Ch 6 The Periodic Table III Periodic Trends
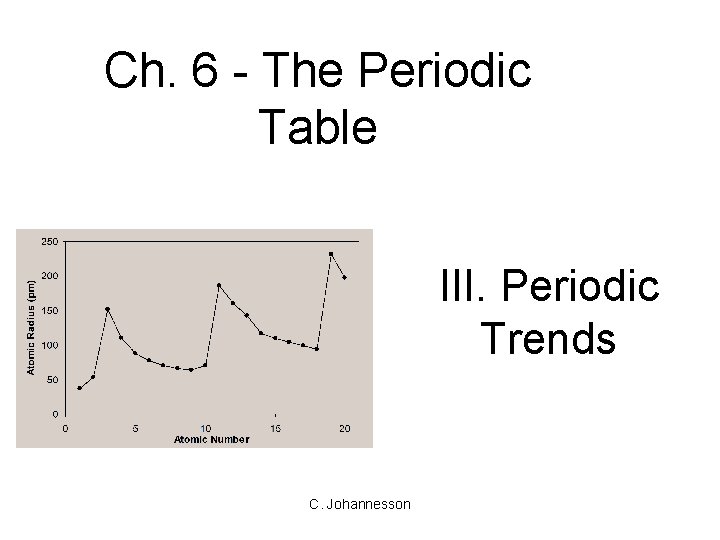
Ch. 6 - The Periodic Table III. Periodic Trends C. Johannesson
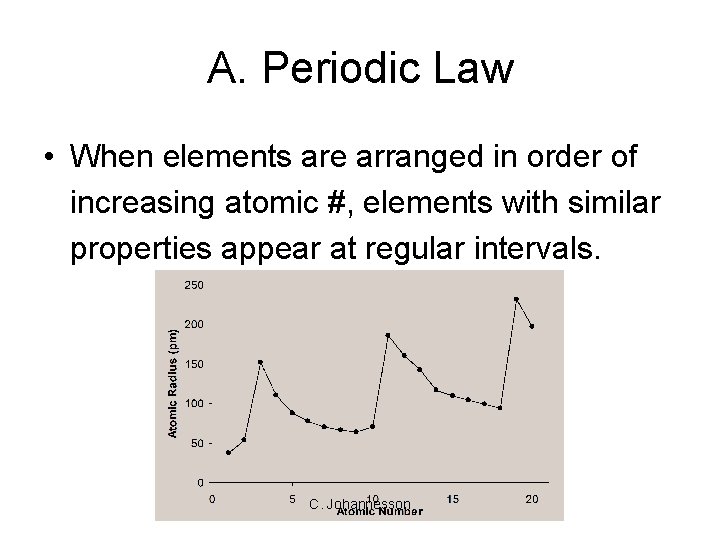
A. Periodic Law • When elements are arranged in order of increasing atomic #, elements with similar properties appear at regular intervals. C. Johannesson
B. Chemical Reactivity • Families/Groups – Similar valence e- within a group result in similar chemical properties C. Johannesson
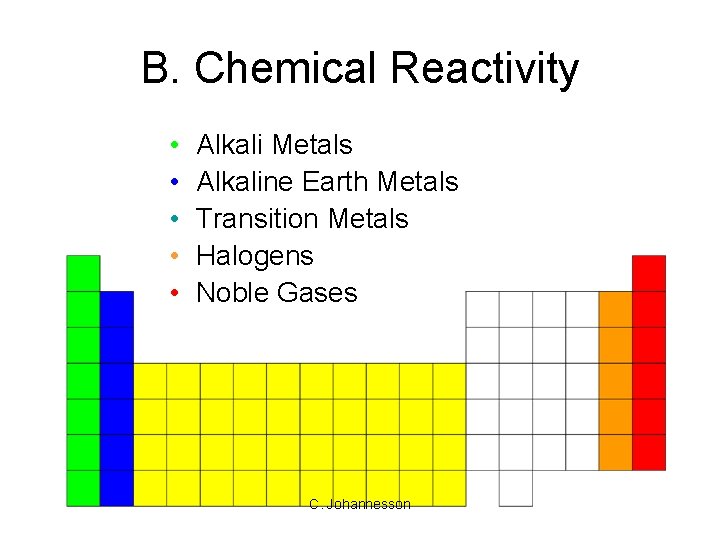
B. Chemical Reactivity • • • Alkali Metals Alkaline Earth Metals Transition Metals Halogens Noble Gases C. Johannesson
C. Valence Electrons and Dot Diagrams • Valence electrons: electrons in outermost energy level – Group number for the representative elements • Don’t do transition elements • Dot diagrams – Use dots to represent valence electrons • Write symbol • Start a dot at the top and go clockwise around – Then make pairs up to a maximum of 8 C. Johannesson
D. Other Properties • Atomic Radius – size of atom • First Ionization Energy © 1998 LOGAL – Energy required to remove one e- from a neutral atom. • Melting/Boiling Point C. Johannesson © 1998 LOGAL
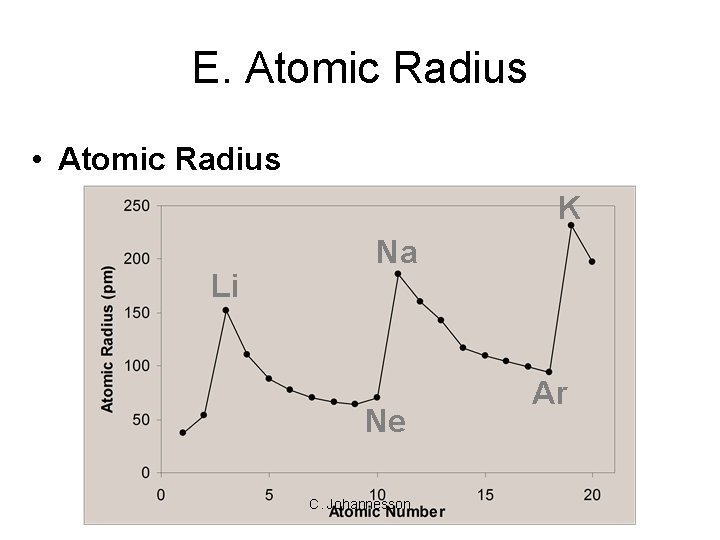
E. Atomic Radius • Atomic Radius K Li Na Ne C. Johannesson Ar
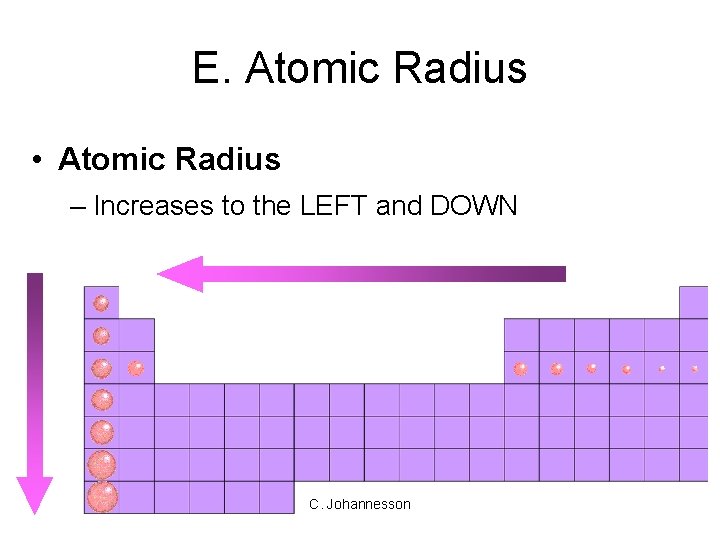
E. Atomic Radius • Atomic Radius – Increases to the LEFT and DOWN C. Johannesson
E. Atomic Radius • Why larger going down? – Higher energy levels have larger orbitals • Each row in the p. t. is called a period – Shielding - core e- block the attraction between the nucleus and the valence e- • Why smaller to the right? – Increased nuclear charge without additional shielding pulls e- in tighter C. Johannesson
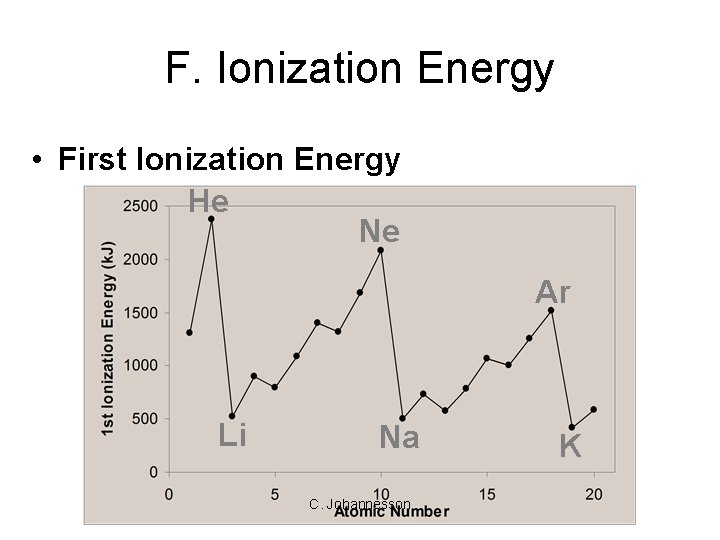
F. Ionization Energy • First Ionization Energy He Ne Ar Li Na C. Johannesson K
F. Ionization Energy • First Ionization Energy – Increases UP and to the RIGHT C. Johannesson
F. Ionization Energy • Why opposite of atomic radius? – In small atoms, e- are close to the nucleus where the attraction is stronger • Why small jumps within each group? – Stable e- configurations don’t want to lose e- C. Johannesson
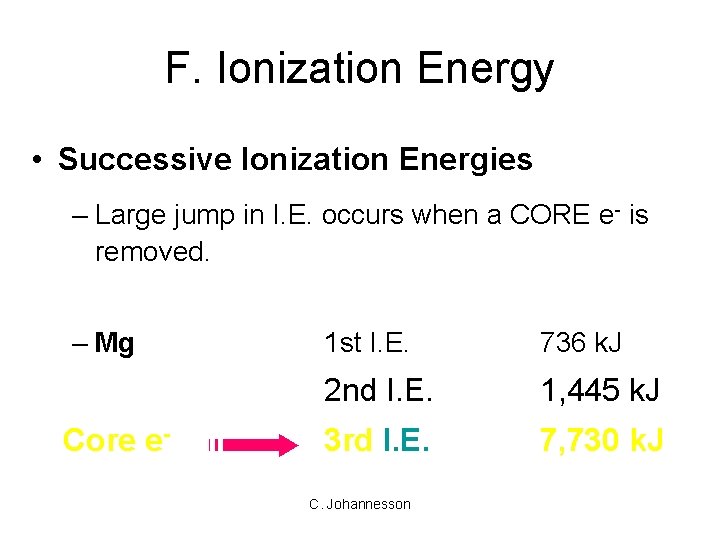
F. Ionization Energy • Successive Ionization Energies – Large jump in I. E. occurs when a CORE e- is removed. – Mg Core e- 1 st I. E. 736 k. J 2 nd I. E. 1, 445 k. J 3 rd I. E. 7, 730 k. J C. Johannesson
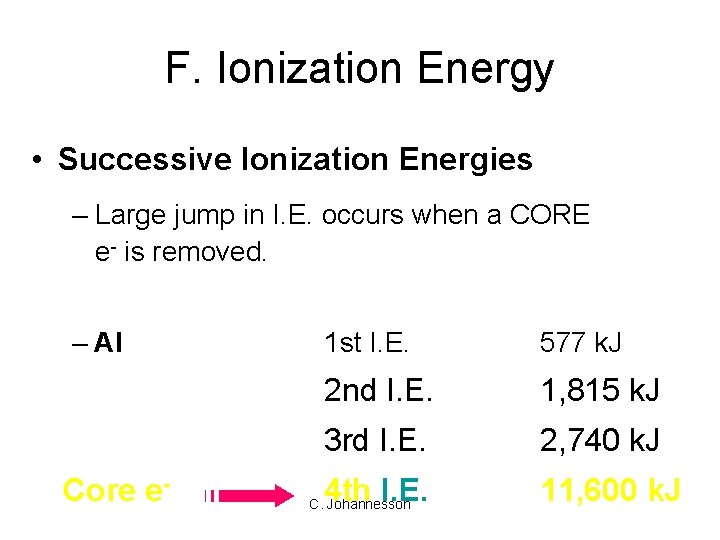
F. Ionization Energy • Successive Ionization Energies – Large jump in I. E. occurs when a CORE e- is removed. – Al Core e- 1 st I. E. 577 k. J 2 nd I. E. 1, 815 k. J 3 rd I. E. 2, 740 k. J 4 th I. E. 11, 600 k. J C. Johannesson
G. Electronegativity • the relative ability of its atoms to attract electrons in a chemical bond. – values are calculated and have a value of 3. 98 or less. (see page 169) y. Increases across y. Decreases down C. Johannesson

G. Electronegativity • Decreases down a group – Shielding of core electrons • Increases left to right across a period – Metals tend to lose e-; nonmetals gain C. Johannesson
G. Electronegativity • F is most electronegative element (most attractive) • Fr is the least electronegative (least attractive) C. Johannesson
H. Melting/Boiling Point • Melting/Boiling Point – Highest in the middle of a period. C. Johannesson
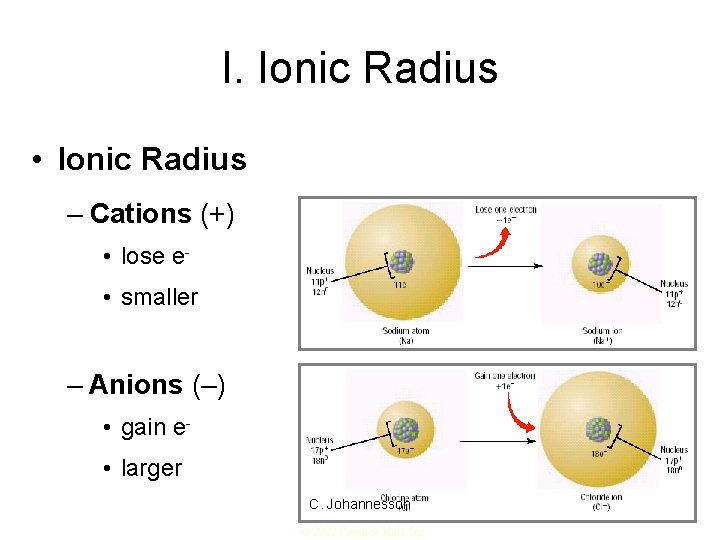
I. Ionic Radius • Ionic Radius – Cations (+) • lose e • smaller – Anions (–) • gain e • larger C. Johannesson © 2002 Prentice-Hall, Inc.
Examples • Which atom has the larger radius? –Be or Ba –Ca or Br C. Johannesson
Examples • Which atom has the higher 1 st I. E. ? –N or Bi –Be or Ne C. Johannesson
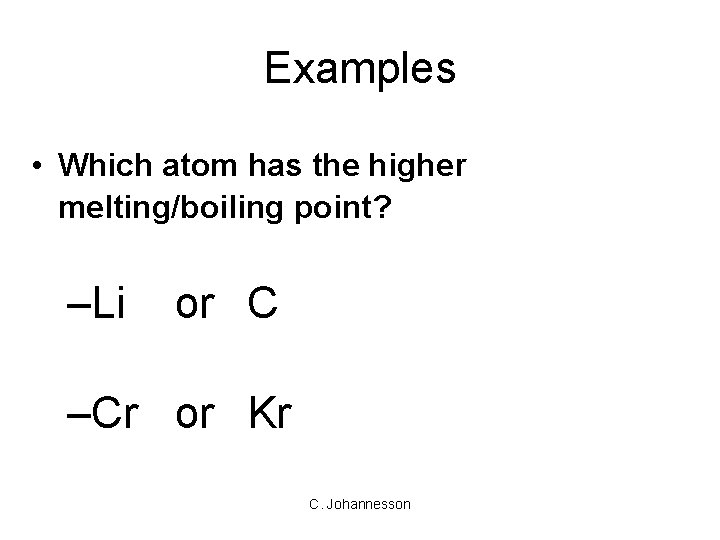
Examples • Which atom has the higher melting/boiling point? –Li or C –Cr or Kr C. Johannesson
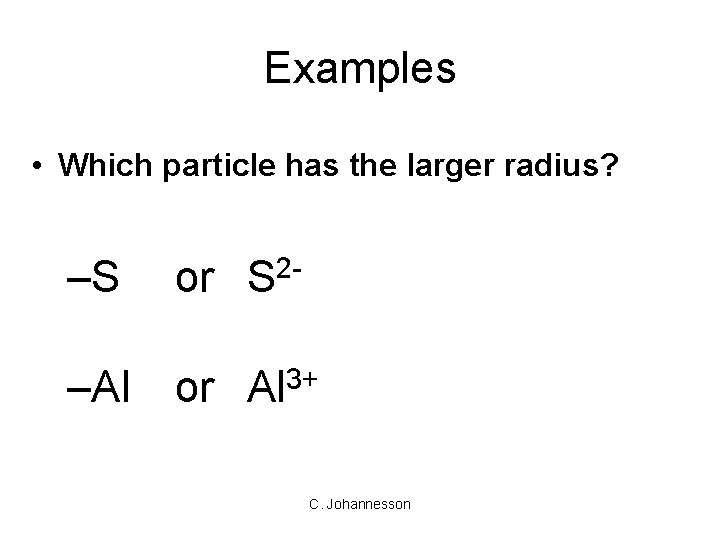
Examples • Which particle has the larger radius? –S or S 2 - –Al or Al 3+ C. Johannesson
- Slides: 23