Ch 30 Compounding of NonSterile Products O Pharmacy



























































- Slides: 82

Ch 30: Compounding of Non-Sterile Products O Pharmacy personnel engage in compounding when a practitioner prescribes a strength or type of medication that is not commercially available O It should never be done to create a product that is already available in the marketplace © 2013 -2015

Compounding of Non-Sterile Products O Compounding can be divided between “sterile” and “non-sterile” depending on the intended use of the product O Examples of non-sterile products may include tablets, capsules, and suppositories (all enteral routes of administration) O This session covers non-sterile products O We will cover sterile products in the future © 2013 -2015

Compounding of Non-Sterile Products O United States Pharmacopeia O Non-profit organization that sets standards O Helps ensure quality and safety O Medications sold and produced must meet these standards O Published in the USP-NF O Cited as Book – Chapter USP ### or USP <###> O Chapter that covers non-sterile compounding is: USP 795 © 2013 -2015

Compounding of Non-Sterile Products O USP Chapter 795 - “Pharmaceutical Compounding Non-Sterile Preparations” O Sets forth the rules for extemporaneous compounding O Defines standard of care O Provides an enforceable set of standards O Outlines responsibility of the compounder O Mandates a compounding record © 2013 -2015

Responsibilities of the Preparer Under USP 795 O USP Chapter puts responsibilities on the preparer of the product: O Have properly trained and capable associates O Appropriate and clean compounding area O Use ingredients that are; O Appropriate identity, quality, and purity O From a reputable source O Appropriate, clean, and well functioning equipment O Only authorized personnel are in the work vicinity O Compounding is reproducible according to the directions recorded – allowing for correction or errors or problems © 2013 -2015

The Compounding Record O The compounding record must contain: O A unique ID number for each product or batch O The date of preparation O The name of the preparer O The name of each ingredient O The lot number & beyond use date of each ingredient O The amount used of each ingredient O The patient’s name O The directions used to produce the compound O MUST be reproducible © 2013 -2015

Assigning a Beyond-Use Date O A Beyond-Use date must be assigned to the compounded product O The rule to remember is set forth by USP 795: “for non-sterile solid and liquid dosage forms that are repackaged in single-unit and unit-dose containers, the beyond-use date shall be one year from the date packaged or the beyond-use date on the manufacturer’s container, whichever is earlier” © 2013 -2015

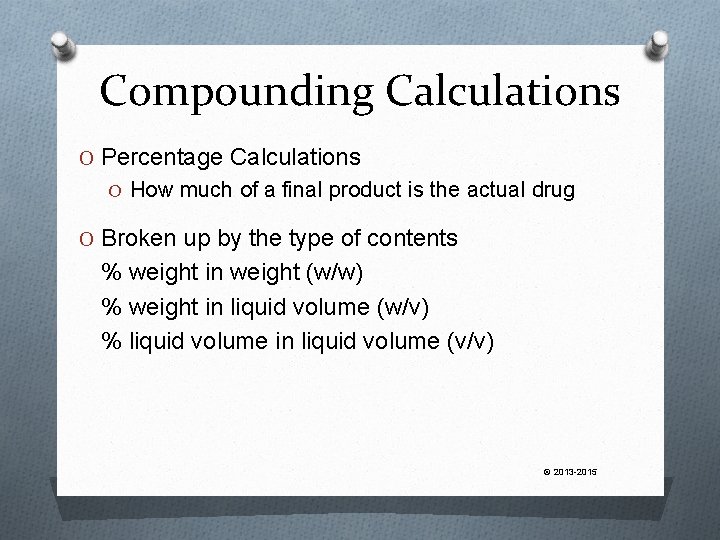
Compounding Calculations O Percentage Calculations O How much of a final product is the actual drug O Broken up by the type of contents % weight in weight (w/w) % weight in liquid volume (w/v) % liquid volume in liquid volume (v/v) © 2013 -2015

Percent weight / weight O Used when both portions are in units of weight O 1% w/w = 1 g of active drug per 100 g of total product (1 g/100 g) © 2013 -2015

Percent weight / volume O Used when the solute is in units of weight and the solvent is in units of volume O 1% w/v = 1 g of active drug per 100 ml of total product (1 g/100 ml) © 2013 -2015

Percent volume / volume O Used when both portions are in the same units of volume O 1% v/v = 1 ml of active drug per 100 ml of total product (1 ml/100 ml) © 2013 -2015

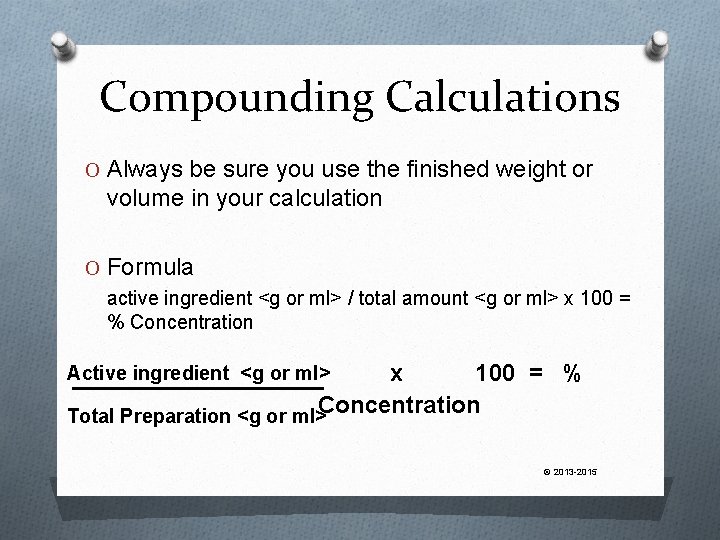
Compounding Calculations O Always be sure you use the finished weight or volume in your calculation O Formula active ingredient <g or ml> / total amount <g or ml> x 100 = % Concentration Total Preparation <g or ml> Active ingredient <g or ml> © 2013 -2015

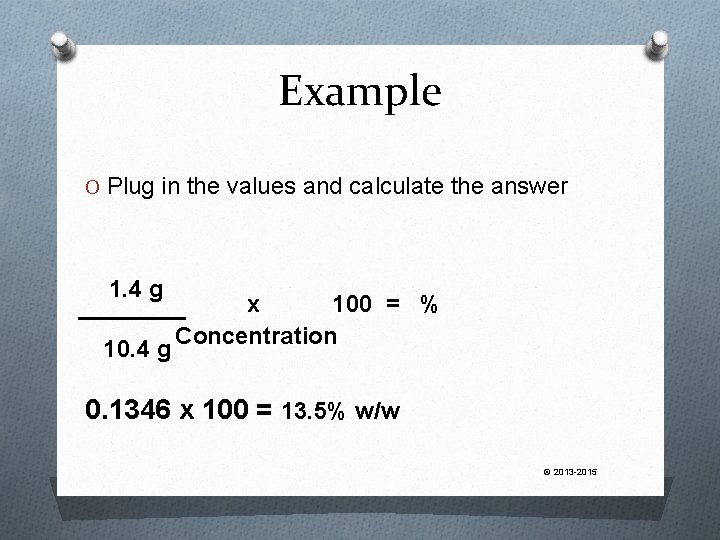
Example 1. 4 g of phenol is mixed with 9. 0 g of glycerin. What is the resulting percentage concentration of phenol? What do we know? O there is 1. 4 g of drug in the product O the final weight of the product will be 10. 4 g (1. 4 g + 9 g = 10. 4 g) © 2013 -2015

Example O Plug in the values and calculate the answer 1. 4 g 10. 4 g x 100 = % Concentration 0. 1346 x 100 = 13. 5% w/w © 2013 -2015

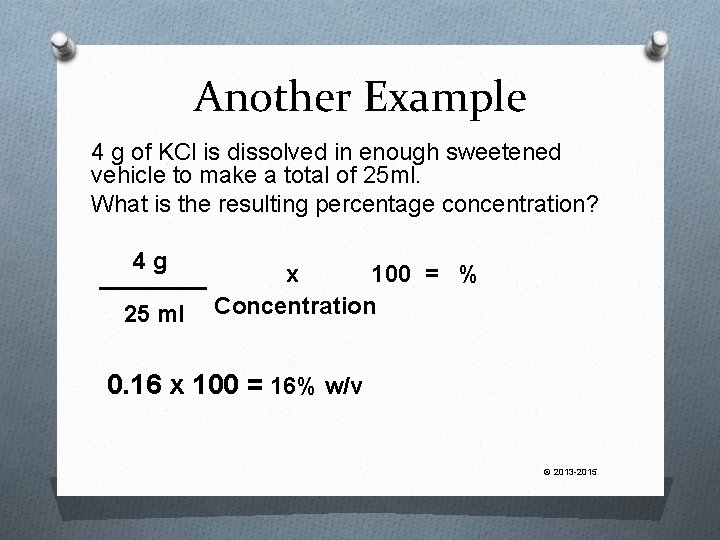
Another Example 4 g of KCl is dissolved in enough sweetened vehicle to make a total of 25 ml. What is the resulting percentage concentration? © 2013 -2015

Another Example 4 g of KCl is dissolved in enough sweetened vehicle to make a total of 25 ml. What is the resulting percentage concentration? 4 g 25 ml x 100 = % Concentration 0. 16 x 100 = 16% w/v © 2013 -2015

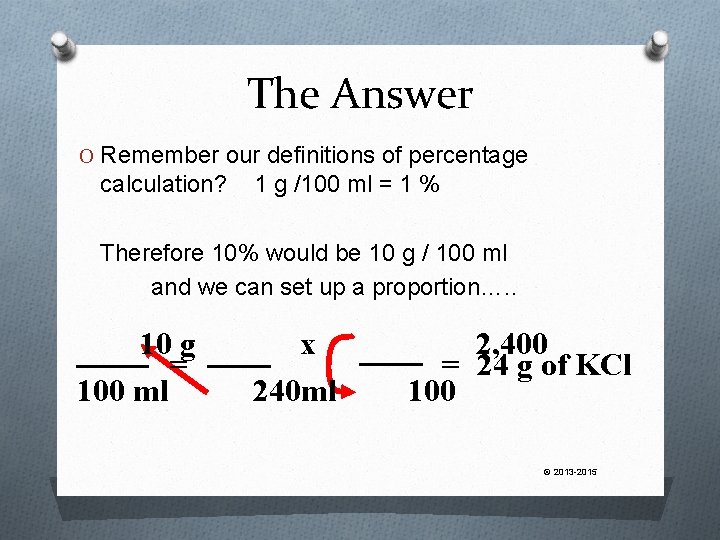
A Twist We can also used percentage concentration expressions as part of proportion calculations ie, How much KCl would be needed to make 240 ml of a 10% solution? © 2013 -2015

The Answer O Remember our definitions of percentage calculation? 1 g /100 ml = 1 % Therefore 10% would be 10 g / 100 ml and we can set up a proportion…. . 10 g = 100 ml x 240 ml 2, 400 = 24 g of KCl 100 © 2013 -2015

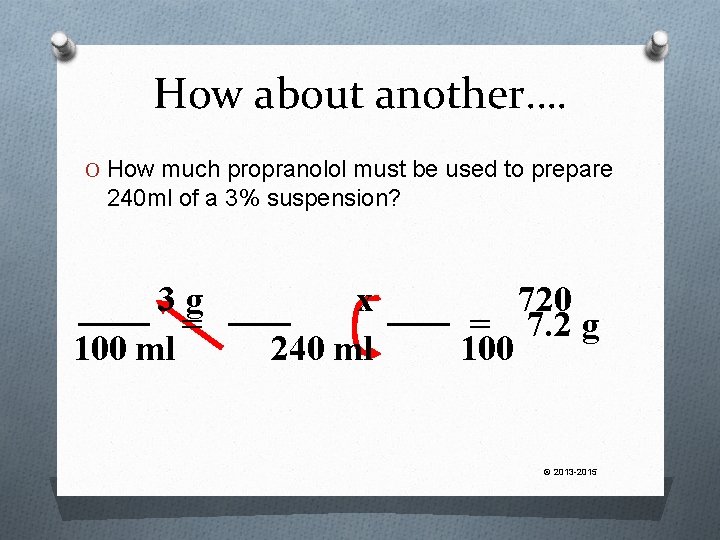
How about another…. How much propranolol must be used to prepare 240 ml of a 3% suspension? © 2013 -2015

How about another…. O How much propranolol must be used to prepare 240 ml of a 3% suspension? 3 g = 100 ml x 240 ml 720 = 7. 2 g 100 © 2013 -2015

Alligation O Alligation is useful whenever we are using two different concentrations of a drug product to arrive at a final concentration between the two components O For instance preparing a 10% product from 20% and 5% components © 2013 -2015

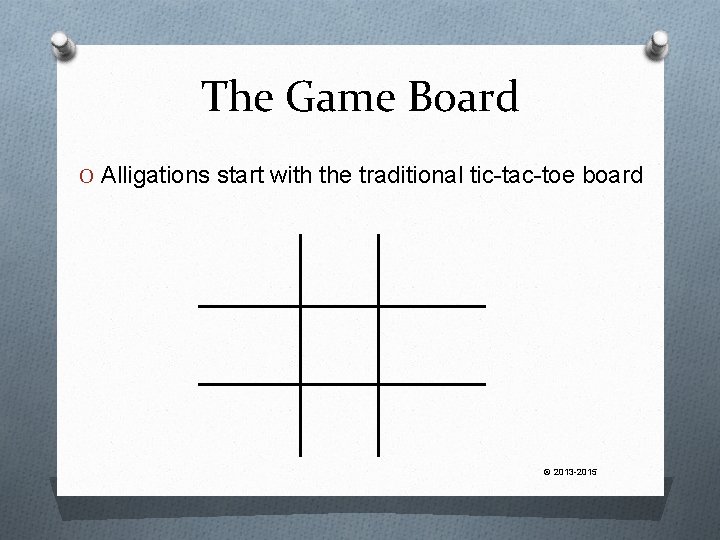
The Game Board O Alligations start with the traditional tic-tac-toe board © 2013 -2015

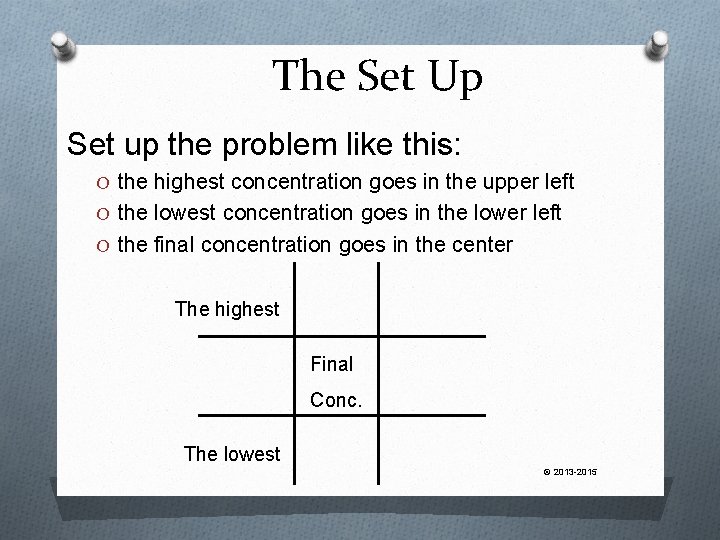
The Set Up Set up the problem like this: O the highest concentration goes in the upper left O the lowest concentration goes in the lower left O the final concentration goes in the center The highest Final Conc. The lowest © 2013 -2015

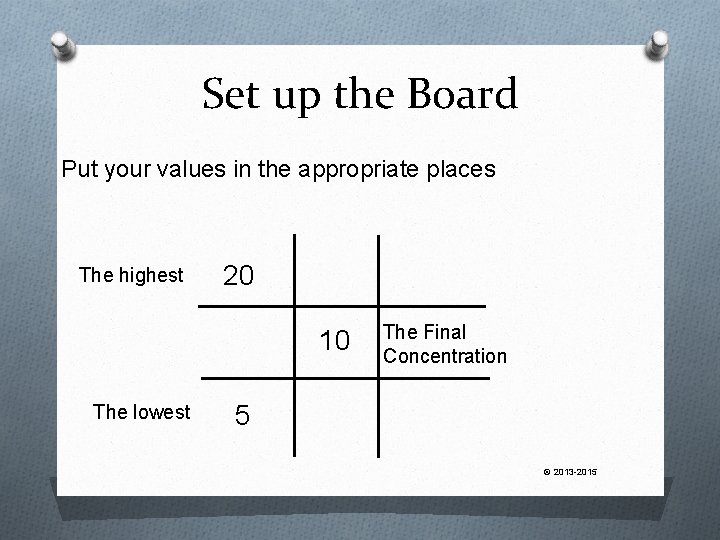
Let’s Try One How much of 20% Drug “A” cream is needed to mix with 5% Drug “A” cream to obtain 240 g of a final 10% concentration? © 2013 -2015

Set up the Board Put your values in the appropriate places The highest 20 10 The lowest The Final Concentration 5 © 2013 -2015

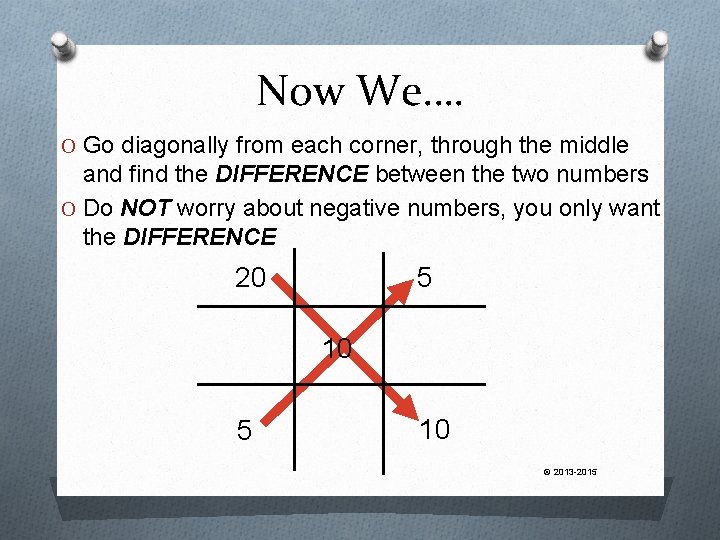
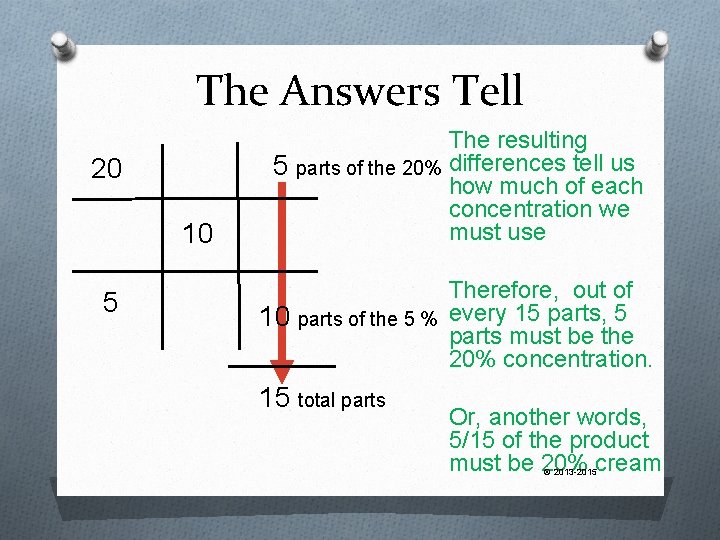
Now We…. O Go diagonally from each corner, through the middle and find the DIFFERENCE between the two numbers O Do NOT worry about negative numbers, you only want the DIFFERENCE 20 5 10 © 2013 -2015

The Answers Tell 20 10 5 The resulting 5 parts of the 20% differences tell us how much of each concentration we must use Therefore, out of 10 parts of the 5 % every 15 parts, 5 parts must be the 20% concentration. 15 total parts Or, another words, 5/15 of the product must be 20% cream © 2013 -2015

Finally O Since we know 5/15 of the product must be the 20% strength, all we do is multiply 5/15 times the amount of product we are making. 5 15 x 20% cream 240 g = 80 g of © 2013 -2015

Not So Bad, But…. . O Be SURE you are solving for the correct concentration O Label all calculations as to which strength you are solving for © 2013 -2015

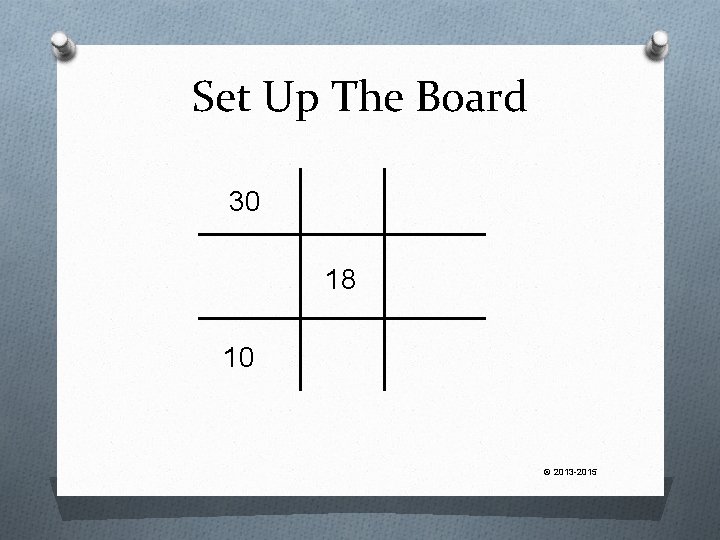
Let’s Try Another We need to compound 300 g of an 18% concentration of Drug “B”. However, Drug “B” is available only in 30% and 10% concentrations. How much of the 10% cream will be needed? © 2013 -2015

Set Up The Board 30 18 10 © 2013 -2015

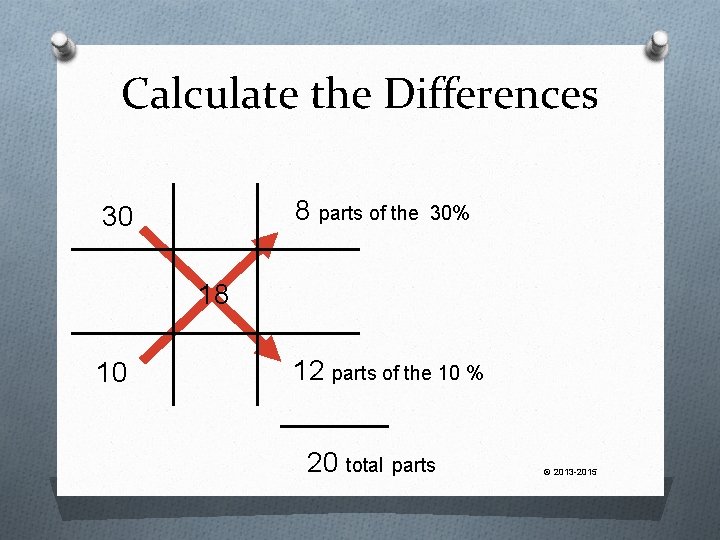
Calculate the Differences 8 parts of the 30 30% 18 10 12 parts of the 10 % 20 total parts © 2013 -2015

Solve We know that 12/20 of the final product must be the 10% 12 20 x 300 g = 180 g of 10% cream © 2013 -2015

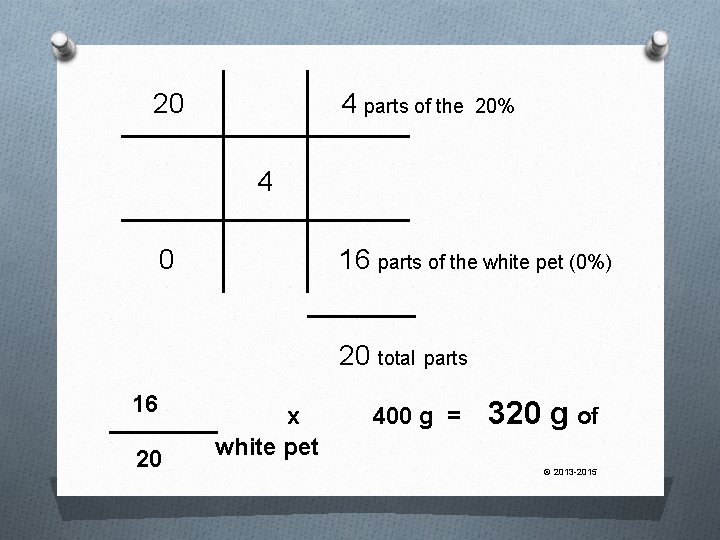
One More, Anyone? How much white petrolatum must be mixed with 20% ichthamol ointment to make 400 g of 4% product? © 2013 -2015

20 4 parts of the 20% 4 0 16 parts of the white pet (0%) 20 total parts 16 20 x white pet 400 g = 320 g of © 2013 -2015

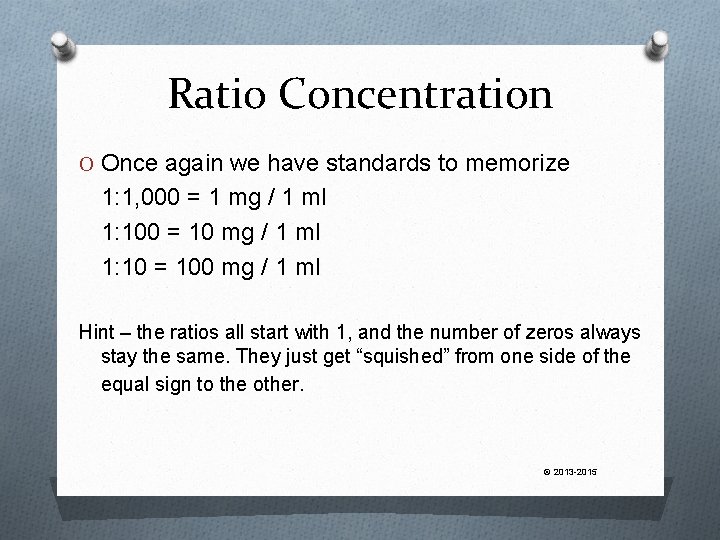
Ratio Concentration O Once again we have standards to memorize 1: 1, 000 = 1 mg / 1 ml 1: 100 = 10 mg / 1 ml 1: 10 = 100 mg / 1 ml Hint – the ratios all start with 1, and the number of zeros always stay the same. They just get “squished” from one side of the equal sign to the other. © 2013 -2015

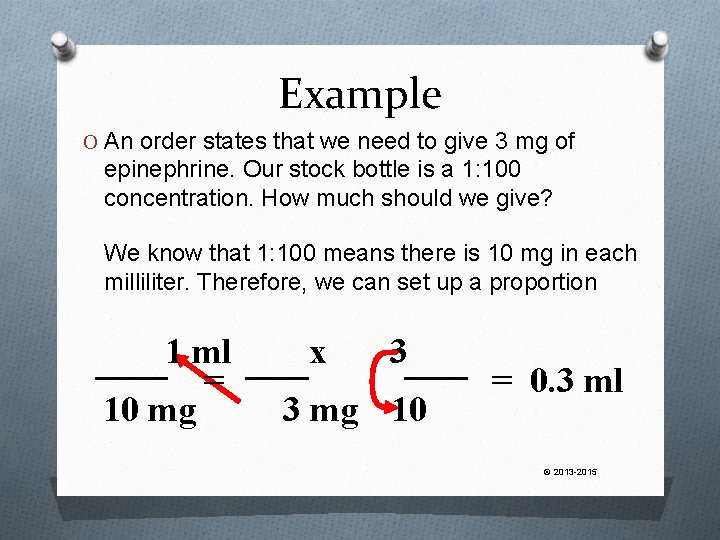
Example An order states that we need to give 3 mg of epinephrine. Our stock bottle is a 1: 100 concentration. How much should we give? © 2013 -2015

Example O An order states that we need to give 3 mg of epinephrine. Our stock bottle is a 1: 100 concentration. How much should we give? We know that 1: 100 means there is 10 mg in each milliliter. Therefore, we can set up a proportion 1 ml = 10 mg x 3 3 mg 10 = 0. 3 ml © 2013 -2015

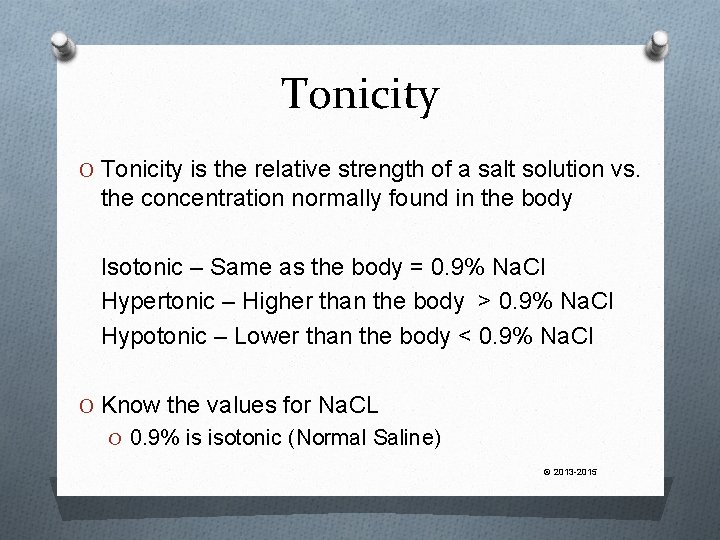
Tonicity O Tonicity is the relative strength of a salt solution vs. the concentration normally found in the body Isotonic – Same as the body = 0. 9% Na. Cl Hypertonic – Higher than the body > 0. 9% Na. Cl Hypotonic – Lower than the body < 0. 9% Na. Cl O Know the values for Na. CL O 0. 9% is isotonic (Normal Saline) © 2013 -2015

Ch 31: Payment for Drug Products O Self Payment O The individual pays for his own medication in the entirety O Third Party Payment O Any party other than the patient who pays all or part of the prescription price © 2013 -2015

Copay & Deductible O Copayment O amount of money the patient is required to pay towards their drug expense O can be a fixed dollar amount or a percentage O Deductible O the amount of money a patient must pay towards their expenses before their insurance company begins to pay © 2013 -2015

Methods of Calculating the Selling Price O Based on the acquisition cost of the product, or O Based on the Average Wholesale Price (AWP) of the product © 2013 -2015

Based on Cost O Formula cost + markup + dispensing fee = selling price O Markup O the amount of profit you expect to make on the item (markup = cost x gross profit expected) O Dispensing Fee O a calculated amount to cover the fixed costs of filling an individual prescription © 2013 -2015

Example 60 tabs of medicine cost us $23. 99. We have a dispensing fee of $4. 00 and a markup of 25%. What is the selling price for the prescription? We know our cost for the amount dispensed = $23. 99 So, using cost + markup + dispensing fee = selling $$ $23. 99 + ($23. 99 x 0. 25) + $4. 00 = $23. 99 + $6. 00 + $4. 00 = $33. 99 © 2013 -2015

Another Example Our pharmacy uses a 30% markup. Our dispensing fee is $3. 25. The drug costs us $45. 99. What is the selling price? © 2013 -2015

The Answer Cost + markup + dispensing fee = selling price $45. 99 + ($45. 99 x 0. 30) + $3. 25 = $45. 99 + $13. 80 + $3. 25 = $63. 04 © 2013 -2015

A Twist Our pharmacy has a dispensing fee of $4. 99 Our markup is 20% The drug costs us $239. 99 for a bottle of 500 tabs The prescription calls for #60 tabs What would our selling price be? © 2013 -2015

The Answer O First you must use a proportion calculation $239. 99 x = 500 tabs 60 tabs $14399. 40 = $28. 80 500 tabs O Next you use the selling price calculation $28. 80 + ($28. 80 x 0. 20) + $4. 99 = $28. 80 + $5. 76 + $4. 99 = $39. 55 © 2013 -2015

AWP Method O Average Wholesale Price O much like a car sticker price O nobody actually pays this price O that’s why there is normally a discount off of AWP involved in this calculation O The formula AWP ± percentage + dispensing fee = selling price © 2013 -2015

AWP Example The AWP for the medication dispensed is $35. 77. The insurance company allows reimbursement at AWP less 15% plus $2. 00 dispensing fee. What is the selling price? We know our AWP for the amount dispensed = $35. 77 So, using AWP ± Percentage + dispensing fee = selling $$ $35. 77 - ($35. 77 x 0. 15) + $2. 00 = $35. 77 - $5. 37 + $2. 00 = $32. 40 © 2013 -2015

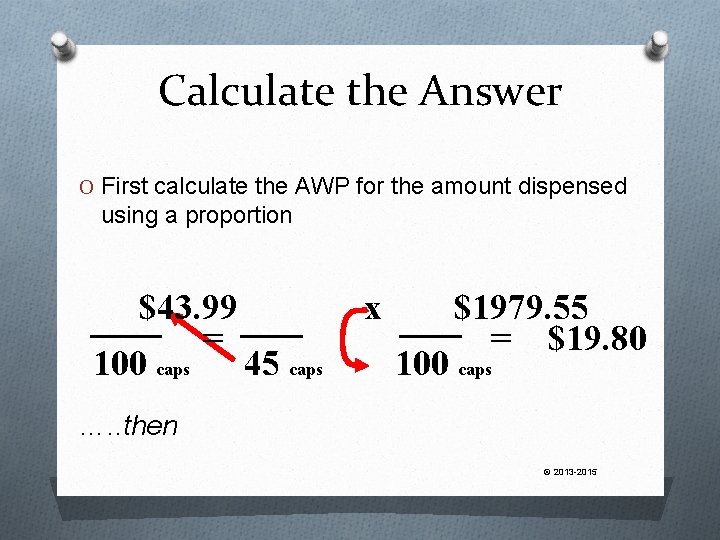
Another Example Our prescription calls for 45 capsules. The AWP for a bottle of 100 caps is $43. 99. The Insurance reimbursement is AWP less 14% plus a $3. 50 dispensing fee. The patient also must pay a $5. 00 copayment. How much should we bill the insurance company? …… Wow! What a Mess! © 2013 -2015

Calculate the Answer O First calculate the AWP for the amount dispensed using a proportion $43. 99 = 100 caps 45 caps x $1979. 55 = $19. 80 100 caps …. . then © 2013 -2015

Calculate the Answer O Use the AWP formula AWP ± Percentage + dispensing fee = selling price $19. 80 – ($19. 80 x 0. 14) + $3. 50 = $19. 80 - $2. 77 + $3. 50 = $20. 53 O BUT DON’T FORGET THE COPAYMENT! $20. 53 - $5. 00 = $15. 53 © 2013 -2015

Ch 32: Third Party Billing Process O Two main types of third party payers: O Private Insurance O Either employer paid or privately purchased O Government O Includes federal, state, or local programs O Biggest are Medicare and Medicaid © 2013 -2015

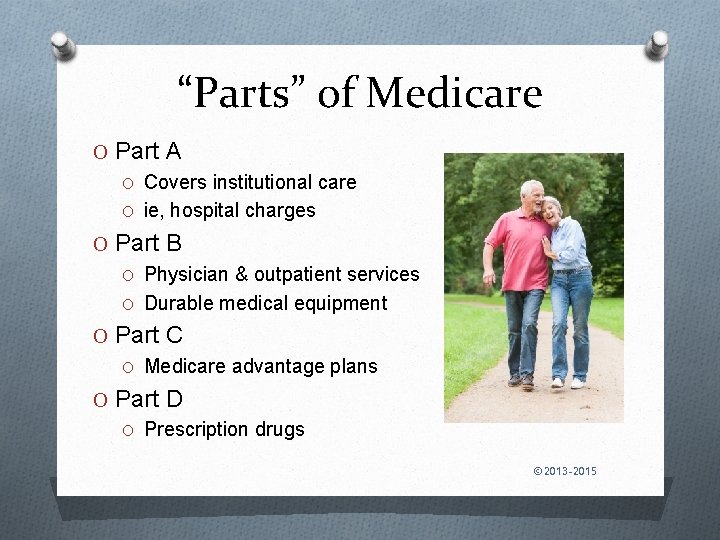
“Parts” of Medicare O Part A O Covers institutional care O ie, hospital charges O Part B O Physician & outpatient services O Durable medical equipment O Part C O Medicare advantage plans O Part D O Prescription drugs © 2013 -2015

Medicaid O Covers indigent patients O By law, must cover hospital, medical, and long term care charges O Prescription drug coverage is actually optional, although most states provide some type of coverage O Amount and type of coverage will vary between states O Coverage is not usually transferrable between states © 2013 -2015

Managed Care O System that integrates both financial and delivery of health care O Managed Care Organizations (MCO) form networks of providers with whom they contract O Some contracts pay providers a set dollar amount per enrolled patient O Means the MCO assumes the financial risk O The MCO then “manages” the care through utilization and cost controls © 2013 -2015

The Pharmacy Benefit Manager (PBM) O PBMs are responsible for the processing and payment of prescription drug claims for the insurance companies O When we submit a claim for the customer, we are sending it to the PBM, not to the insurance company O PBMs follow the directions from the insurance company’s contract with the insured, but they also are charged with reducing health costs when possible © 2013 -2015

Methods to Reduce Third Party Prescription Drug Costs O Preferred provider networks O Negotiated price discounts from pharmacies O Negotiated rebates from manufacturers O Mandatory Mail Order O Deductibles and Copayments O Formularies O Tier structures O Prior Authorizations O Medication Therapy Management O Mandatory Education © 2013 -2015

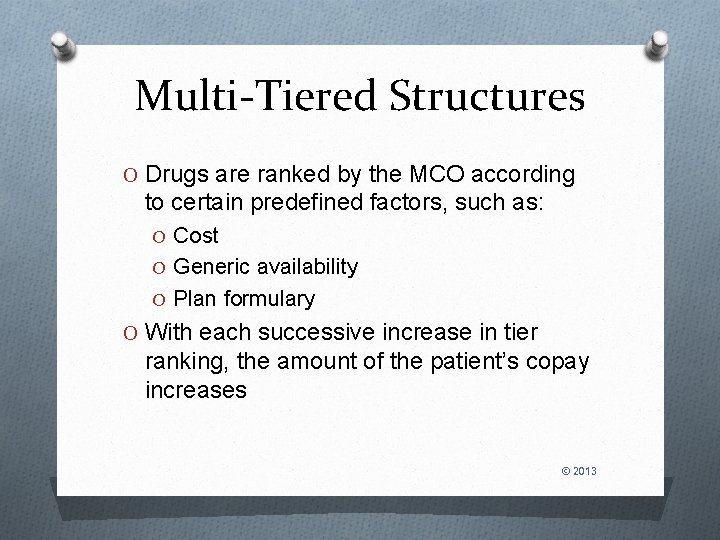
Multi-Tiered Structures O Drugs are ranked by the MCO according to certain predefined factors, such as: O Cost O Generic availability O Plan formulary O With each successive increase in tier ranking, the amount of the patient’s copay increases © 2013

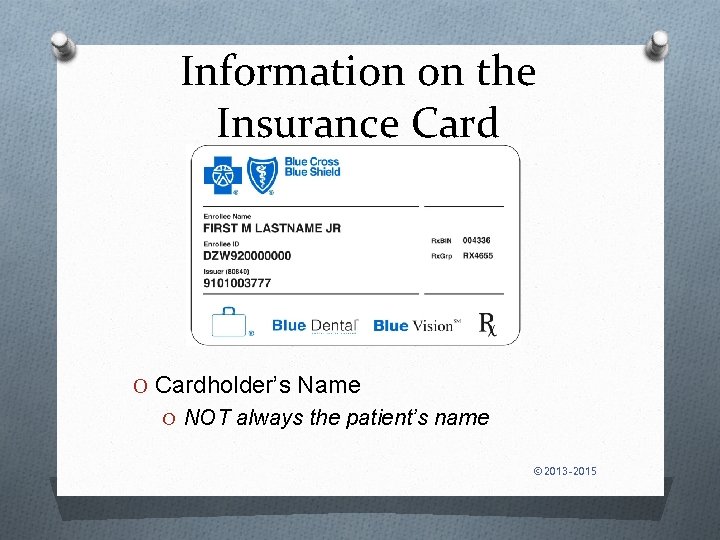
Information on the Insurance Card O Cardholder’s Name O NOT always the patient’s name © 2013 -2015

Information on the Insurance Card O Cardholder’s ID Number O Used for all covered individuals on the card O Also requires a dependent number that is rarely on the card © 2013 -2015

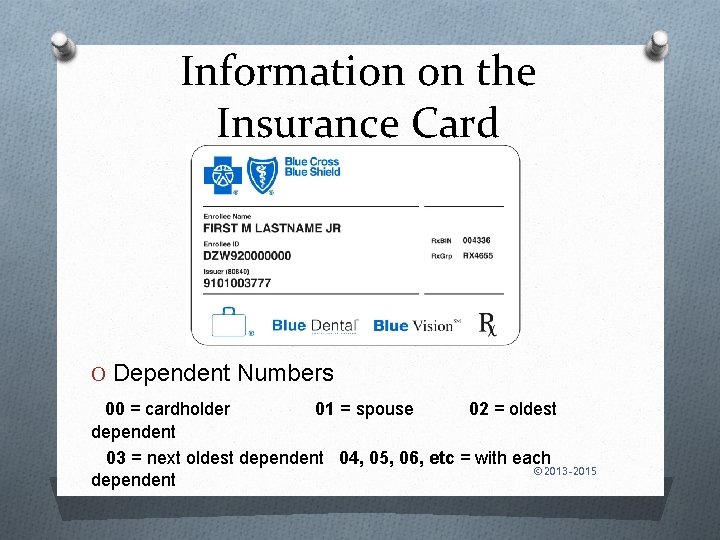
Information on the Insurance Card O Dependent Numbers 00 = cardholder 01 = spouse 02 = oldest dependent 03 = next oldest dependent 04, 05, 06, etc = with each © 2013 -2015 dependent

Information on the Insurance Card O BIN Number O Tells us who to bill the claim to © 2013 -2015

Information on the Insurance Card O Group Number O Identifies which plan the cardholder belongs to © 2013 -2015

Required Information When Billing Third Party Claims O In addition to all of the required information for a prescription to be valid, the following items must also be present on the prescription: O Dispense as Written Codes (DAW) O Day Supply O Actual Package Size Used O Diagnosis Codes © 2013 -2015

Dispense as Written Codes O Determined by prescriber’s notation on the prescription O Common DAW Codes: 0 = no product selection indicated (may use generic) 1 = Prescriber wants the brand name product 2 = Patient wants the brand name product 3 = Pharmacist wants the brand name product 4 = generic not in stock 5 = brand drug dispensed as generic 6 = special override 7 = brand mandated by law 8 = generic not available in marketplace © 2013 -2015

Day Supply O The day supply is calculated using the amount of medication dispensed divided by the daily expected usage of the medication O Monitoring the day supply can give several pieces of information to the PBM and the pharmacy: O Is the drug being prescribed correctly? O Is the patient taking too much or too little of the medication based on expected use? (called “adherence”) O The PBM can then approve or deny the © 2013 -2015 claim based on use

Actual Package Size Used O Integrity of the billing system is dependent on accurate information being submitted O The NDC number of the product used is submitted with the claim O NDC indicates package size used O Acquisition cost varies by the product size O Payment is based on the acquisition cost O You must use the correct NDC for the item used O It can be considered insurance fraud to use an incorrect NDC © 2013 -2015

Diagnosis Codes O ICD-10 codes O Identifies main disease state, plus: O Disease variants, unusual characteristics, and cause of the condition O Codes can contain letters in addition to numbers O Diagnosis codes are required on all Medicare Part B prescriptions © 2013 -2015

Third Party Claim Rejections O When a claim is submitted, the PBM will conduct a drug utilization review (DUR) O Common reasons for rejections: O Refill too soon O Invalid day supply O Prior authorization needed O Rejections require investigation and resolution (when possible) by pharmacy staff O Online documentation through DUR override codes © 2013 -2015

Reconciliation and Audits O Reconciliation is the act of comparing submitted insurance claims with amounts actually paid O May require claim correction and resubmission O Audits are conducted by the PBMs O Do the prescriptions actually exist? O Did the patient pick up the prescription? O Does the pharmacy stock the package size they billed for? O Audit exceptions can trigger forfeited money to PBM © 2013 -2015

Ch 33: Return of Pharmaceuticals O Reasons for returns O returns from patients O over stock O ordered by mistake O expired drugs O recalls O What we can do with the product depends on many factors © 2013 -2015

Returns from Outpatients O We have lost control over the product O where was it stored? O was it contaminated? O is it even the same product that you dispensed? O Never place the drug back in stock O do not risk other patients health O Controlled substances O recommendation is not to take controlled substances back. O let the patient destroy the drugs © 2013 -2015 O give them a refund if desired

Returns from Inpatient Areas O We had control over the medication while it was gone O We can return unit dosed medications back into stock O Exemption exists for bulk drugs O Sent home with patient or destroyed © 2013 -2015

Manufacturer Recalls O FDA or manufacturer can issue recalls O FDA has the power to seize affected medicine O Rated into Classes by the severity of damage that could be caused with use © 2013 -2015

FDA Recall Classes O Class I O serious health problems or death O Class II O temporary health problems or slight risk of serious health problem O Class III O unlikely to cause serious health problem but still violates FDA rules © 2013 -2015

Returns to Wholesalers for Credit O Each will have their own rules for returns O Credit invoices must be filed with your other invoices from the wholesaler O Returns of schedule 2 drugs require the use of a 222 form from the wholesaler © 2013 -2015

Return of Drugs for Destruction O Most returns will be of drugs that should no longer be used O Beyond-use date O Recalls O Returns from customers O Returned to “reverse distributors” O Company contracted who handles all returns at a central location O Advantages: O Simplifies O Makes obtaining and credits easy O Helps stop diversion of any beyond-use drugs © 2013 -2015

Returns of Controlled Substances for Destruction O Many return centers exist O Schedule 2 drugs require a 222 form from the return center O Sent through reverse distributor or in rare cases to the DEA O Maintain return paperwork with your invoices © 2013 -2015

Hazardous Substance Returns O Must be returned to a licensed Hazardous Waste Disposal Company O Process O Receipt for waste when picked up (“cradle”) O Confirmation sent to pharmacy when the substance is destroyed (“grave”) O BOTH copies must be kept on file O Gives “cradle to grave” accountability © 2013 -2015

Questions? © 2013 -2015
 Pharmacy compounding calculations
Pharmacy compounding calculations Lloyd center compounding pharmacy
Lloyd center compounding pharmacy Functional vs innovative products
Functional vs innovative products Marketing mix of pepsi and coca cola
Marketing mix of pepsi and coca cola Sterile compounding calculations
Sterile compounding calculations Pvc twin screw extruder factory
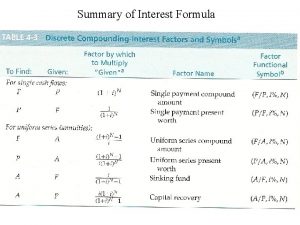
Pvc twin screw extruder factory Discrete compounding formula
Discrete compounding formula Master formula sheet compounding
Master formula sheet compounding Insect bite
Insect bite 3-6 continuous compounding
3-6 continuous compounding Compounding spatula
Compounding spatula Low and medium risk sterile compounding quiz
Low and medium risk sterile compounding quiz Compounding periods
Compounding periods Adelphi coldstream
Adelphi coldstream Compounding daily formula
Compounding daily formula Example of word formation process
Example of word formation process Compounding pompen
Compounding pompen Fundamental operation in compounding
Fundamental operation in compounding Non coring technique
Non coring technique Non sterile preparation example
Non sterile preparation example Dual air brake system diagram
Dual air brake system diagram Compounding interest rate swap
Compounding interest rate swap Sterile technique quiz
Sterile technique quiz Non sterile compounding examples
Non sterile compounding examples What is levigation in compounding
What is levigation in compounding Compounding refers to
Compounding refers to Usaha konsep compound
Usaha konsep compound Morphological processing definition
Morphological processing definition Idaily semi
Idaily semi Buss kneader technology
Buss kneader technology Compounding refers to
Compounding refers to 3-6 continuous compounding answer key
3-6 continuous compounding answer key Parklands hospital pharmacy
Parklands hospital pharmacy 7 principles of pharmacy
7 principles of pharmacy Vafb pharmacy
Vafb pharmacy Consultation skills for pharmacy practice
Consultation skills for pharmacy practice Ozemedicine
Ozemedicine Waterloo pharmacy prerequisites
Waterloo pharmacy prerequisites Community pharmacy topics
Community pharmacy topics Mccabes pharmacy discount code
Mccabes pharmacy discount code New mexico board of pharmacy license verification
New mexico board of pharmacy license verification Pliva 433 street value
Pliva 433 street value Colees pharmacy
Colees pharmacy Isotonic solution definition
Isotonic solution definition Alligation pharmacy
Alligation pharmacy Ventegra
Ventegra Shaunaks pharmacy westbury
Shaunaks pharmacy westbury Dusting powder pass through sieve no
Dusting powder pass through sieve no Examples of unplanned cpd pharmacy
Examples of unplanned cpd pharmacy Pharmacy practice pearls
Pharmacy practice pearls Volatile oils - pharmacognosy
Volatile oils - pharmacognosy Sixth avenue medical pharmacy
Sixth avenue medical pharmacy University of medicine and pharmacy timisoara
University of medicine and pharmacy timisoara Applications of molality in pharmacy
Applications of molality in pharmacy Pharmacy rebates 101
Pharmacy rebates 101 Ncbop renewal
Ncbop renewal Hospital pharmacy layout
Hospital pharmacy layout Schema://reminder_special?days=33
Schema://reminder_special?days=33 Divided powders examples
Divided powders examples Fhcp pharmacy port orange
Fhcp pharmacy port orange Reducing and enlarging formula
Reducing and enlarging formula Costco questions answers
Costco questions answers Washington homeopathic remedies
Washington homeopathic remedies Pharmacy inspector
Pharmacy inspector Unit dose drug distribution system flowchart
Unit dose drug distribution system flowchart Jrmc pharmacy
Jrmc pharmacy Arcw pharmacy
Arcw pharmacy Garbing hand
Garbing hand Pharmacy order 2010
Pharmacy order 2010 Pharmacy audit ideas
Pharmacy audit ideas Granules pharmacy
Granules pharmacy What is adsorption in physical pharmacy
What is adsorption in physical pharmacy Objectives of pharmacy
Objectives of pharmacy Preformulation studies in industrial pharmacy
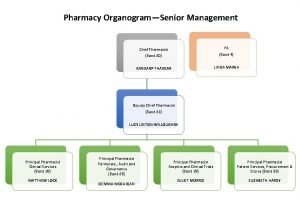
Preformulation studies in industrial pharmacy Pharmacy organogram
Pharmacy organogram Hospital organizational structure
Hospital organizational structure What is forensic pharmacy
What is forensic pharmacy Industrial pharmacy lab
Industrial pharmacy lab Specific gravity in pharmacy
Specific gravity in pharmacy Ltc pharmacy gpo
Ltc pharmacy gpo Osphe pharmacy
Osphe pharmacy Father of modern pharmacy
Father of modern pharmacy Osotsala pharmacy
Osotsala pharmacy