Chapter 15 Classification of Matter Matter anything that











- Slides: 17

Chapter 15: Classification of Matter

Matter: anything that has mass and takes up space 4 types: solid: definite shape & volume liquid: no definite shape but definite volume gas: no definite shape or volume plasma: electrically charged particles

Composition of Matter Atoms: the smallest particle that makes up all matter Element: substance made up of the same type of atoms all are listed in the periodic table. Substance: pure matter-either an element or compound

Composition of Matter Compound: two or more elements chemically combined, always in the same ratio; ex. Na. Cl; H 2 O Mixture: a material made up of 2 or more substances that can be separated by physical means. ex. Italian dressing; sand in water; air

Types of Mixtures homogeneous: a mixture where the substances are evenly distributed throughout ex. air; milk, salt water solution: a homogeneous mixture of particles so small they cannot be seen and will not settle.

Types of Mixtures heterogeneous: a mixture where the materials separate upon standing and keep their individual properties. ex. chocolate milk, trail mix, soda suspension: a heterogeneous mixture containing a liquid where visible particles settle, ex: Italian dressing

Types of Mixtures Colloid: a mixture with particles that are larger than those in a solution but not heavy enough to settle out. ex: paint; fog; smoke Tyndall Effect: scattering of light seen in a colloid

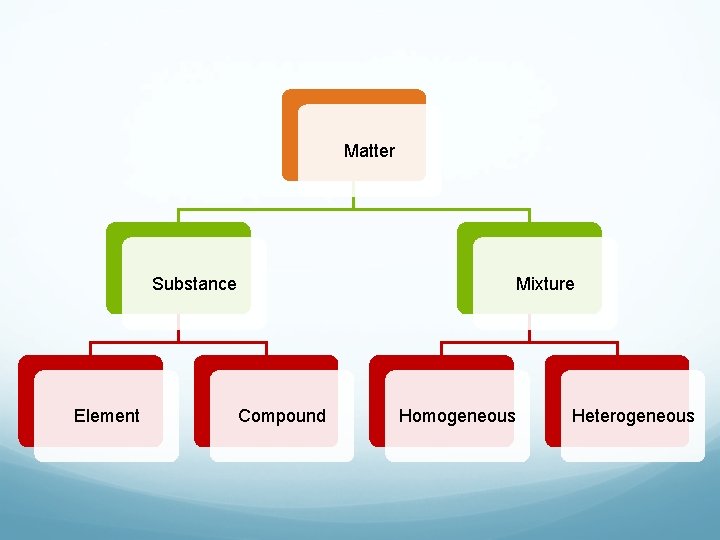
Matter Substance Element Mixture Compound Homogeneous Heterogeneous

Properties of Matter 1. Physical property: a characteristic of a material that you can observe without changing the identity of the substances that make up the material. ex: appearance; behavior; separation by size, magnetism, filtering, boiling Physical Change: a change in size, shape, or state of matter - but the identity stays the same. ex. change of state; density; distillation. density: mass per unit volume of material; m ÷ v distillation: separating substances by evaporating a liquid and recondensing its vapor.

Properties of Matter 2. Chemical Property: a characteristic of a substance that indicates whether it can undergo a certain chemical change. ex. tendency to burn; oxidize Chemical Change: a change of one substance into another ex: iron-rust; cooking Detection of chemical changes: ü Foaming ✓ Bubbling ü Heat ✓ Light ü Sound

Weathering: the wearing away of Earth’s surface Chemical change: calcium carbonate + acidic rain Physical change: cracking of rocks; streams wearing away rock canyons

Law of Conservation of Mass: the mass of all substances that are present before a chemical change = the mass of all the substances that remain after the change. Chemical Reactions: when one or more substances changes into a new and different substance.

Writing a Chemical Equation Uses a. Chemical formulas- shows elements; # atoms/molecules 1. Reactants: substances that combine/change 2. Products: the new substances that are formed b. Symbols- shows state of reactants/products (solid (s), aqueous (aq); gas (g); precipitate (p) c. Subscripts- numbers that tell # atoms of each element present d. Coefficients- numbers that tell # molecules of each substance present.

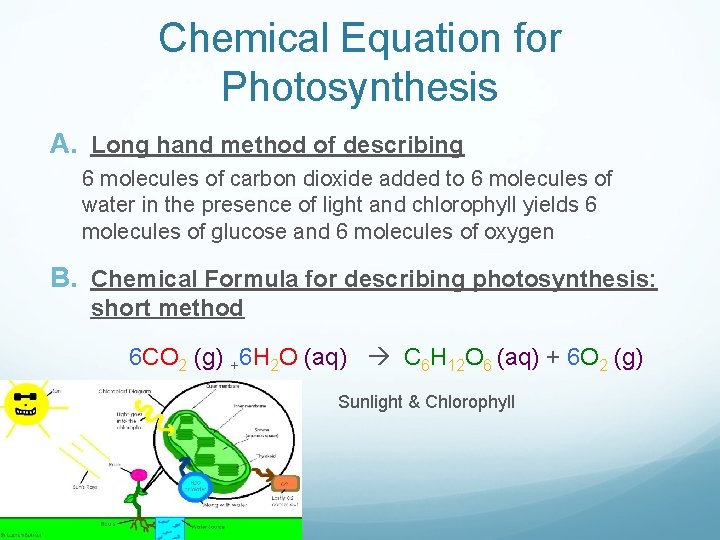
Chemical Equation for Photosynthesis A. Long hand method of describing 6 molecules of carbon dioxide added to 6 molecules of water in the presence of light and chlorophyll yields 6 molecules of glucose and 6 molecules of oxygen B. Chemical Formula for describing photosynthesis: short method 6 CO 2 (g) +6 H 2 O (aq) C 6 H 12 O 6 (aq) + 6 O 2 (g) Sunlight & Chlorophyll

The law of conservation of mass states that matter cannot be created or destroyed, THEREFORE, all chemical equations must be balanced when written. BOTH SIDES OF AN EQUATION MUST HAVE THE SAME NUMBER OF ATOMS OF EACH ELEMENT OR THE EQUATION IS NOT WRITTREN CORRECTLY OR BALANCED. To balance an equation you must choose only the coefficients that will help you get the same number of atoms on each side of the equation

Steps to Balance an Equation: 1. Identify the names of elements present in the reaction. 2. Draw a wall in between the reactants and products. 3. Under each element write the number of each present. 4. Add coefficients where needed for balance.

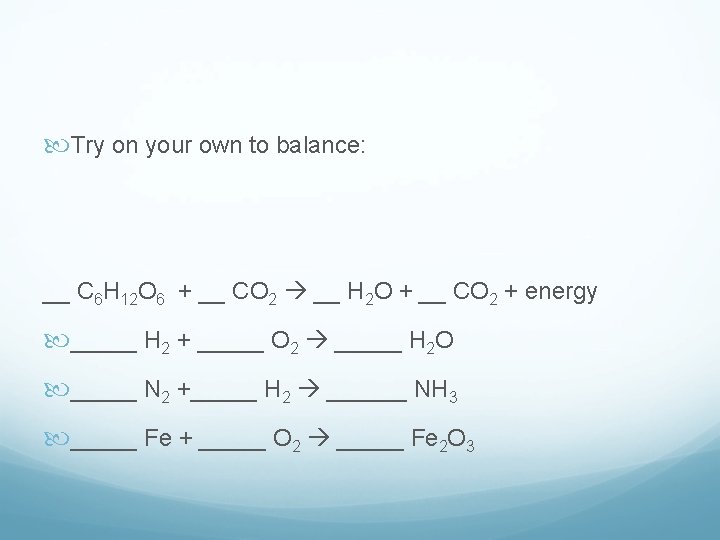
Try on your own to balance: __ C 6 H 12 O 6 + __ CO 2 __ H 2 O + __ CO 2 + energy _____ H 2 + _____ O 2 _____ H 2 O _____ N 2 +_____ H 2 ______ NH 3 _____ Fe + _____ O 2 _____ Fe 2 O 3