Chapter 1 Chemical Foundations Scanning Tunneling Microscope Uses












- Slides: 28

Chapter 1 Chemical Foundations

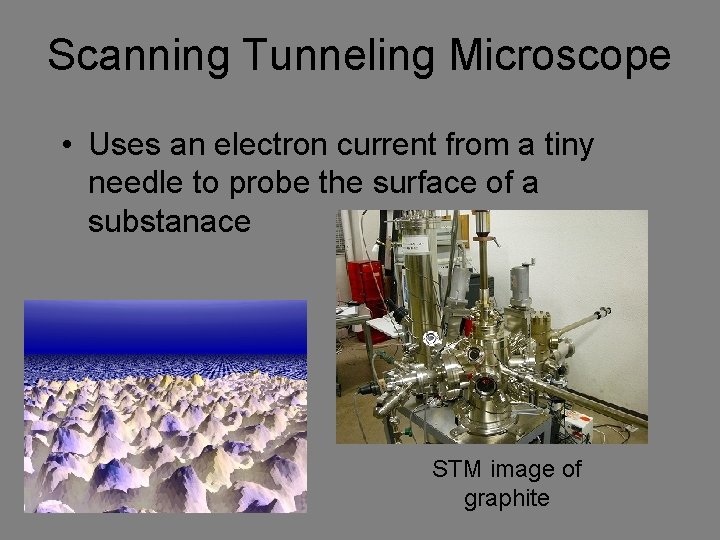
Scanning Tunneling Microscope • Uses an electron current from a tiny needle to probe the surface of a substanace STM image of graphite

Macroscopic vs. Microscopic • Macroscopic: General outlook, wide-scope view • Microscopic: Atomic/molecular view

Diatomic Molecules • Molecules that consist of two atoms • Diatomic elements: H, N, O, F, Cl, Br, I

Fundamental concepts of chemistry: • *Matter is composed of various types of atoms • *One substance changes into another by reorganizing the way the atoms are attached to each other.

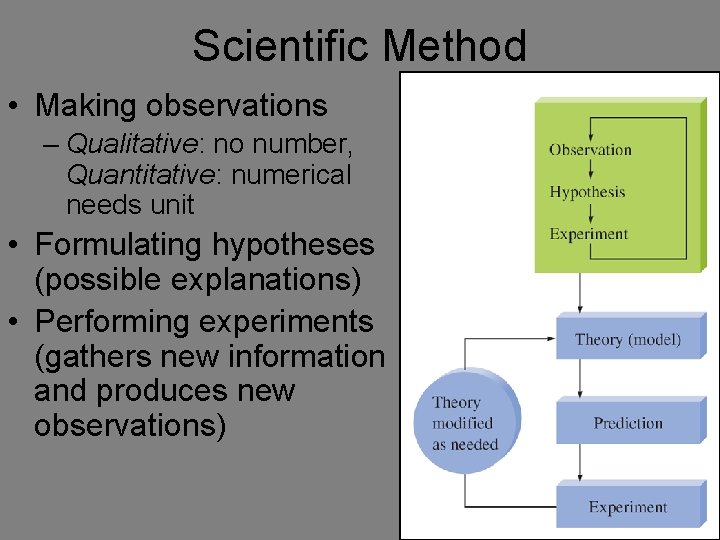
Fundamental Elements of Science • Making Observations (collecting data) • Suggesting a possible explanation (formulating a hypothesis) • Doing experiments to test the possible explanation (testing the hypothesis) • AKA: Scientific Method

Scientific Method • Making observations – Qualitative: no number, Quantitative: numerical needs unit • Formulating hypotheses (possible explanations) • Performing experiments (gathers new information and produces new observations)

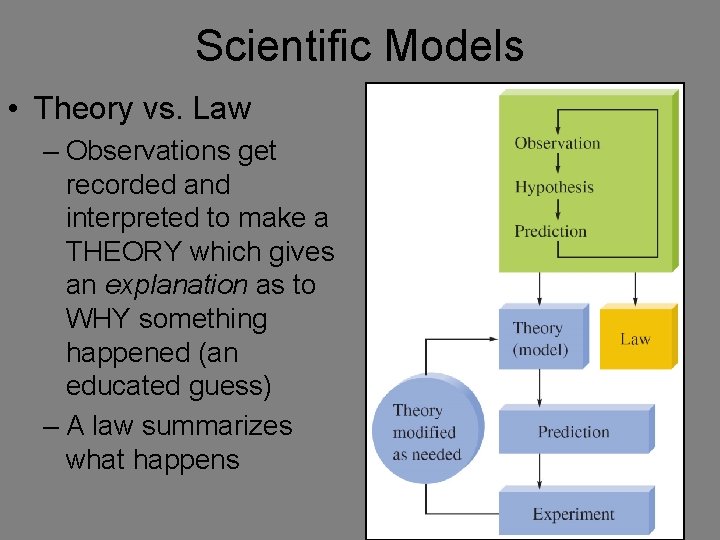
Scientific Models • Theory vs. Law – Observations get recorded and interpreted to make a THEORY which gives an explanation as to WHY something happened (an educated guess) – A law summarizes what happens

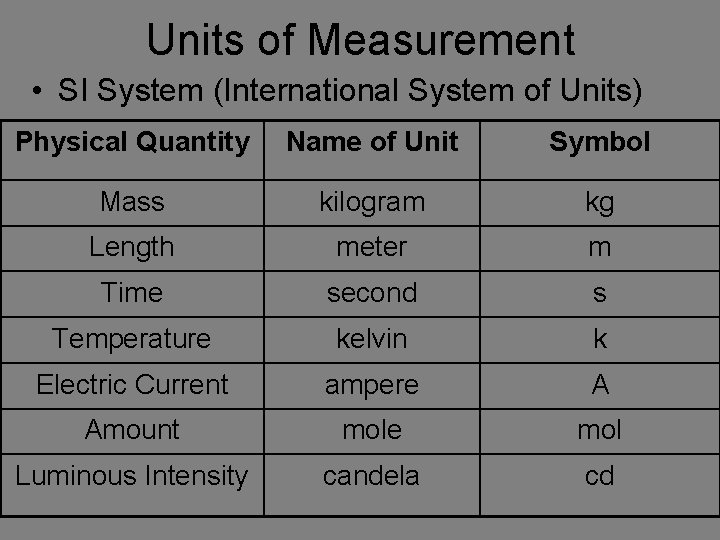
Units of Measurement • SI System (International System of Units) Physical Quantity Name of Unit Symbol Mass kilogram kg Length meter m Time second s Temperature kelvin k Electric Current ampere A Amount mole mol Luminous Intensity candela cd

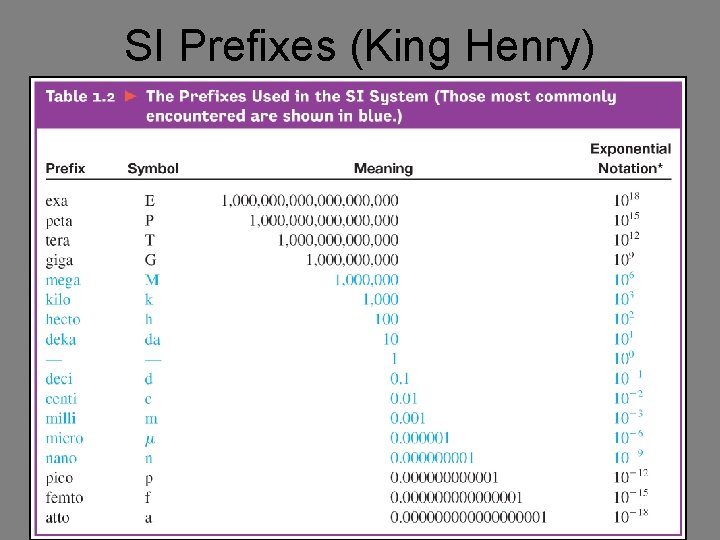
SI Prefixes (King Henry)

Common Units • Volume: length X width X height • MASS: a measure of the resistance of an object to a change in its state of motion vs. WEIGHT: the force that gravity exerts on an object

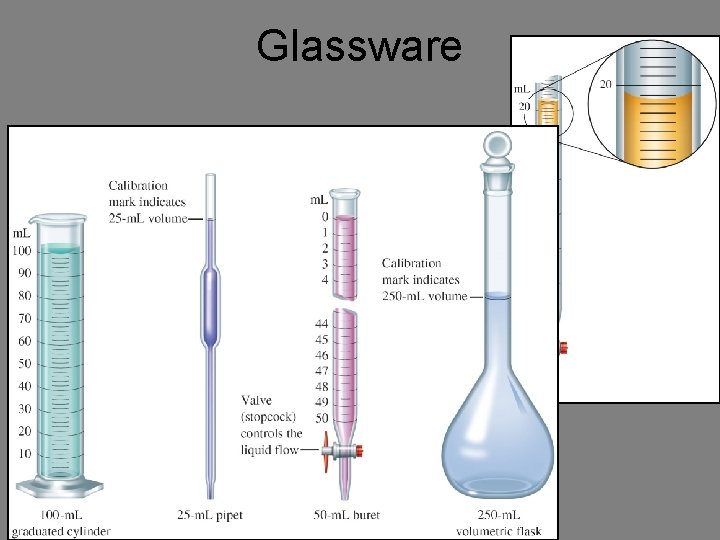
Glassware

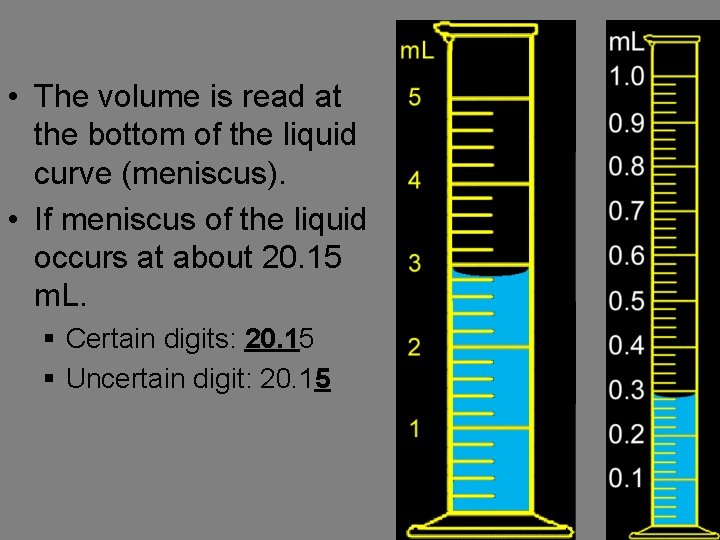
• The volume is read at the bottom of the liquid curve (meniscus). • If meniscus of the liquid occurs at about 20. 15 m. L. § Certain digits: 20. 15 § Uncertain digit: 20. 15

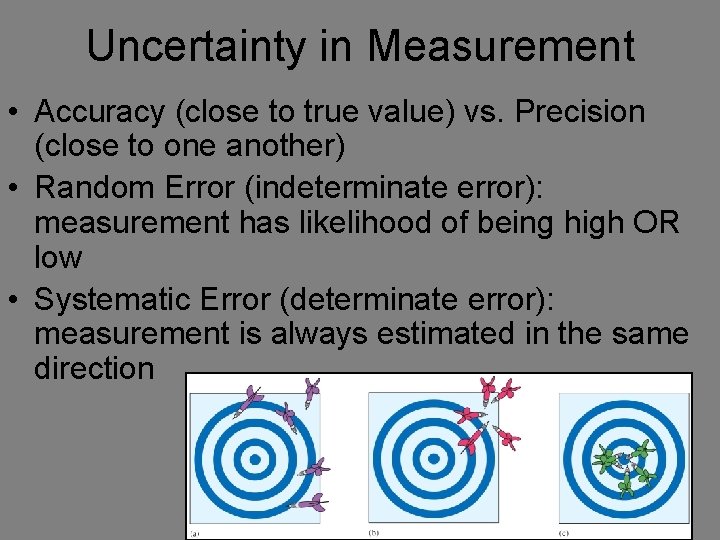
Uncertainty in Measurement • Accuracy (close to true value) vs. Precision (close to one another) • Random Error (indeterminate error): measurement has likelihood of being high OR low • Systematic Error (determinate error): measurement is always estimated in the same direction

Significant Figures • Nonzero numbers are significant • Zeros in between nonzeros ARE significant • Leftmost zeros are NOT • Rightmost zeros are IF there is a decimal point present • Exact numbers have infinite number of significant figures

Significant Figures in Math • Multiplication/Division: answer has the same as the least number of TOTAL significant figures • Addition/Subtraction: answer has the same number of decimals as the number with the least number of digits after the decimal

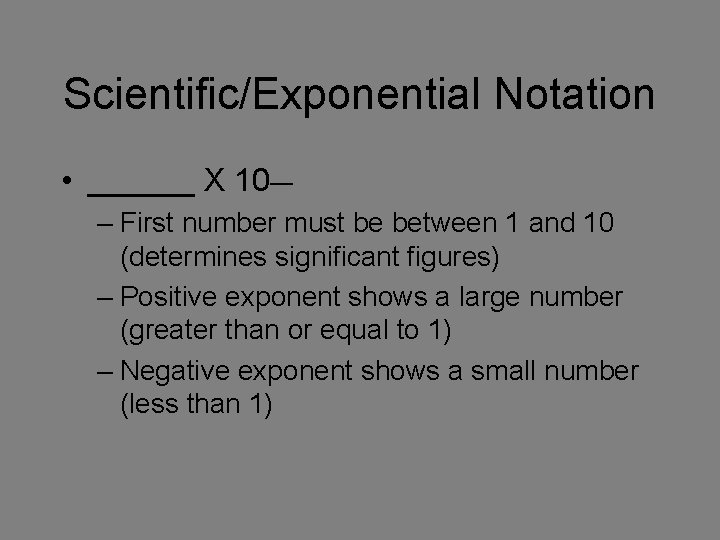
Scientific/Exponential Notation • ______ X 10__ – First number must be between 1 and 10 (determines significant figures) – Positive exponent shows a large number (greater than or equal to 1) – Negative exponent shows a small number (less than 1)

Rounding • When you have a multi-step problem, don’t stop midway through • 5 or more, raise the score • 4 or less, let it rest • Do NOT double round

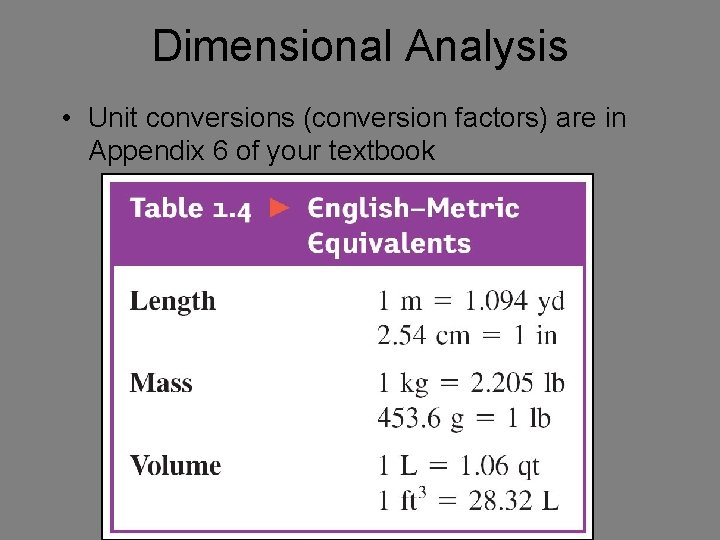
Dimensional Analysis • Unit conversions (conversion factors) are in Appendix 6 of your textbook

Dimensional Analysis • Determine unknown (? = _____) • Determine your knowns (some given in problem, some assumed that you know - table in book) • Determine what you’re starting with (usually the number/unit given) • Use your knowns to cancel units diagonally until you arrive at your unknown unit • REMEMBER SIGNIFCANT FIGURES AND UNITS IN YOUR ANSWERS!!

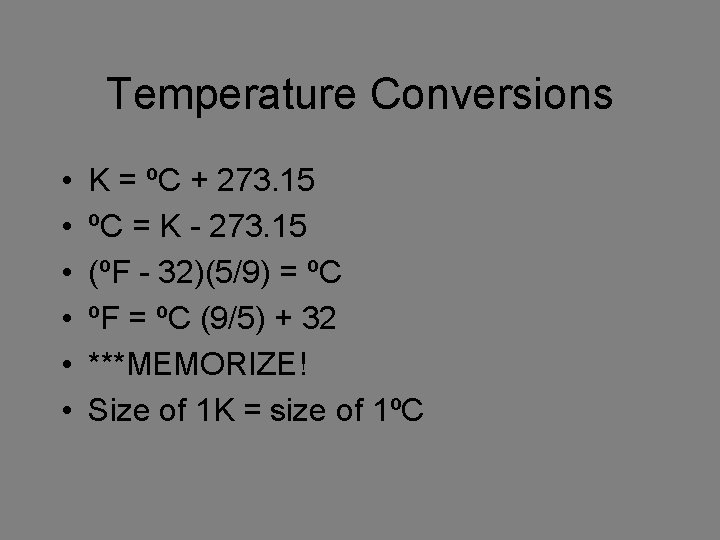
Temperature Conversions • • • K = ºC + 273. 15 ºC = K - 273. 15 (ºF - 32)(5/9) = ºC ºF = ºC (9/5) + 32 ***MEMORIZE! Size of 1 K = size of 1ºC

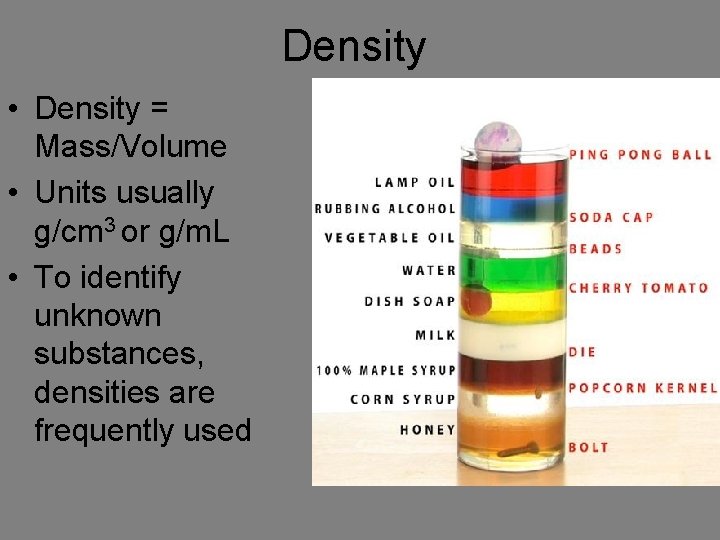
Density • Density = Mass/Volume • Units usually g/cm 3 or g/m. L • To identify unknown substances, densities are frequently used

Matter • Definition: anything that occupies space and has mass • States: solid, liquid, gas SOLID Volume? Shape? Compress? Attraction? fixed no strong LIQUID fixed GAS fixed not fixed yes weak none

Mixtures • Mixture has variable composition – Homogeneous (aka solution): indistinguishable parts – Heterogeneous: visibly distinguishable parts • Pure Substance: has constant composition (elements and compounds - broken to elements by chemical processes)

Physical Change • Physical: phase changes, changes the form of the substance, but not its chemical composition – Distillation (separates liquid in liquid): mixture is heated and condensed back to a liquid to purify – Filtration (separates solid in liquid): Solution poured onto mesh-like material to allow liquid to pass through and solid to be left behind – Chromatography: involves a mobile (liquid or gas) and stationary phase (solid)

Paper Chromatography • Strip of filter paper (stationary phase) is used with a drop of the mixture to be separated on it • It is placed with the edge near the drop touching a liquid (mobile phase) which travels up the paper taking different parts of the mixture with it based on their affinities

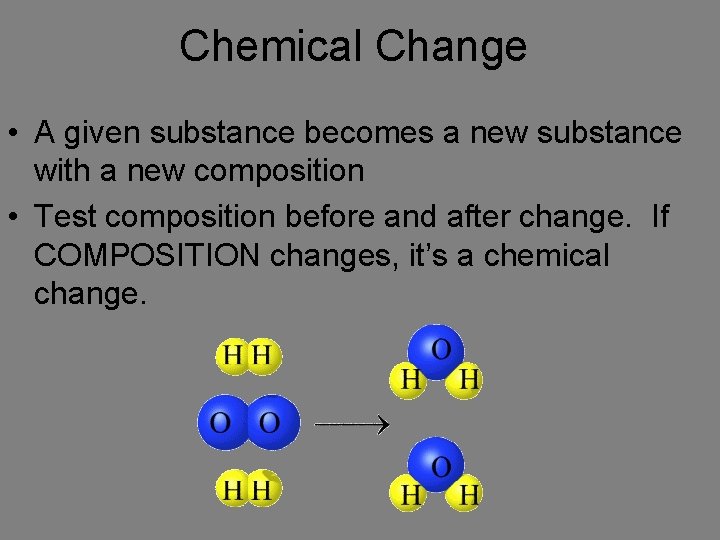
Chemical Change • A given substance becomes a new substance with a new composition • Test composition before and after change. If COMPOSITION changes, it’s a chemical change.

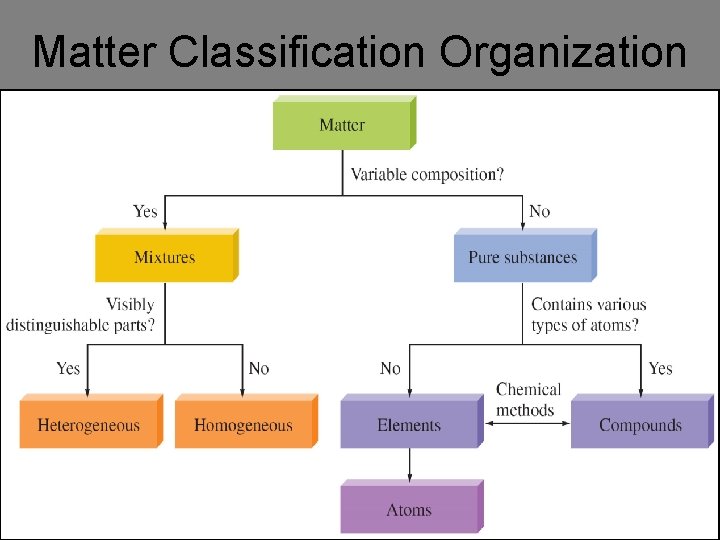
Matter Classification Organization