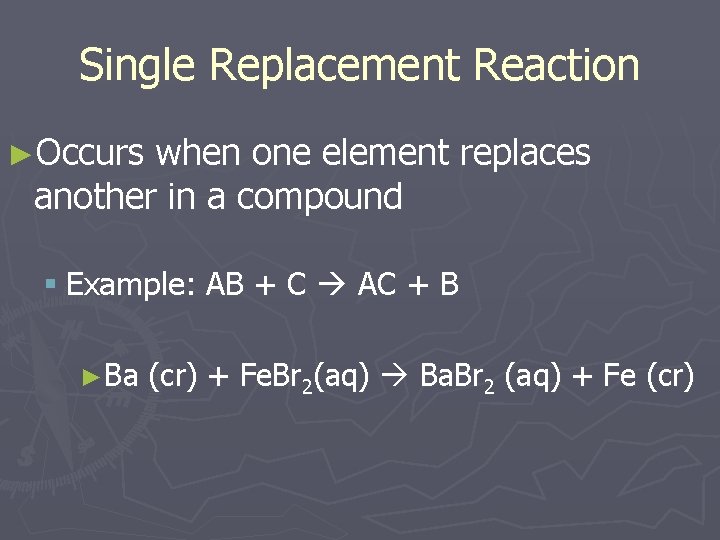
Single Replacement Reaction ►Occurs when one element replaces another in a compound § Example: AB + C AC + B ►Ba (cr) + Fe. Br 2(aq) Ba. Br 2 (aq) + Fe (cr)

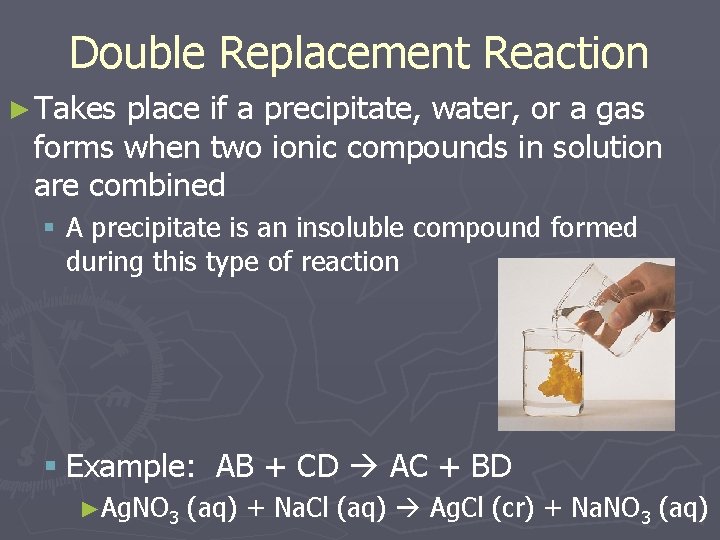
Double Replacement Reaction ► Takes place if a precipitate, water, or a gas forms when two ionic compounds in solution are combined § A precipitate is an insoluble compound formed during this type of reaction § Example: AB + CD AC + BD ►Ag. NO 3 (aq) + Na. Cl (aq) Ag. Cl (cr) + Na. NO 3 (aq)

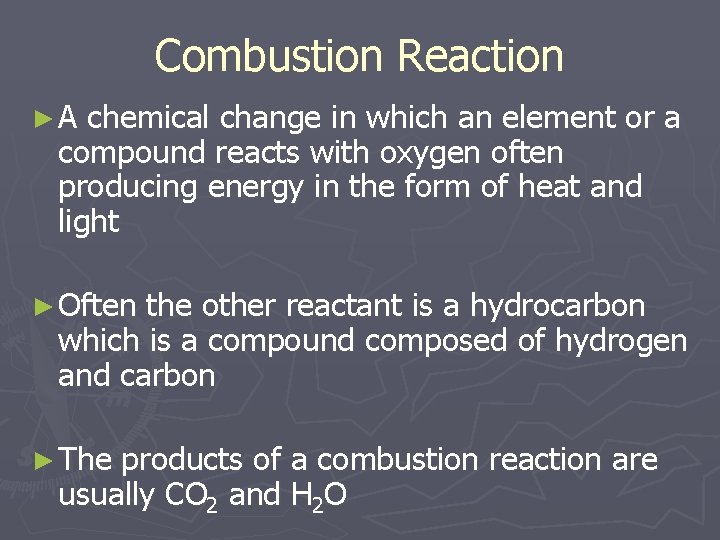
Combustion Reaction ►A chemical change in which an element or a compound reacts with oxygen often producing energy in the form of heat and light ► Often the other reactant is a hydrocarbon which is a compound composed of hydrogen and carbon ► The products of a combustion reaction are usually CO 2 and H 2 O

Example ► 2 C 8 H 18(l) + 25 O 2(g) 16 CO 2(g) + 18 H 2 O(l) ► Notice the reactants and products § Reactants – Hydrocarbon reacting with Oxygen § Products – CO 2 and H 2 O ► Is this a combustion reaction § 2 Mg(s) + O 2(g) 2 Mg. O (s) ►Yes, because it reacts with oxygen producing heat and light ►It can also be classified as a synthesis or combination reaction

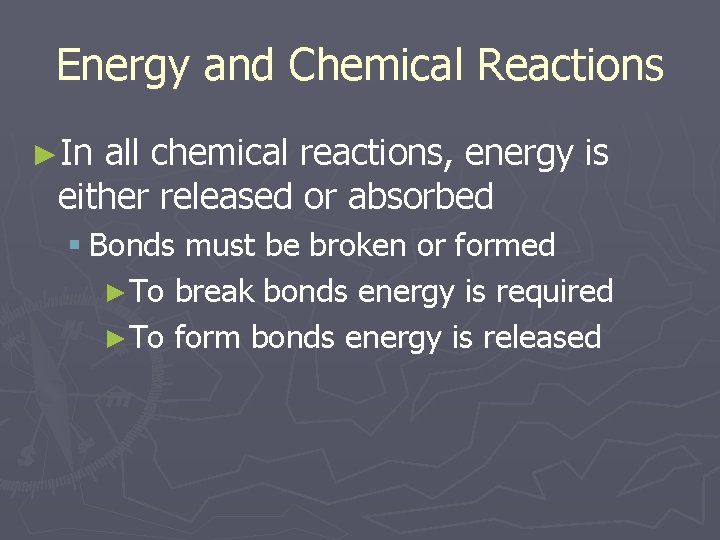
Energy and Chemical Reactions ►In all chemical reactions, energy is either released or absorbed § Bonds must be broken or formed ►To break bonds energy is required ►To form bonds energy is released

Exothermic Reaction ► In many chemical reactions, less energy is required to break the original bonds than is released with the formation of new bonds § Heat is given off ►Examples: ►Reactants Burning of wood; Explosion Products + Energy

Endothermic Reaction ► More energy is required to break the original chemical bonds than is given off. § Heat is absorbed so the container feels colder § Reactants + Energy Products

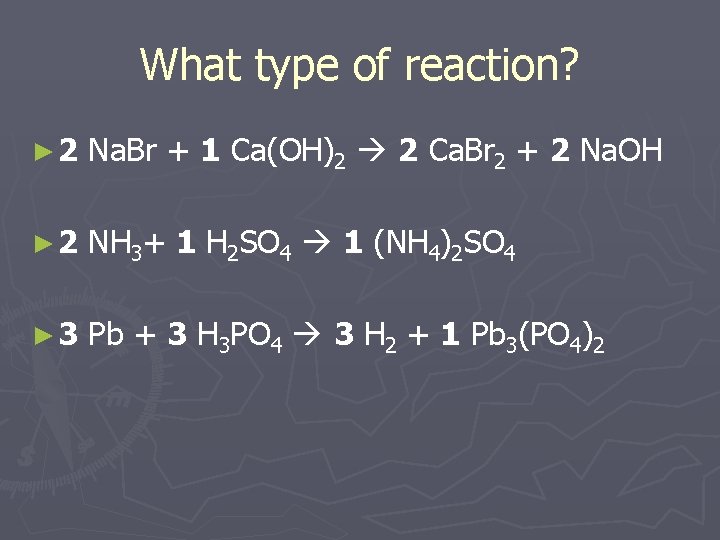
What type of reaction? ► 2 Na. Br + 1 Ca(OH)2 2 Ca. Br 2 + 2 Na. OH ► 2 NH 3+ 1 H 2 SO 4 1 (NH 4)2 SO 4 ► 3 Pb + 3 H 3 PO 4 3 H 2 + 1 Pb 3(PO 4)2
 Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Section 1 chemical changes
Section 1 chemical changes Are kc and kp equal
Are kc and kp equal Different types of redox reactions
Different types of redox reactions How to identify types of chemical reactions
How to identify types of chemical reactions Types of reactions chemistry
Types of reactions chemistry Types of chemical reactions
Types of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions Five chemical changes
Five chemical changes 5 general types of chemical reactions
5 general types of chemical reactions























