Challenge To determine the melting point of water





















- Slides: 31

• Challenge: To determine the melting point of water. • Your write up should include the following components: • I. TITLE • II. PURPOSE III. MATERIALS • IV. PROCEDURE • V. DATA • VI. CALCULATIONS/GRAPH • VII. CONCLUSION (including a discussion of sources of error. ) •

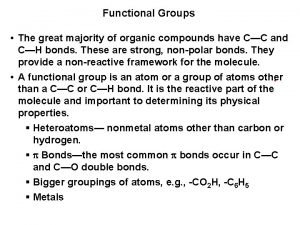
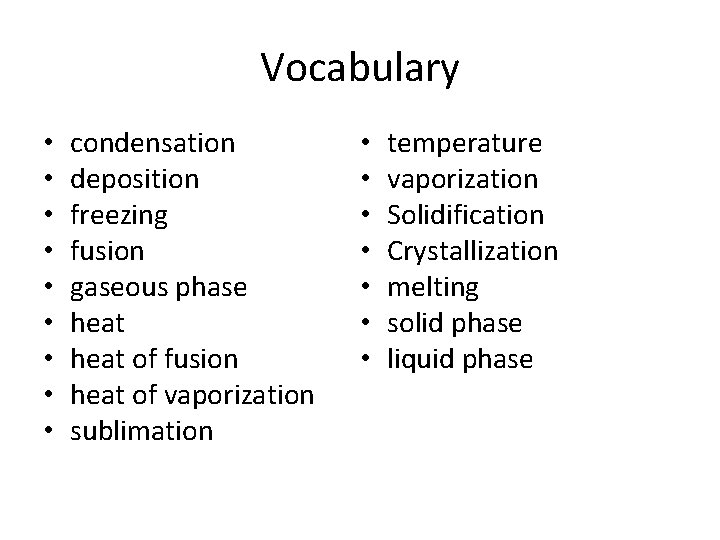
Vocabulary • • • condensation deposition freezing fusion gaseous phase heat of fusion heat of vaporization sublimation • • temperature vaporization Solidification Crystallization melting solid phase liquid phase

• Types of energy • POTENTIAL ENERGY : • STORED ENERGY. The energy inside the substance. • KINETIC ENERGY : Associated with motion. • Average KE = TEMPERATURE

HEAT OF FUSION FOR WATER (TABLE B) • Amount of heat needed to completely melt 1 g of water (ice!). • 334 J/g • 334 Joules of heat are necessary to completely melt 1 g of water. • HOW MUCH HEAT IS NEEDED TO MELT 10 g OF WATER?

HEAT OF VAPORIZATION FOR WATER (TABLE T) • The amount of heat needed to completely vaporize one g of water at its boiling point. • 2260 J/g • Water needs 2260 J of heat per gram to convert to gas!

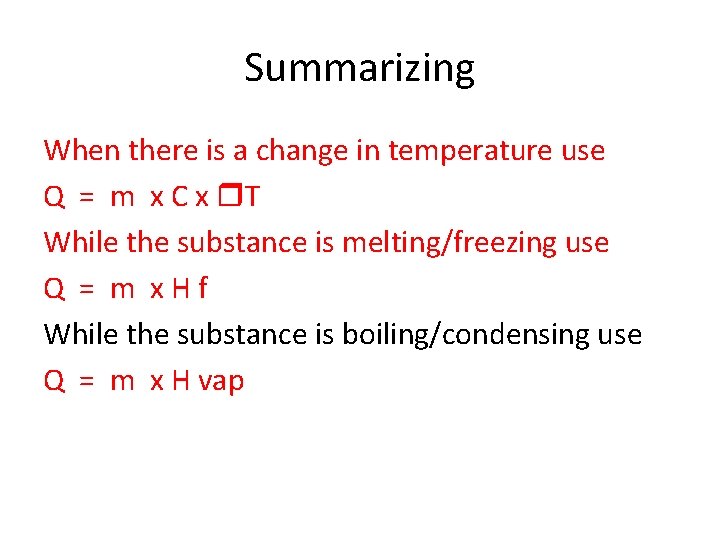
Summarizing When there is a change in temperature use Q = m x C x T While the substance is melting/freezing use Q = m x. Hf While the substance is boiling/condensing use Q = m x H vap

Do now! • How much heat is needed to completely melt 10 g of ice at 0 0 C ? • How much heat is needed to vaporize 10 g of water at 100 0 C ?

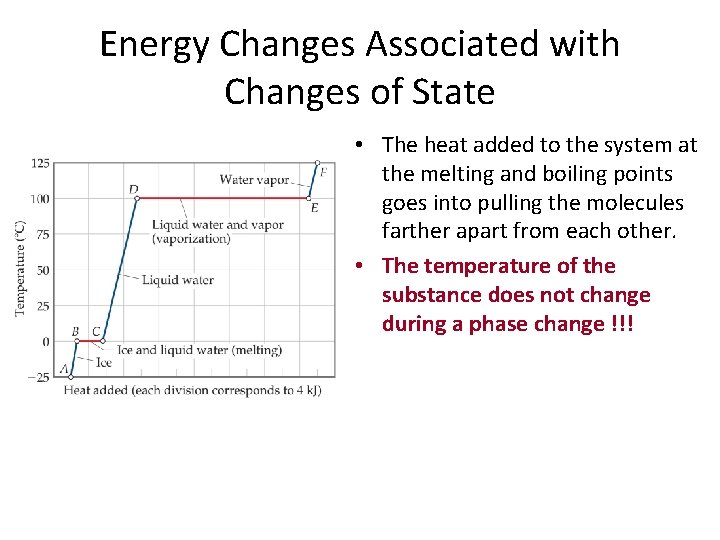
Energy Changes Associated with Changes of State • The heat added to the system at the melting and boiling points goes into pulling the molecules farther apart from each other. • The temperature of the substance does not change during a phase change !!!

Objectives • Describe what happens with PE and KE as phase changes • Identify MP and BP from heating curve • MP=FP and BP=condensation point • Calculate the Hfus and Hvap from the heating curves • Cooling curves

Objective: How to calculate heat from heating curves Do now: Draw a heating curve for the heating of 2 grams of substance that are being heated at a rate of 50 J/min. Melting Point 20 C Boiling Point 80 C Substance begins to melt at minute 2 and melts for 1 minute. It begins to boil at minute 8 and it takes 3 minutes to completely boil.

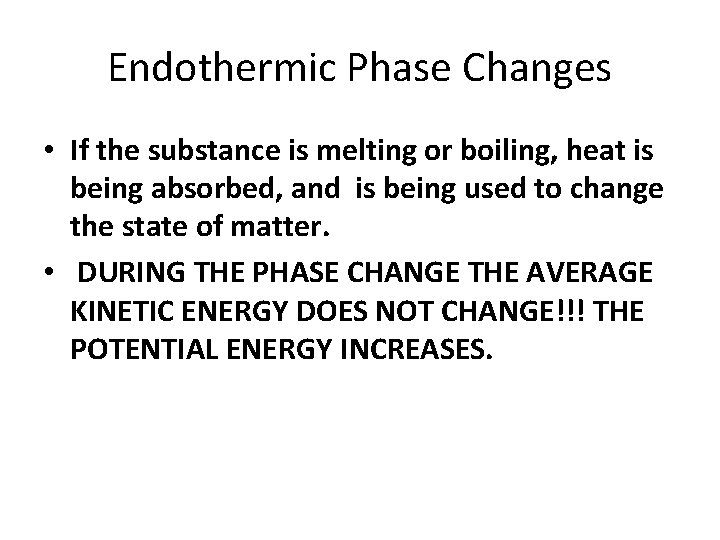
Endothermic Phase Changes • If the substance is melting or boiling, heat is being absorbed, and is being used to change the state of matter. • DURING THE PHASE CHANGE THE AVERAGE KINETIC ENERGY DOES NOT CHANGE!!! THE POTENTIAL ENERGY INCREASES.

Melting Point and Freezing Point • The temperature at which a substance melts. Is the same temperature at which the substance freeze. • Boiling Point and Condensation Point are the same temperature. Normal boiling point is the temperature at which a liquid boils at normal pressure.

Exothermic changes • If the substance is undergoing condensation or freezing then heat energy is being released. The potential energy is decreasing and the TEMPERATURE REMAINS CONSTANT!!!

Objective: How to represent heat changes Do now: Draw a cooling curve for the heating of 50 grams of substance that are being heated at a rate of 200 J/min. At minute 0 the substance is a gas. Substance begins to condense at minute 2 and it takes 7 minutes to turn completely to liquid at 70 C. At minute 16 it begins to freeze and it takes 3 minutes till it solidifies completely at 20 C. Indicate Boiling Point, Melting Point and calculate heat of fusion and heat of vaporization.

VAPORIZATION IS ENDOTHERMIC • In hot climates drinking water is cooled by evaporating water from the surfaces of porous clay pots. As water evaporates it ABSORBS heat from the water inside the container which is maintained cool. • Like cooling yourself off on a hot day by pouring water over your body. As water evaporates it absorbs heat



FREEZING IS EXOTHERMIC • In freezing weather, citrus crops are sprayed with water to protect the fruit from frost damage. As the water freezes (around the fruit-outside the fruit!) it releases heat, which helps to prevent the fruit from freezing.


How to calculate the amount of heat that a substance absorbs while it is being heated up. • DO NOW • 1. -Calculate the amount of heat needed to increase the temperature of 200 g of water from 0 0 C to 100 0 C. The heating rate is 100 J/min. • 2. -How much heat is needed to completely vaporize 200 g of water at 100 0 C

Heating curve Homework answers 1) 20 C 2) b and d 3) KE (temperature) remains constant PE increases 3) S and L 4) 20 C 5) 60 C 6) Gas 7) Increases (is T!) 8) E

TOPICS FOR TEST TEMPERATURE SCALES/CONVERSIONS HEAT TRANSFER CALORIMETRY PROBLEMS • SPECIFIC HEAT • • • PHASES OF MATTER • HEAT OF VAPORIZATION AND HEAT OF FUSION • ENDO AND EXO CHANGES • HEATING AND COOLING CURVES • BRING PENCIL AND REFERENCE TABLES • AND CALCULATOR!!!!!

HEATING/COOLING CURVE WORKSHEET ANSWERS 1. 2. 3. 4. 5. 6. 7. 40 C BC AND DE AB, CD AND EF 9 min x 60 k. J/min= 540 k. J 7 minx 60 k. J/min= 420 k. J 24 minx 60 k. J/min = 140 k. J 8 minx 60 k. J/min=480 k. J Hvap= 480 k. J/10 kg Hvap=48 J/g 8. AB 9. CD 10. EF 11. DE

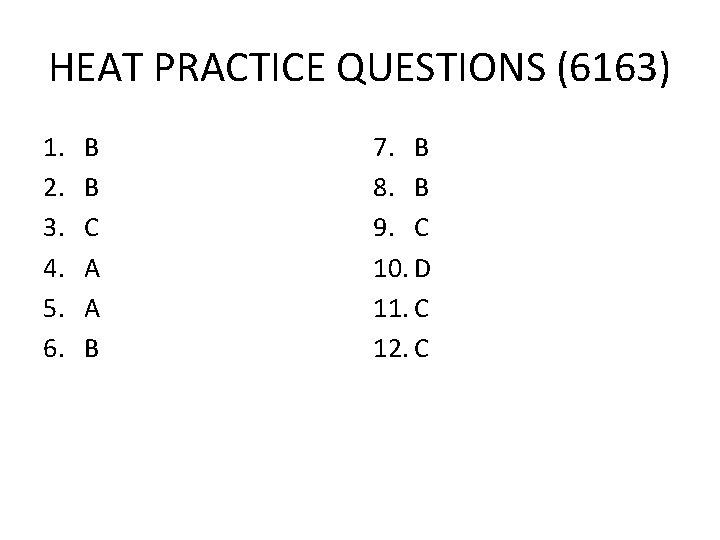
HEAT PRACTICE QUESTIONS (6163) 1. 2. 3. 4. 5. 6. B B C A A B 7. B 8. B 9. C 10. D 11. C 12. C

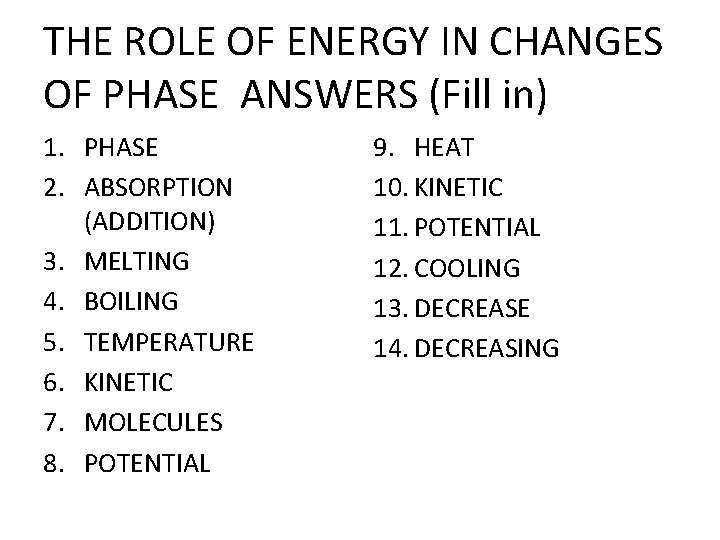
THE ROLE OF ENERGY IN CHANGES OF PHASE ANSWERS (Fill in) 1. PHASE 2. ABSORPTION (ADDITION) 3. MELTING 4. BOILING 5. TEMPERATURE 6. KINETIC 7. MOLECULES 8. POTENTIAL 9. HEAT 10. KINETIC 11. POTENTIAL 12. COOLING 13. DECREASE 14. DECREASING

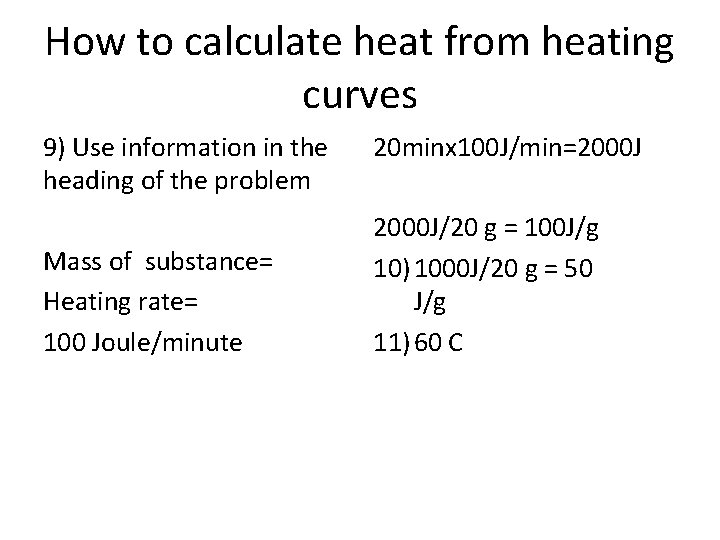
How to calculate heat from heating curves 9) Use information in the heading of the problem Mass of substance= Heating rate= 100 Joule/minute 20 minx 100 J/min=2000 J/20 g = 100 J/g 10) 1000 J/20 g = 50 J/g 11) 60 C

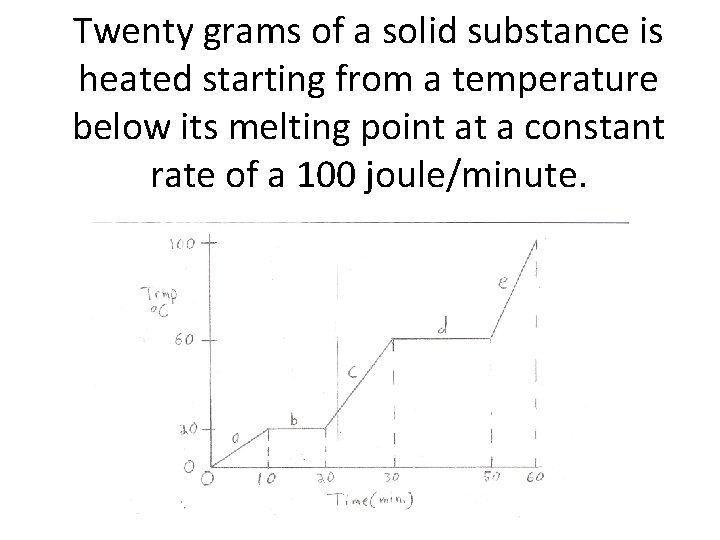
Twenty grams of a solid substance is heated starting from a temperature below its melting point at a constant rate of a 100 joule/minute.

Heat Answers • SET 1 1. 4 2. 4 3. 4 4. 4 5. 1 6. 2 7. 2 8. 3 9. 3 • • • SET 2 13 3 14 4 15 4 16 3 17 3 18 2 19 2 20 3 21 122 J/g x 7. 5 g= 915 J

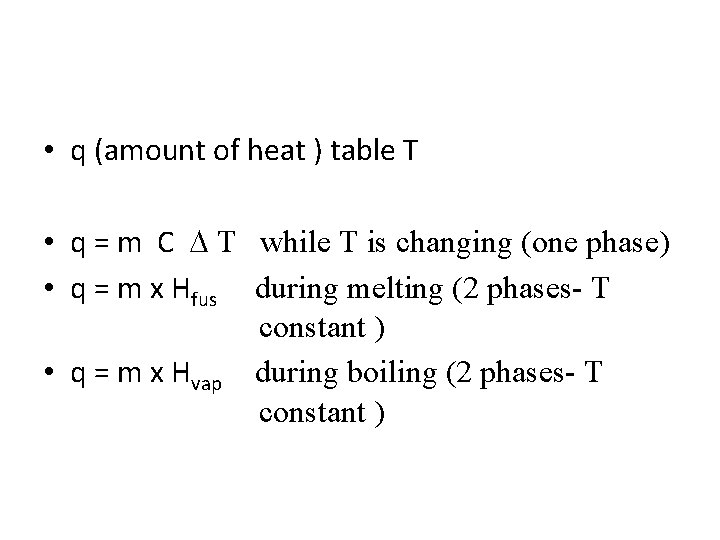
• q (amount of heat ) table T • q = m C T while T is changing (one phase) • q = m x Hfus during melting (2 phases- T constant ) • q = m x Hvap during boiling (2 phases- T constant )

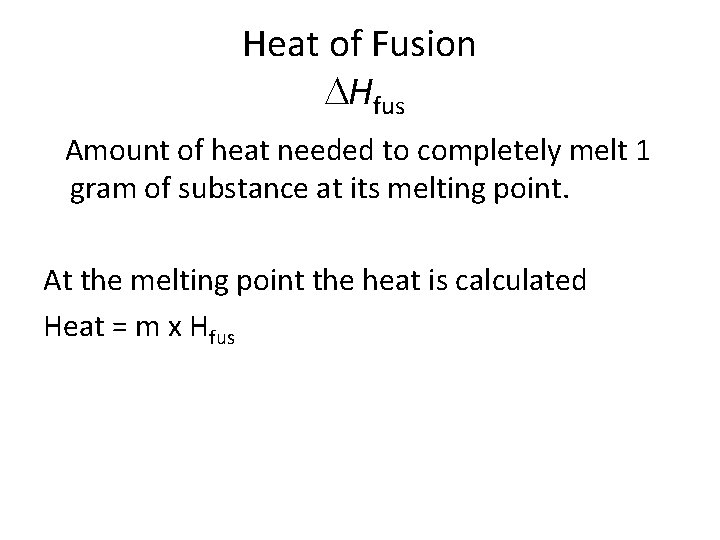
Heat of Fusion Hfus Amount of heat needed to completely melt 1 gram of substance at its melting point. At the melting point the heat is calculated Heat = m x Hfus

Heat of Vaporization Amount of heat needed to completely boil off 1 gram of substance at its boiling point. At the boiling point the heat is calculated Heat = m x Hvap
 What is congruent and incongruent melting point
What is congruent and incongruent melting point Melting point of water
Melting point of water Water and water and water water
Water and water and water water Solid to liquid
Solid to liquid Nonpolar functional groups
Nonpolar functional groups Fallon sherrock smoking
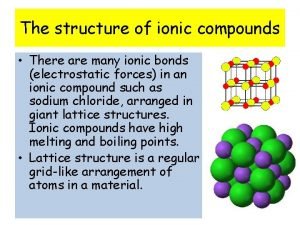
Fallon sherrock smoking Ionic compounds at room temperature
Ionic compounds at room temperature Group 1
Group 1 Melting point of group 2
Melting point of group 2 Phase diagram of ferric chloride water system
Phase diagram of ferric chloride water system Melting point of group 2
Melting point of group 2 Compare the properties of metals and nonmetals
Compare the properties of metals and nonmetals Ionic bond melting point
Ionic bond melting point Which chocolate melts faster experiment
Which chocolate melts faster experiment Hoch2oh boiling point
Hoch2oh boiling point Melting point periodic trend
Melting point periodic trend Congruent melting point
Congruent melting point Melting point of carboxylic acid derivatives
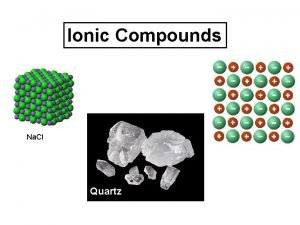
Melting point of carboxylic acid derivatives Ionic compound high melting point
Ionic compound high melting point Covalently bonded substances
Covalently bonded substances Ppo
Ppo Is melting point intensive or extensive
Is melting point intensive or extensive Mass defintion
Mass defintion Olivine melting point
Olivine melting point 1,2,3,4-tetraphenylnaphthalene
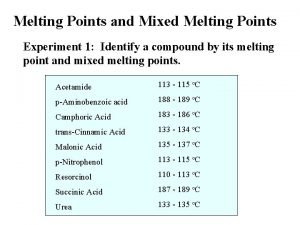
1,2,3,4-tetraphenylnaphthalene What is mixed melting point
What is mixed melting point Water tower challenge
Water tower challenge How to find stationary points using differentiation
How to find stationary points using differentiation Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Frameset trong html5
Frameset trong html5 Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em