4 INTRODUCTION TO QUANTUM MECHANICS CHAPTER 4 1


- Slides: 50

4 INTRODUCTION TO QUANTUM MECHANICS CHAPTER 4. 1 Preliminaries: Wave Motion and Light 4. 2 Evidence for Energy Quantization in Atoms 4. 3 The Bohr Model: Predicting Discrete Energy Levels in Atoms 4. 4 Evidence for Wave-Particle Duality 4. 5 The Schrödinger Equation 4. 6 Quantum mechanics of Particle-in-a-Box Models General Chemistry I

Nanometer-Sized Crystals of Cd. Se General Chemistry I

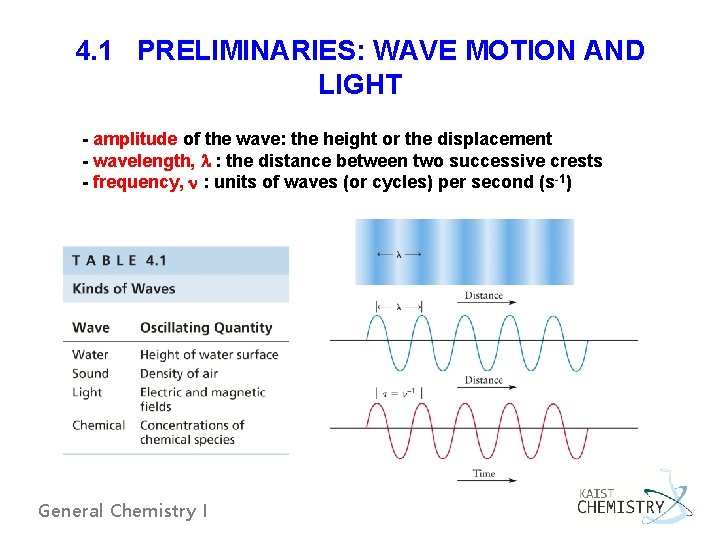
4. 1 PRELIMINARIES: WAVE MOTION AND LIGHT - amplitude of the wave: the height or the displacement - wavelength, l : the distance between two successive crests - frequency, n : units of waves (or cycles) per second (s-1) General Chemistry I

General Chemistry I

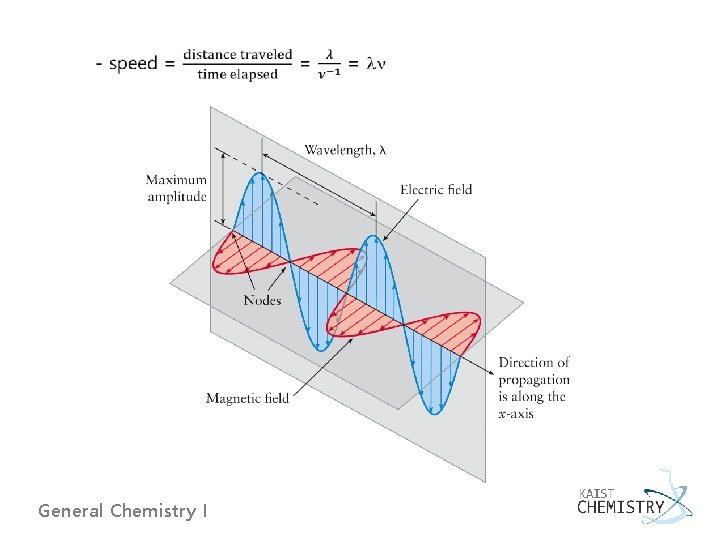
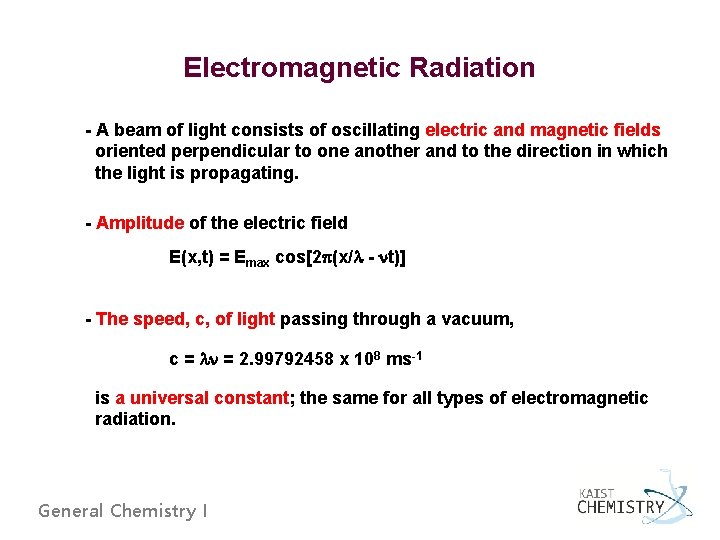
Electromagnetic Radiation - A beam of light consists of oscillating electric and magnetic fields oriented perpendicular to one another and to the direction in which the light is propagating. - Amplitude of the electric field E(x, t) = Emax cos[2 p(x/l - nt)] - The speed, c, of light passing through a vacuum, c = ln = 2. 99792458 x 108 ms-1 is a universal constant; the same for all types of electromagnetic radiation. General Chemistry I

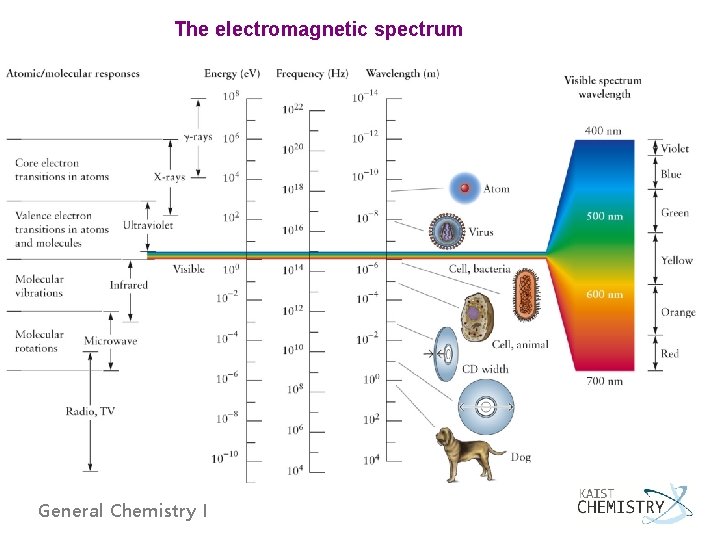
The electromagnetic spectrum General Chemistry I

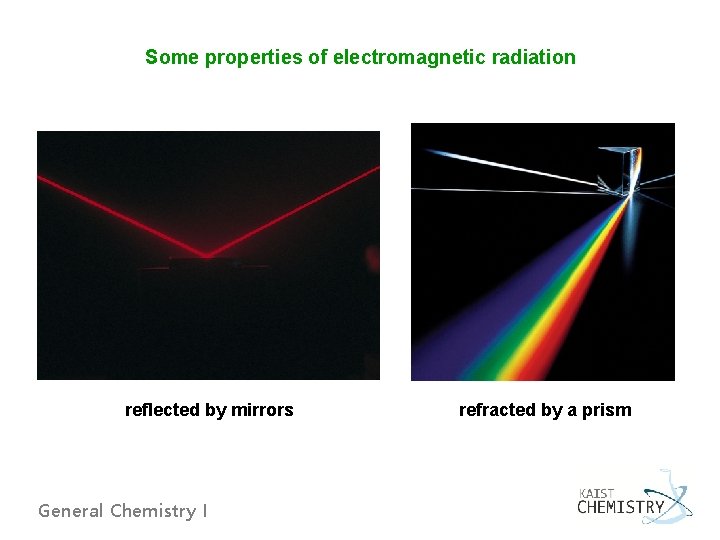
Some properties of electromagnetic radiation reflected by mirrors General Chemistry I refracted by a prism

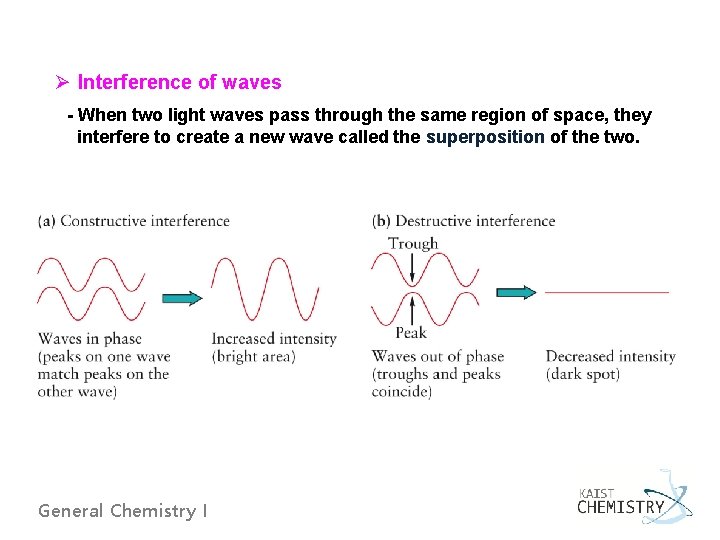
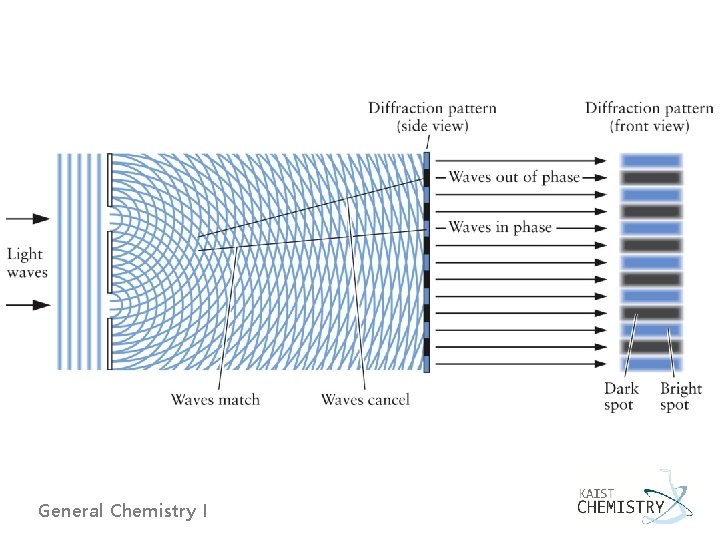
Ø Interference of waves - When two light waves pass through the same region of space, they interfere to create a new wave called the superposition of the two. General Chemistry I

General Chemistry I

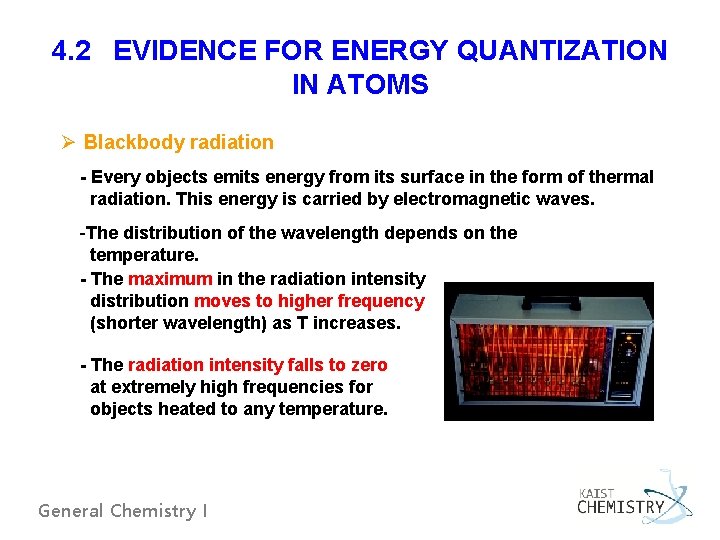
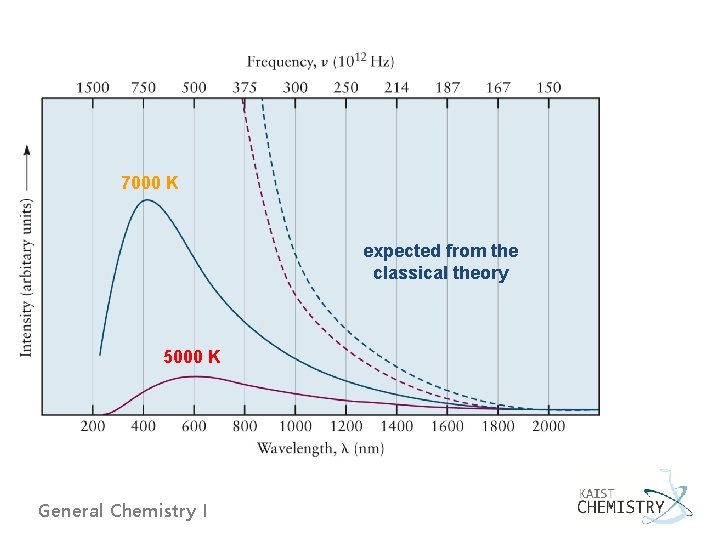
4. 2 EVIDENCE FOR ENERGY QUANTIZATION IN ATOMS Ø Blackbody radiation - Every objects emits energy from its surface in the form of thermal radiation. This energy is carried by electromagnetic waves. -The distribution of the wavelength depends on the temperature. - The maximum in the radiation intensity distribution moves to higher frequency (shorter wavelength) as T increases. - The radiation intensity falls to zero at extremely high frequencies for objects heated to any temperature. General Chemistry I

7000 K expected from the classical theory 5000 K General Chemistry I

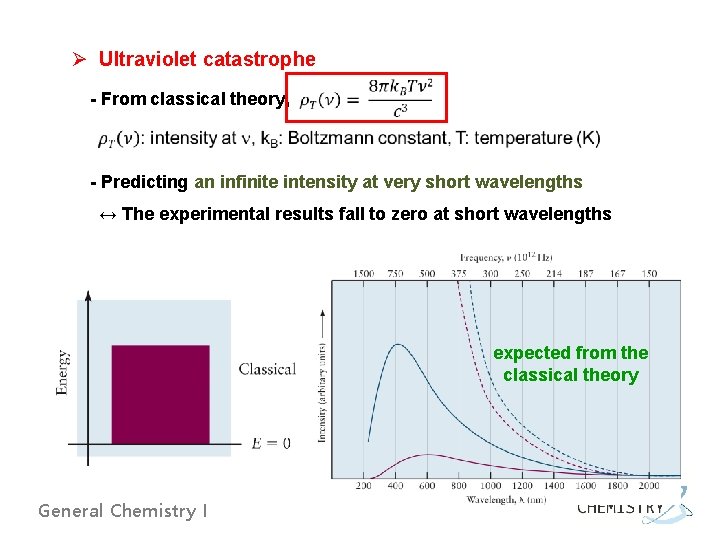
Ø Ultraviolet catastrophe - From classical theory, - Predicting an infinite intensity at very short wavelengths ↔ The experimental results fall to zero at short wavelengths expected from the classical theory General Chemistry I

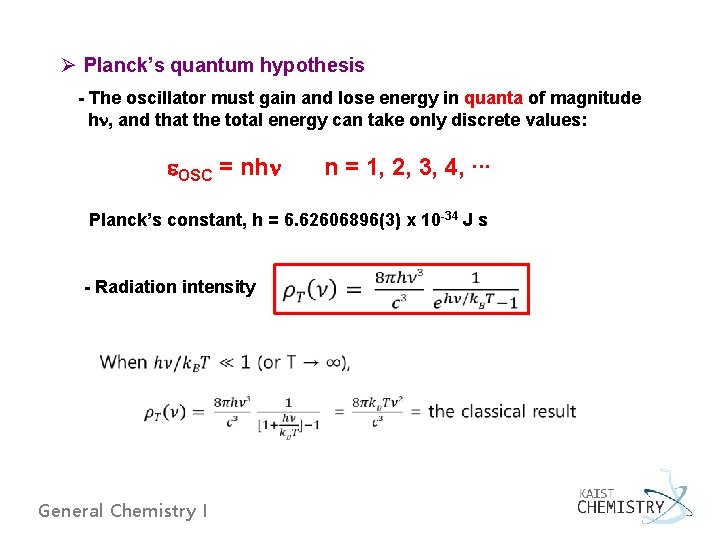
Ø Planck’s quantum hypothesis - The oscillator must gain and lose energy in quanta of magnitude hn, and that the total energy can take only discrete values: e. OSC = nhn n = 1, 2, 3, 4, ∙∙∙ Planck’s constant, h = 6. 62606896(3) x 10 -34 J s - Radiation intensity General Chemistry I

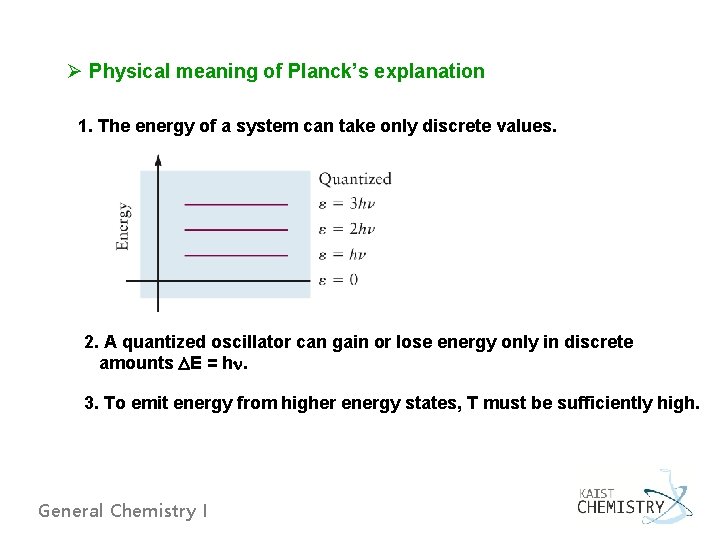
Ø Physical meaning of Planck’s explanation 1. The energy of a system can take only discrete values. 2. A quantized oscillator can gain or lose energy only in discrete amounts DE = hn. 3. To emit energy from higher energy states, T must be sufficiently high. General Chemistry I

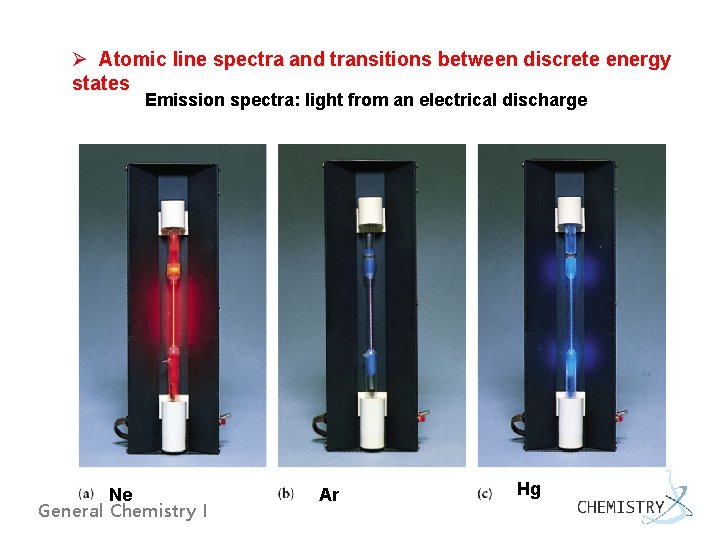
Ø Atomic line spectra and transitions between discrete energy states Emission spectra: light from an electrical discharge Ne General Chemistry I Ar Hg

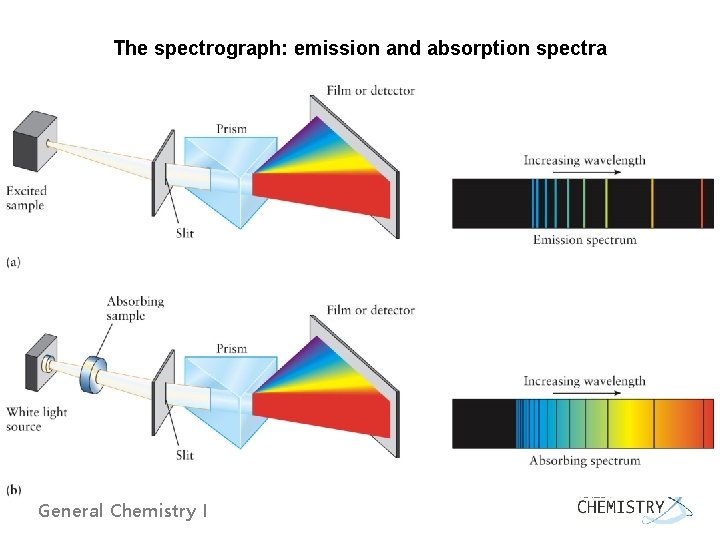
The spectrograph: emission and absorption spectra General Chemistry I

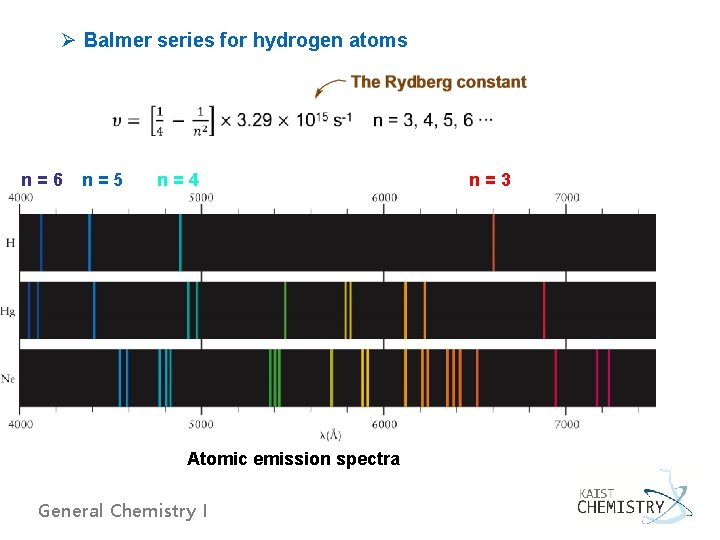
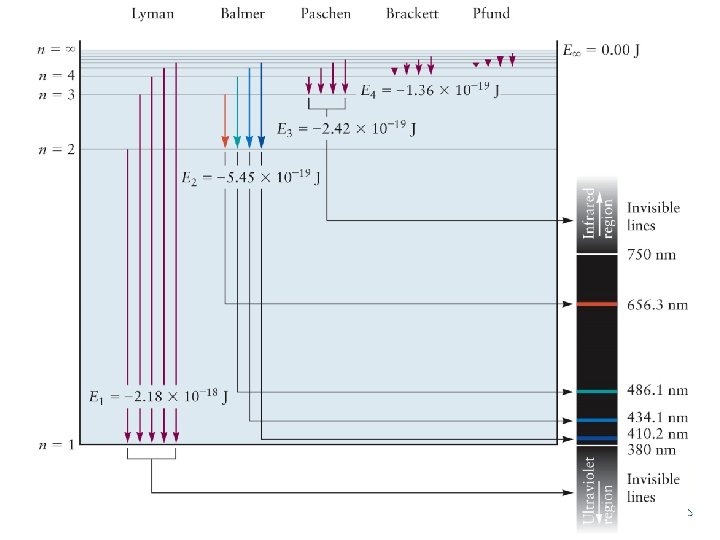
Ø Balmer series for hydrogen atoms n=6 n=5 n=4 Atomic emission spectra General Chemistry I n=3

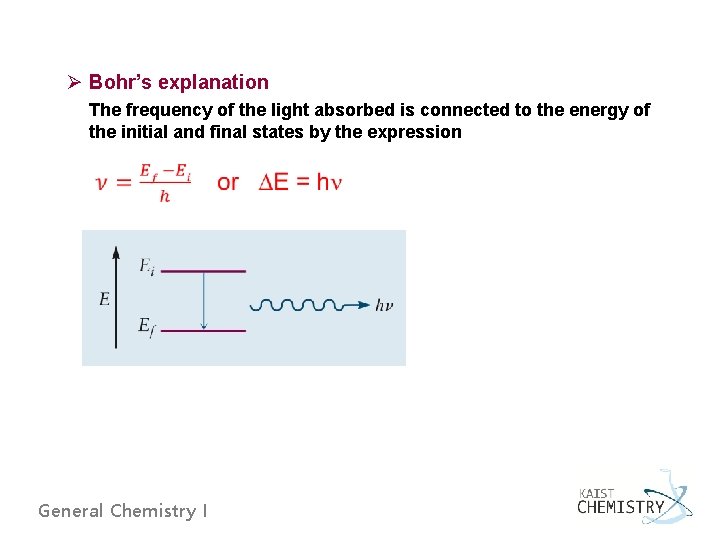
Ø Bohr’s explanation The frequency of the light absorbed is connected to the energy of the initial and final states by the expression General Chemistry I

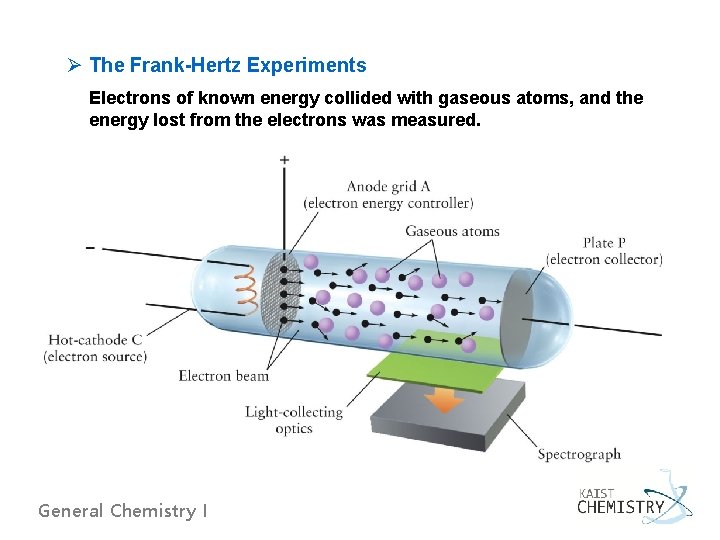
Ø The Frank-Hertz Experiments Electrons of known energy collided with gaseous atoms, and the energy lost from the electrons was measured. General Chemistry I

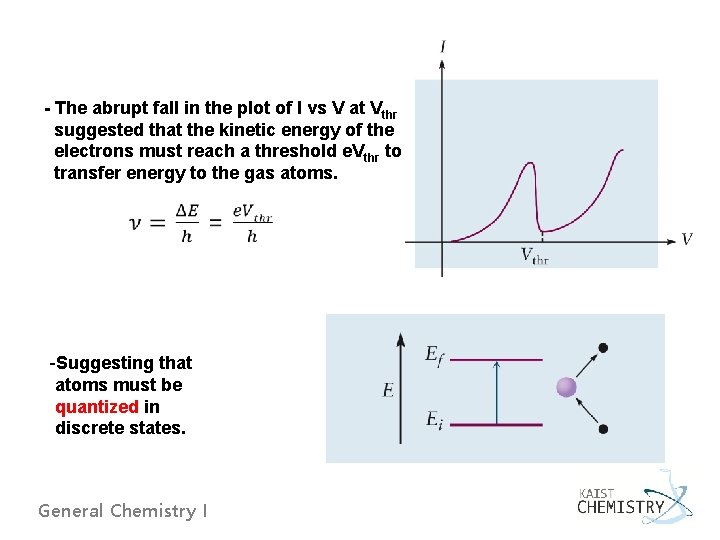
- The abrupt fall in the plot of I vs V at Vthr suggested that the kinetic energy of the electrons must reach a threshold e. Vthr to transfer energy to the gas atoms. -Suggesting that atoms must be quantized in discrete states. General Chemistry I

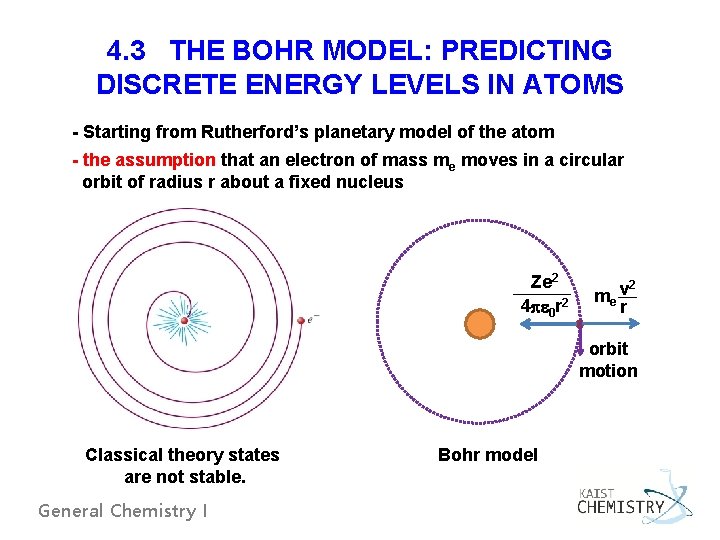
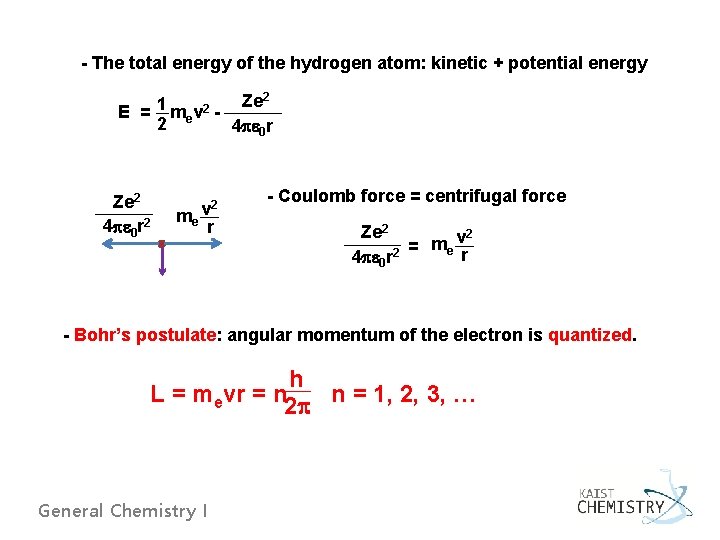
4. 3 THE BOHR MODEL: PREDICTING DISCRETE ENERGY LEVELS IN ATOMS - Starting from Rutherford’s planetary model of the atom - the assumption that an electron of mass me moves in a circular orbit of radius r about a fixed nucleus Ze 2 4 pe 0 r 2 me v r 2 orbit motion Classical theory states are not stable. General Chemistry I Bohr model

- The total energy of the hydrogen atom: kinetic + potential energy Ze 2 1 2 E = m ev 2 4 pe 0 r Ze 2 4 pe 0 r 2 2 me v r - Coulomb force = centrifugal force Ze 2 v 2 m = e r 4 pe 0 r 2 - Bohr’s postulate: angular momentum of the electron is quantized. h L = mevr = n 2 p n = 1, 2, 3, … General Chemistry I

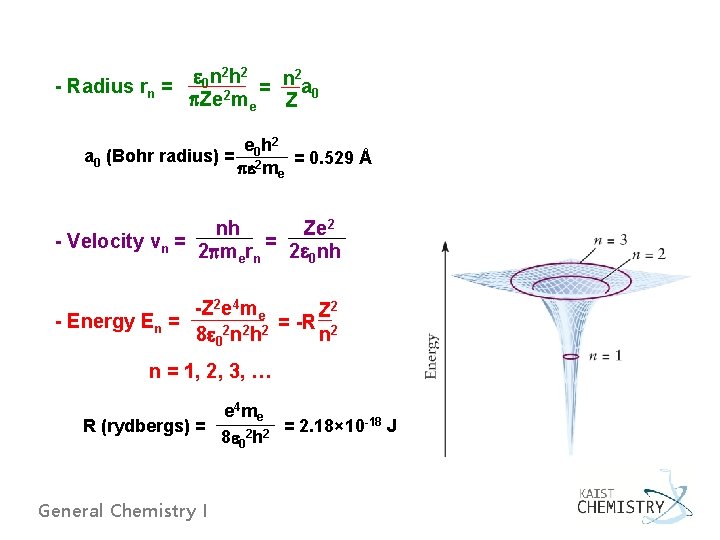
2 h 2 2 e n n 0 - Radius rn = a = p. Ze 2 me Z 0 e 0 h 2 a 0 (Bohr radius) = 2 = 0. 529 Å pe me Ze 2 nh = - Velocity vn = 2 pmern 2 e 0 nh -Z 2 e 4 me Z 2 - Energy En = = -R 2 8 e 02 n 2 h 2 n n = 1, 2, 3, … e 4 me R (rydbergs) = = 2. 18× 10 -18 J 2 2 8 e 0 h General Chemistry I

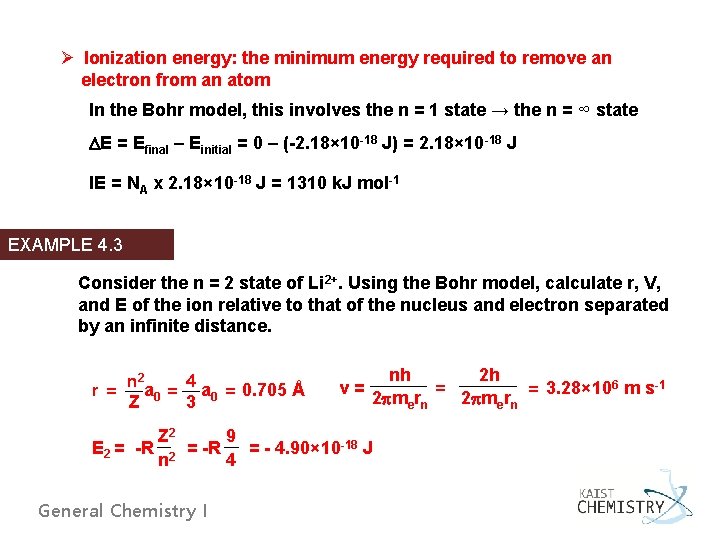
Ø Ionization energy: the minimum energy required to remove an electron from an atom In the Bohr model, this involves the n = 1 state → the n = ∞ state DE = Efinal – Einitial = 0 – (-2. 18× 10 -18 J) = 2. 18× 10 -18 J IE = NA x 2. 18× 10 -18 J = 1310 k. J mol-1 EXAMPLE 4. 3 Consider the n = 2 state of Li 2+. Using the Bohr model, calculate r, V, and E of the ion relative to that of the nucleus and electron separated by an infinite distance. n 2 4 r = a 0 = 0. 705 Å Z 3 v= nh 2 h = = 3. 28× 106 m s-1 2 pmern Z 2 9 E 2 = -R = - 4. 90× 10 -18 J n 4 General Chemistry I

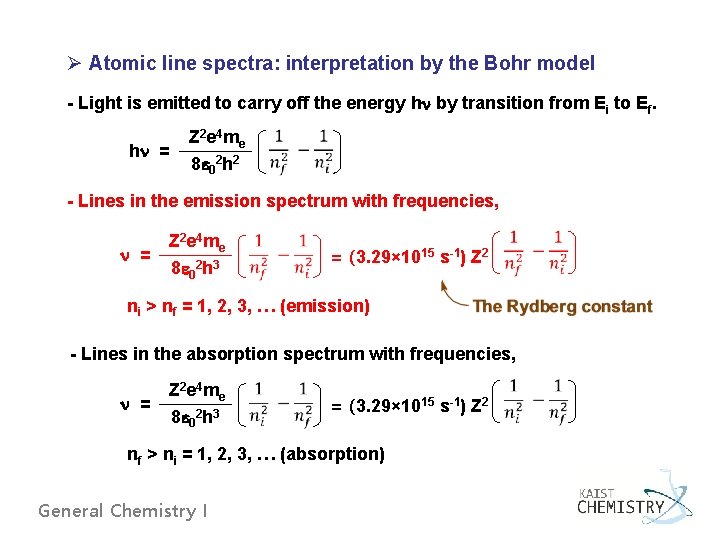
Ø Atomic line spectra: interpretation by the Bohr model - Light is emitted to carry off the energy hn by transition from Ei to Ef. hn = Z 2 e 4 me 8 e 02 h 2 - Lines in the emission spectrum with frequencies, n = Z 2 e 4 me 8 e 02 h 3 = (3. 29× 1015 s-1) Z 2 ni > nf = 1, 2, 3, … (emission) - Lines in the absorption spectrum with frequencies, n = Z 2 e 4 me 8 e 02 h 3 = (3. 29× 1015 s-1) Z 2 nf > ni = 1, 2, 3, … (absorption) General Chemistry I

General Chemistry I

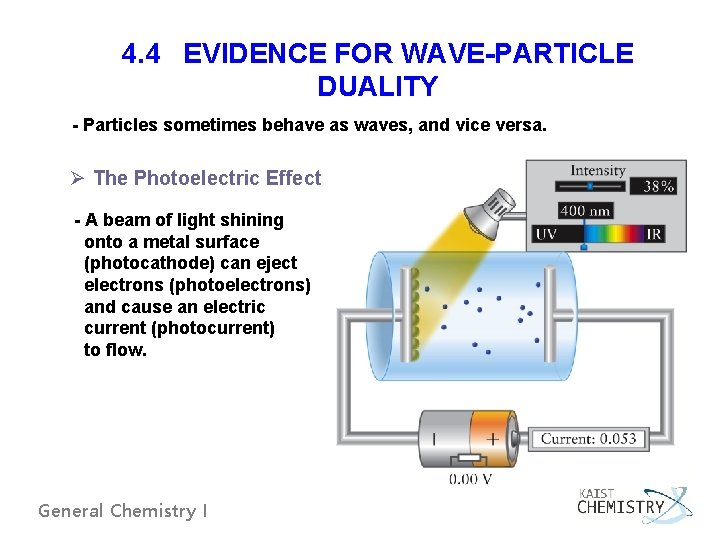
4. 4 EVIDENCE FOR WAVE-PARTICLE DUALITY - Particles sometimes behave as waves, and vice versa. Ø The Photoelectric Effect - A beam of light shining onto a metal surface (photocathode) can eject electrons (photoelectrons) and cause an electric current (photocurrent) to flow. General Chemistry I

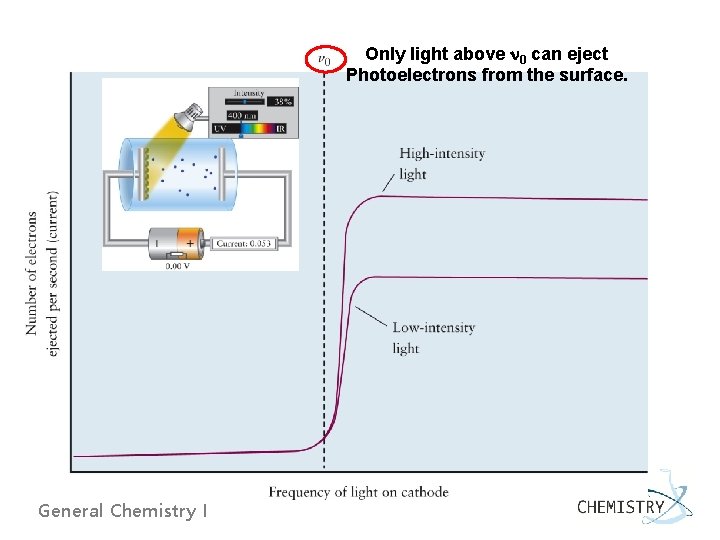
Only light above n 0 can eject Photoelectrons from the surface. General Chemistry I

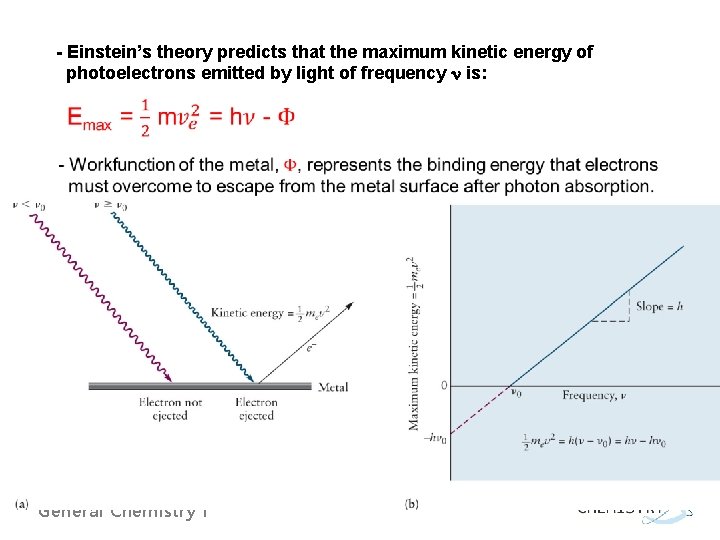
- Einstein’s theory predicts that the maximum kinetic energy of photoelectrons emitted by light of frequency n is: General Chemistry I

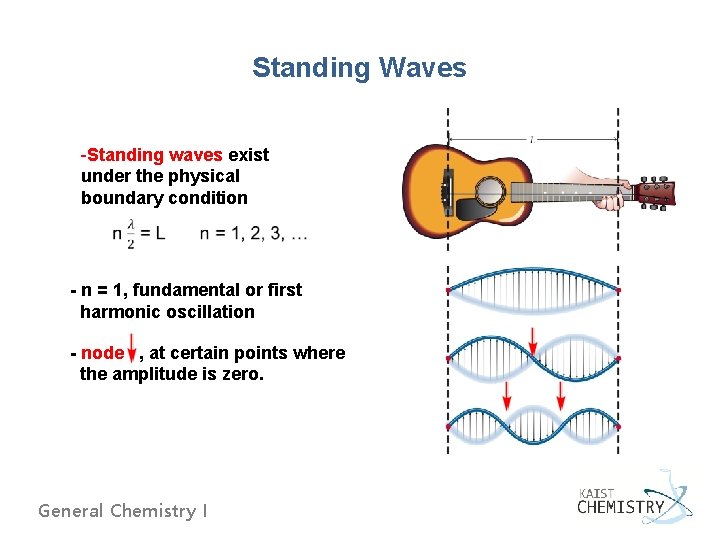
Standing Waves -Standing waves exist under the physical boundary condition - n = 1, fundamental or first harmonic oscillation - node , at certain points where the amplitude is zero. General Chemistry I

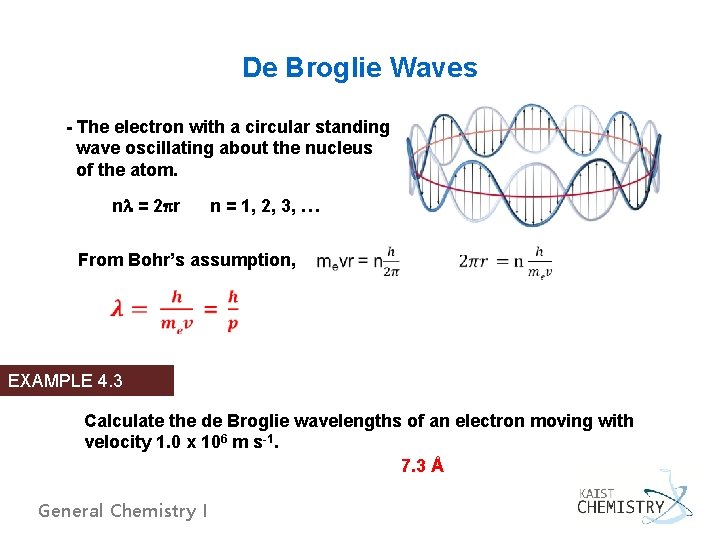
De Broglie Waves - The electron with a circular standing wave oscillating about the nucleus of the atom. nl = 2 pr n = 1, 2, 3, … From Bohr’s assumption, EXAMPLE 4. 3 Calculate the de Broglie wavelengths of an electron moving with velocity 1. 0 x 106 m s-1. 7. 3 Å General Chemistry I

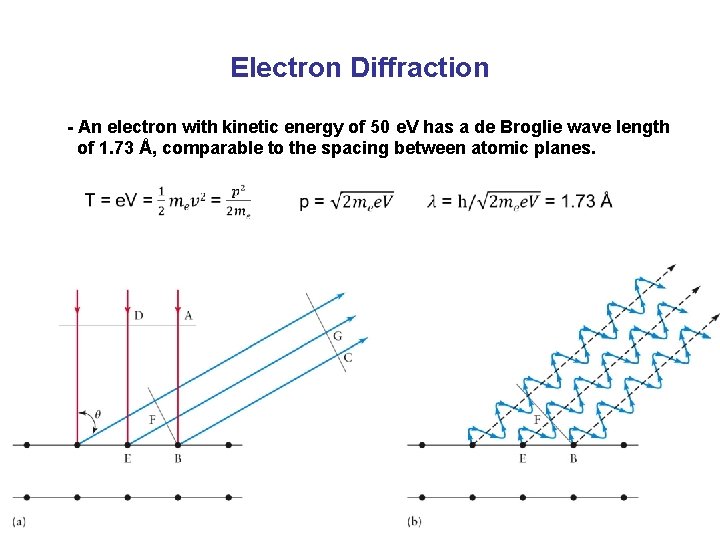
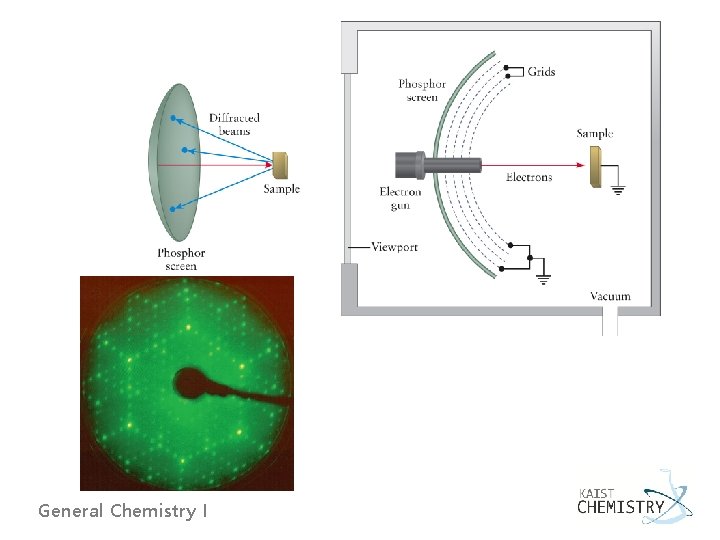
Electron Diffraction - An electron with kinetic energy of 50 e. V has a de Broglie wave length of 1. 73 Å, comparable to the spacing between atomic planes. General Chemistry I

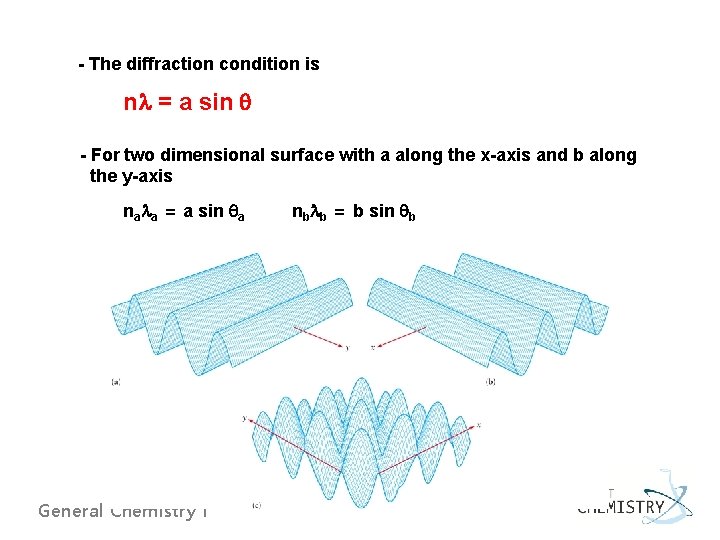
- The diffraction condition is nl = a sin q - For two dimensional surface with a along the x-axis and b along the y-axis nala = a sin qa General Chemistry I nblb = b sin qb

General Chemistry I

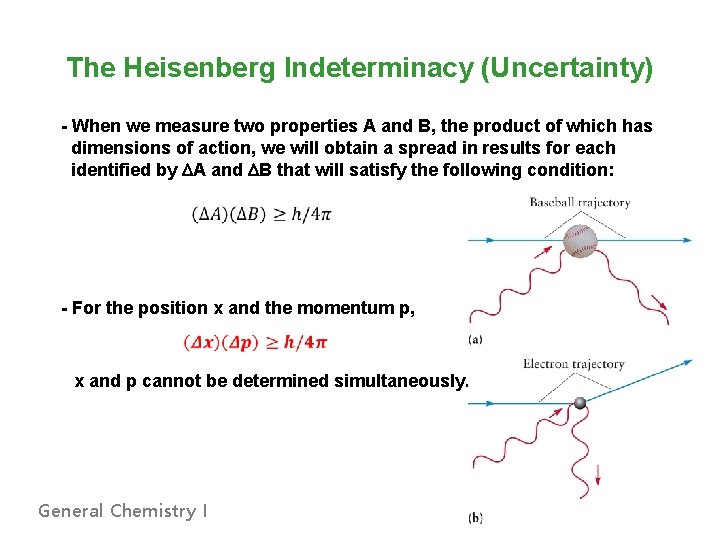
The Heisenberg Indeterminacy (Uncertainty) - When we measure two properties A and B, the product of which has dimensions of action, we will obtain a spread in results for each identified by DA and DB that will satisfy the following condition: - For the position x and the momentum p, x and p cannot be determined simultaneously. General Chemistry I

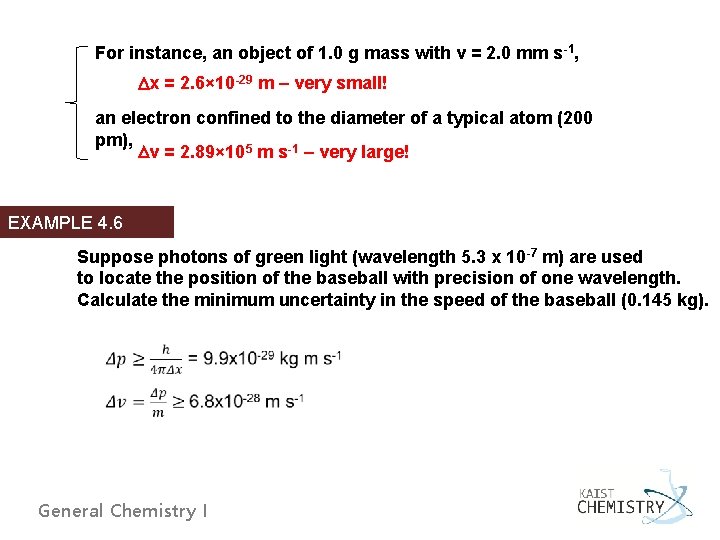
For instance, an object of 1. 0 g mass with v = 2. 0 mm s-1, Dx = 2. 6× 10 -29 m – very small! an electron confined to the diameter of a typical atom (200 pm), Dv = 2. 89× 105 m s-1 – very large! EXAMPLE 4. 6 Suppose photons of green light (wavelength 5. 3 x 10 -7 m) are used to locate the position of the baseball with precision of one wavelength. Calculate the minimum uncertainty in the speed of the baseball (0. 145 kg). General Chemistry I

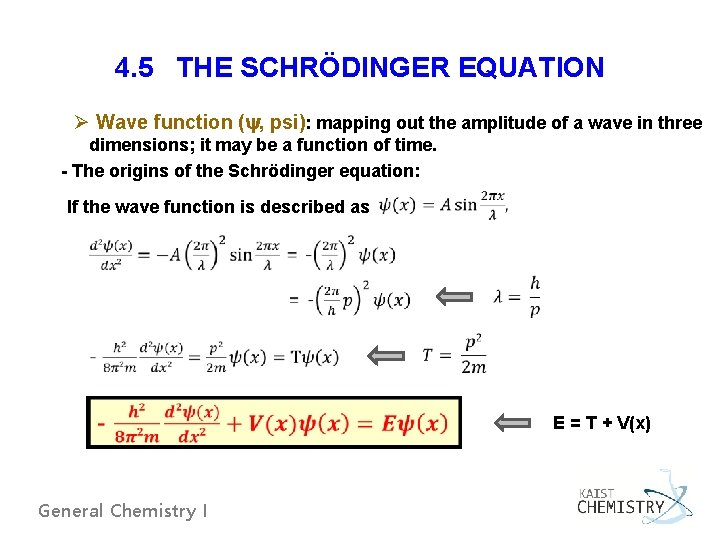
4. 5 THE SCHRÖDINGER EQUATION Ø Wave function (y, psi): mapping out the amplitude of a wave in three dimensions; it may be a function of time. - The origins of the Schrödinger equation: If the wave function is described as General Chemistry I E = T + V(x)

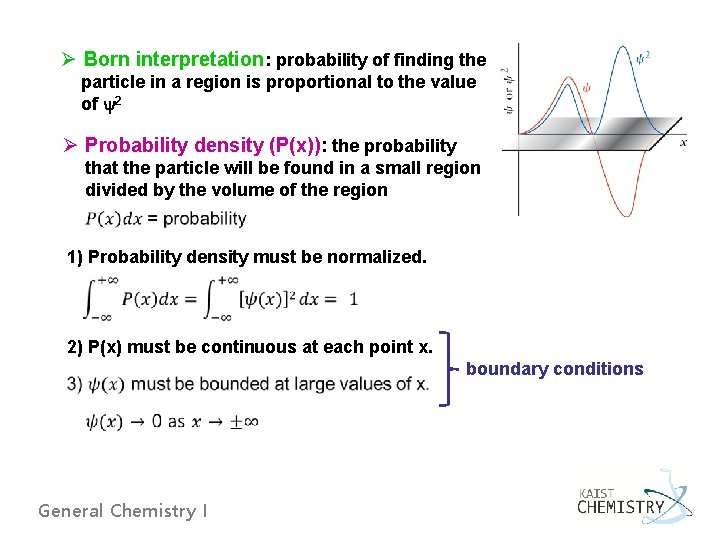
Ø Born interpretation: probability of finding the particle in a region is proportional to the value of y 2 Ø Probability density (P(x)): the probability that the particle will be found in a small region divided by the volume of the region 1) Probability density must be normalized. 2) P(x) must be continuous at each point x. boundary conditions General Chemistry I

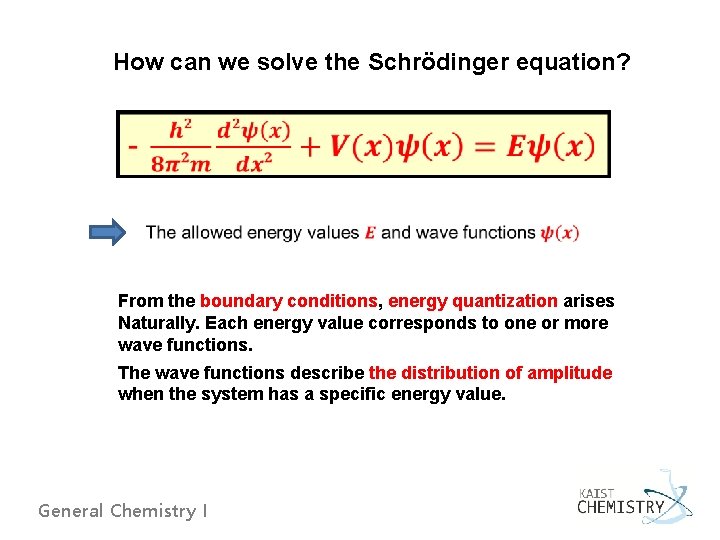
How can we solve the Schrödinger equation? From the boundary conditions, energy quantization arises Naturally. Each energy value corresponds to one or more wave functions. The wave functions describe the distribution of amplitude when the system has a specific energy value. General Chemistry I

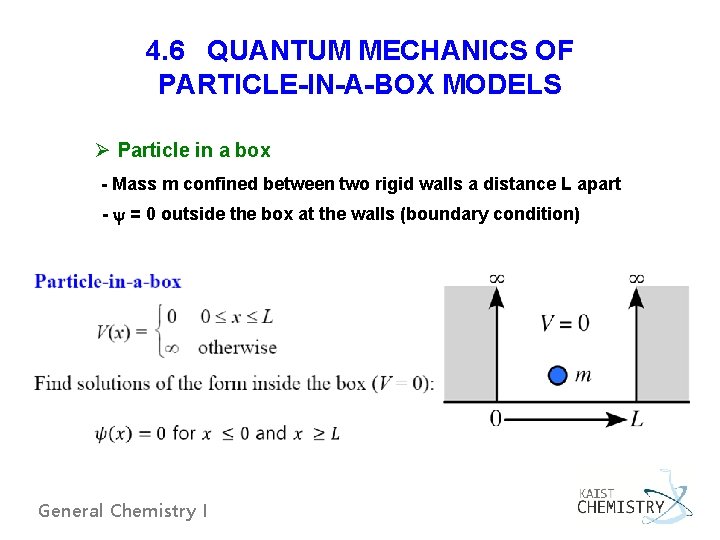
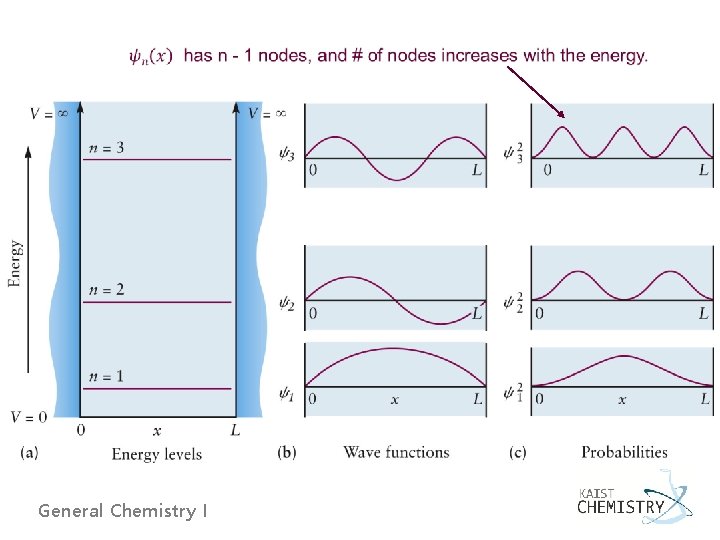
4. 6 QUANTUM MECHANICS OF PARTICLE-IN-A-BOX MODELS Ø Particle in a box - Mass m confined between two rigid walls a distance L apart - y = 0 outside the box at the walls (boundary condition) General Chemistry I

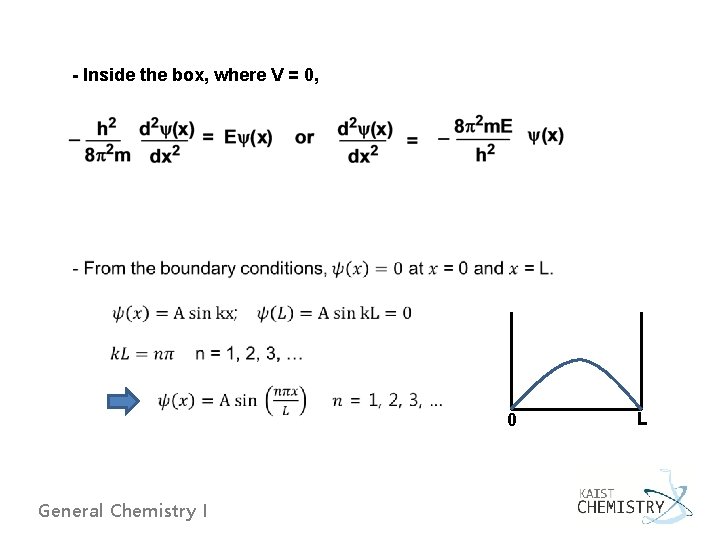
- Inside the box, where V = 0, 0 General Chemistry I L

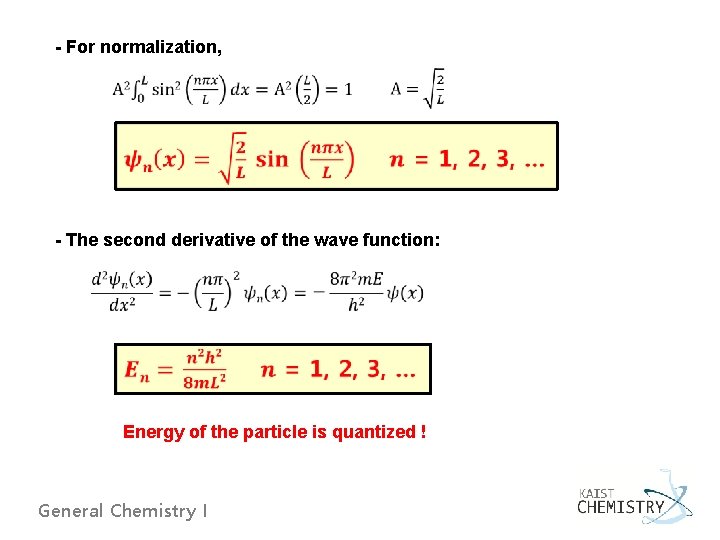
- For normalization, - The second derivative of the wave function: Energy of the particle is quantized ! General Chemistry I

General Chemistry I

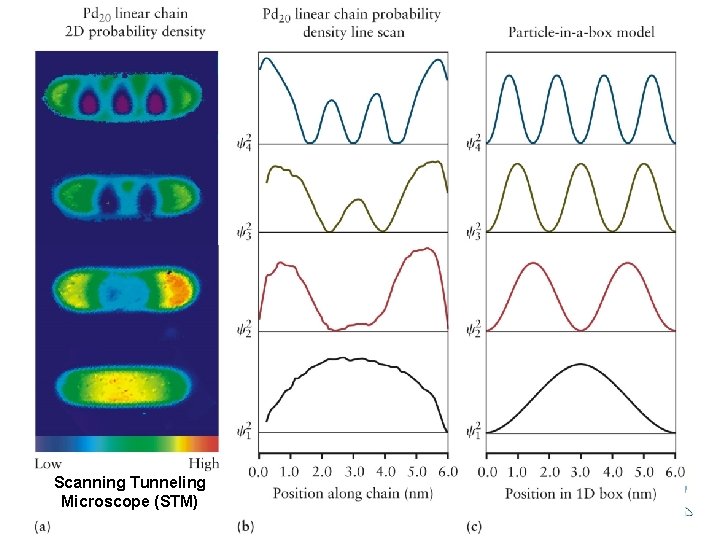
Scanning Tunneling Microscope (STM) General Chemistry I

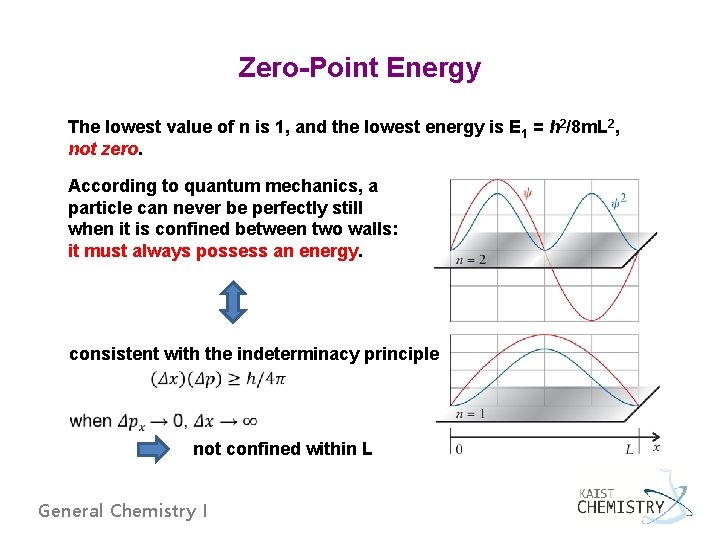
Zero-Point Energy The lowest value of n is 1, and the lowest energy is E 1 = h 2/8 m. L 2, not zero. According to quantum mechanics, a particle can never be perfectly still when it is confined between two walls: it must always possess an energy. consistent with the indeterminacy principle not confined within L General Chemistry I

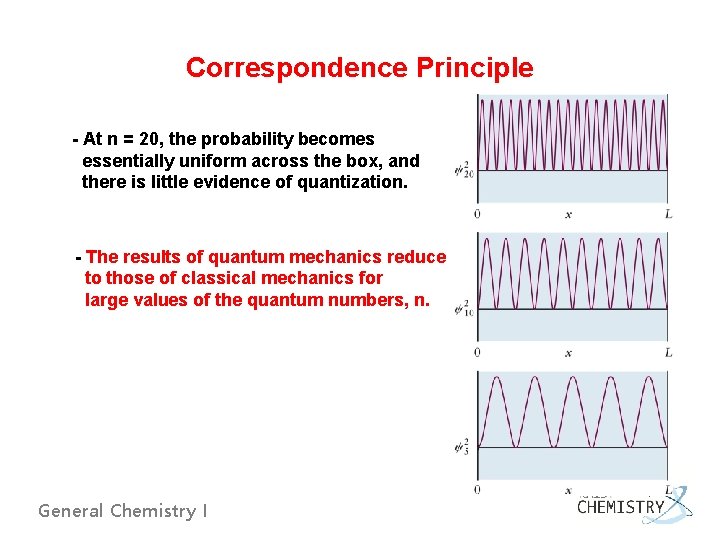
Correspondence Principle - At n = 20, the probability becomes essentially uniform across the box, and there is little evidence of quantization. - The results of quantum mechanics reduce to those of classical mechanics for large values of the quantum numbers, n. General Chemistry I

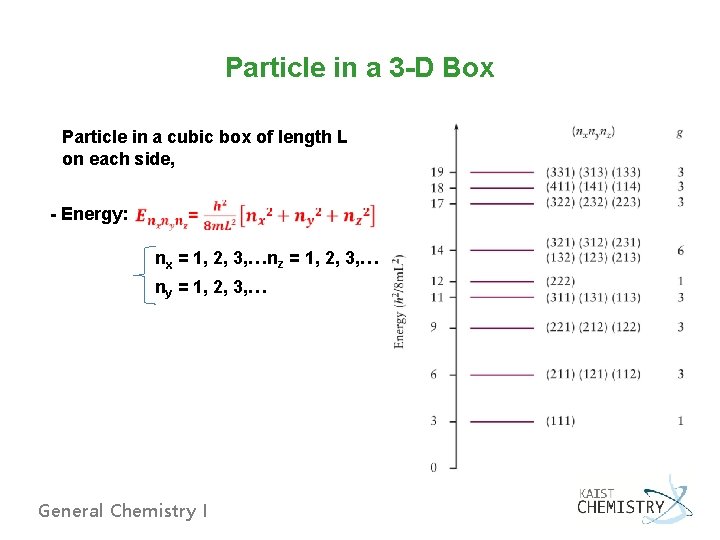
Particle in a 3 -D Box Particle in a cubic box of length L on each side, - Energy: nx = 1, 2, 3, … nz = 1, 2, 3, … ny = 1, 2, 3, … General Chemistry I

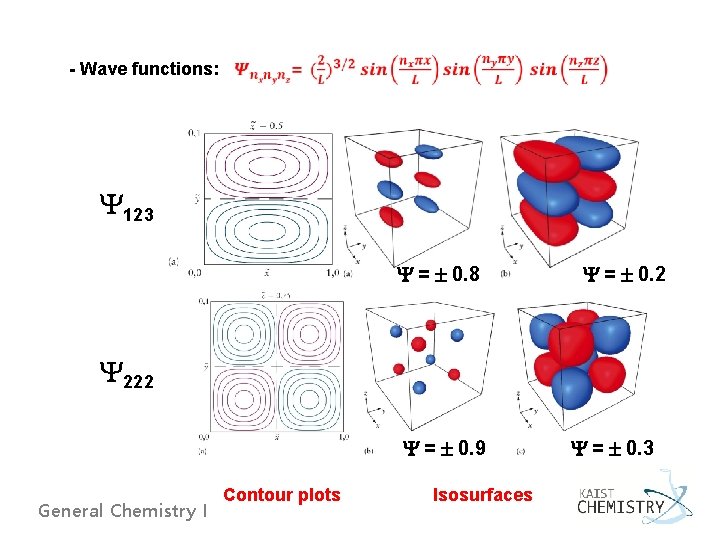
- Wave functions: Y 123 Y = 0. 8 Y = 0. 2 Y 222 Y = 0. 9 General Chemistry I Contour plots Isosurfaces Y = 0. 3

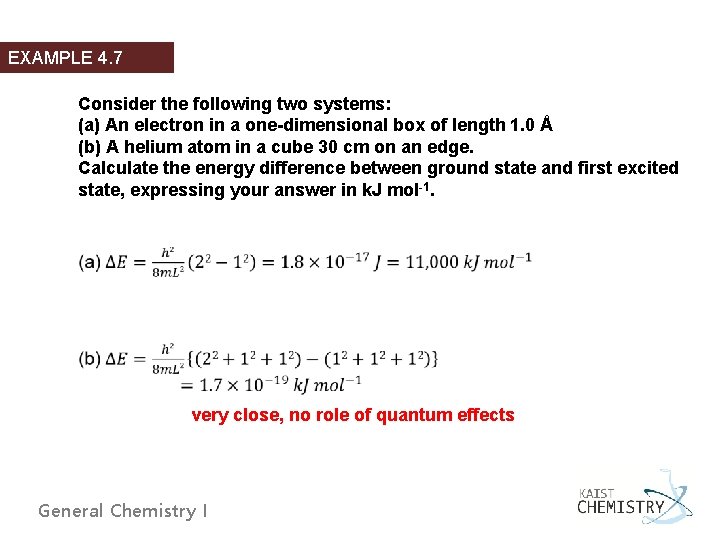
EXAMPLE 4. 7 Consider the following two systems: (a) An electron in a one-dimensional box of length 1. 0 Å (b) A helium atom in a cube 30 cm on an edge. Calculate the energy difference between ground state and first excited state, expressing your answer in k. J mol-1. very close, no role of quantum effects General Chemistry I

10 Problem Sets For Chapter 4, 6, 10, 28, 32, 38, 40, 48, 50, 58 General Chemistry I