Density Specific Gravity and Specific Volume Summer 2017





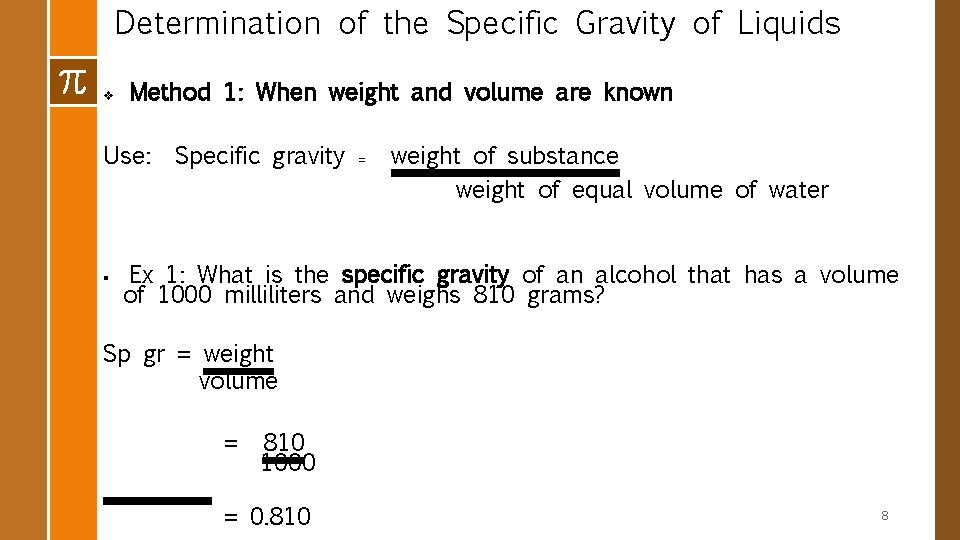



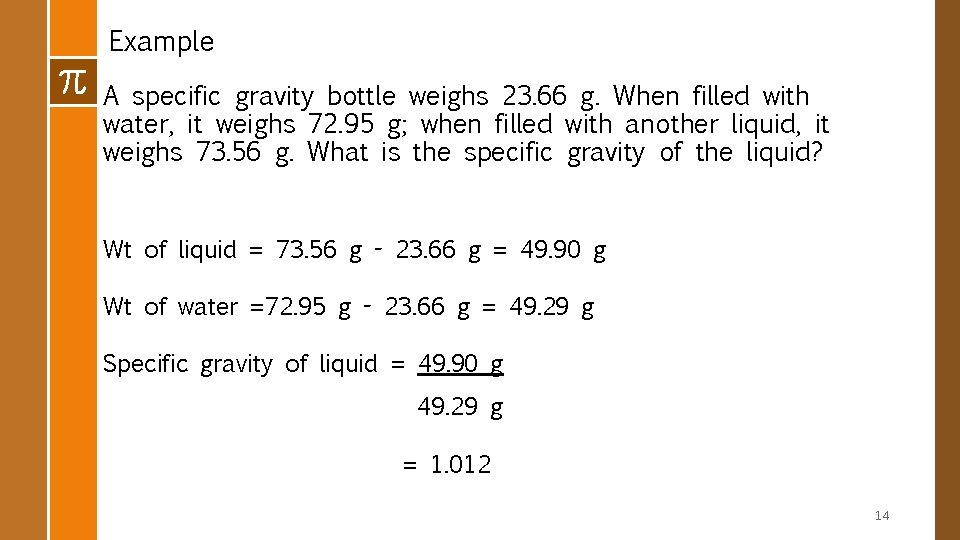





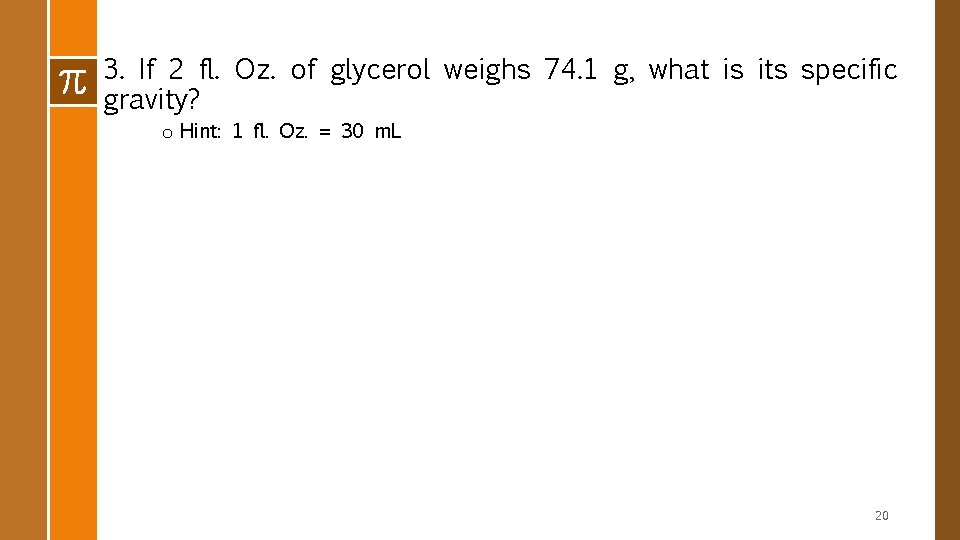



- Slides: 24

Density, Specific Gravity and Specific Volume Summer 2017 Augustina Kwevie Pharm. D Candidate 1

Lecture Objectives v. Understand define density, specific gravity, specific volume v. Know the appropriate calculation for each concept v. Apply specific gravity correctly in converting weight to volume and volume to weight 2

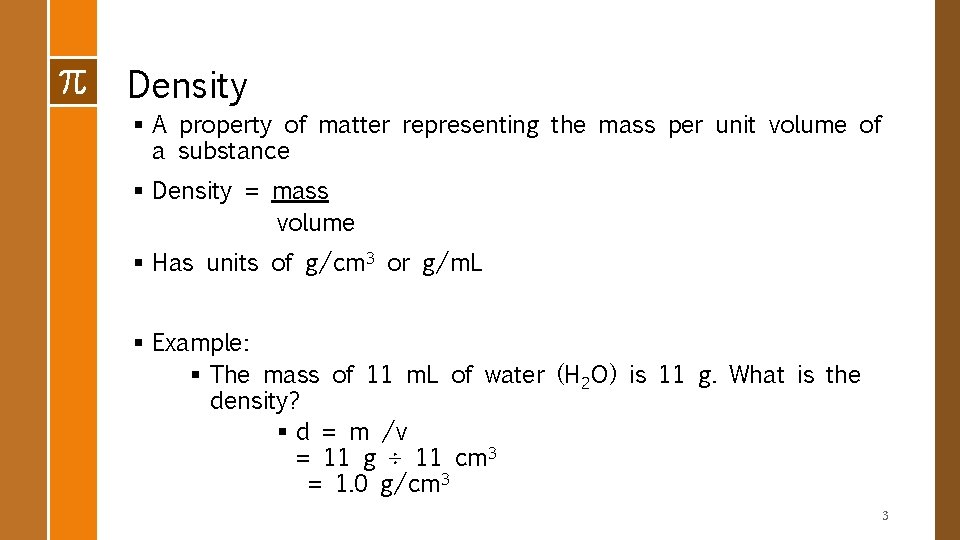
Density § A property of matter representing the mass per unit volume of a substance § Density = mass volume § Has units of g/cm 3 or g/m. L § Example: § The mass of 11 m. L of water (H 2 O) is 11 g. What is the density? § d = m /v = 11 g ÷ 11 cm 3 = 1. 0 g/cm 3 3

Specific Gravity (sp gr) § Sp gr is a ratio expressed decimally, of the weight of a substance to the weight of an equal volume of a substance chosen as a standard. § For liquids and solids: standard is water For gases: standard is hydrogen § Specific gravity = weight of substance weight of equal volume of water § Therefore, sp gr is a factor that expresses how much heavier or lighter a substance is – when compared to the standard (water) § Ex. a liquid with sp gr of 1. 25 is 1. 25 times as heavy as water etc 4

Why is specific gravity important? Substances that have sp gr <1 are lighter than water. Substances with sp gr >1 are heavier than water. 5

Examples of Specific Gravity of Different Liquids 6

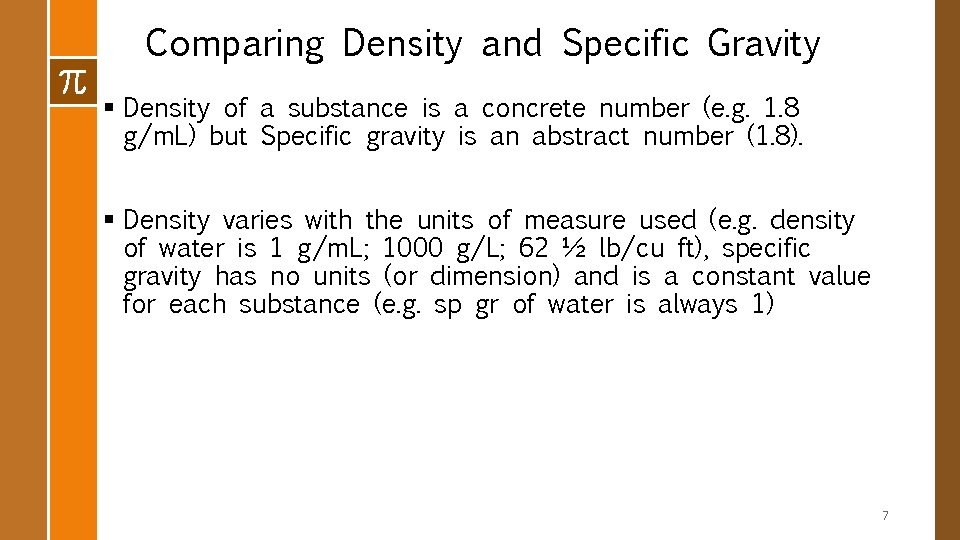
Comparing Density and Specific Gravity § Density of a substance is a concrete number (e. g. 1. 8 g/m. L) but Specific gravity is an abstract number (1. 8). § Density varies with the units of measure used (e. g. density of water is 1 g/m. L; 1000 g/L; 62 ½ lb/cu ft), specific gravity has no units (or dimension) and is a constant value for each substance (e. g. sp gr of water is always 1) 7
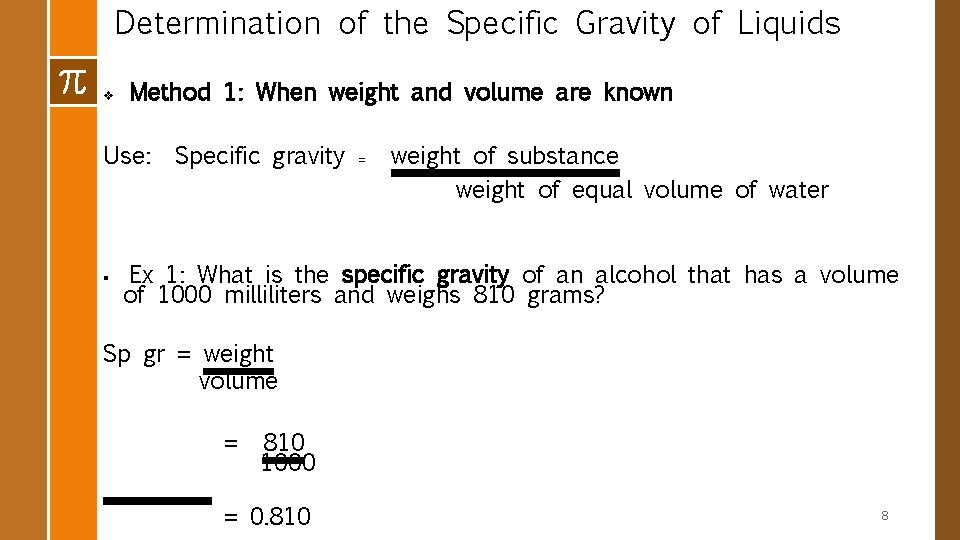
Determination of the Specific Gravity of Liquids v Method 1: When weight and volume are known Use: § Specific gravity = weight of substance weight of equal volume of water Ex 1: What is the specific gravity of an alcohol that has a volume of 1000 milliliters and weighs 810 grams? Sp gr = weight volume = 810 1000 = 0. 810 8

Ex 2: What is the weight in grams of 240 milliliters of light liquid petrolatum having a specific gravity of 0. 81? Sp gr = weight wt. of eq. volume of water Weight = Sp gr × volume of water (substance) = 0. 81 × 240 = 194. 4 g 9

Ex 3: What is the volume in pints, of 50 lbs of glycerin having a specific gravity of 1. 25? 50 lbs = 22, 700 g Sp gr = 1. 25 Volume =m. L = g 22, 700 sp gr 1. 25 = 18, 160 m. L § for the volume in pints 1 pint = 473 m. L X pint = 18160 m. L X = 18160/473 = 38. 4 pints 10

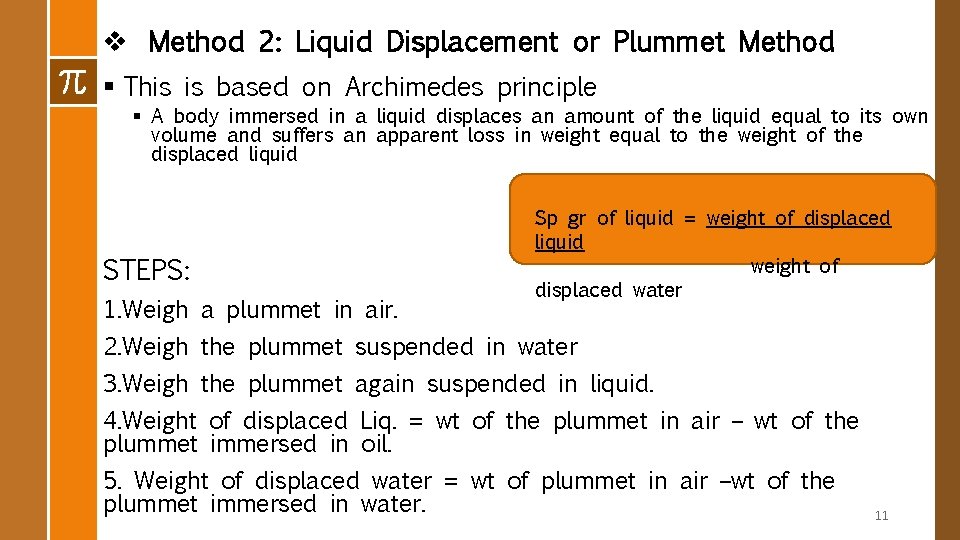
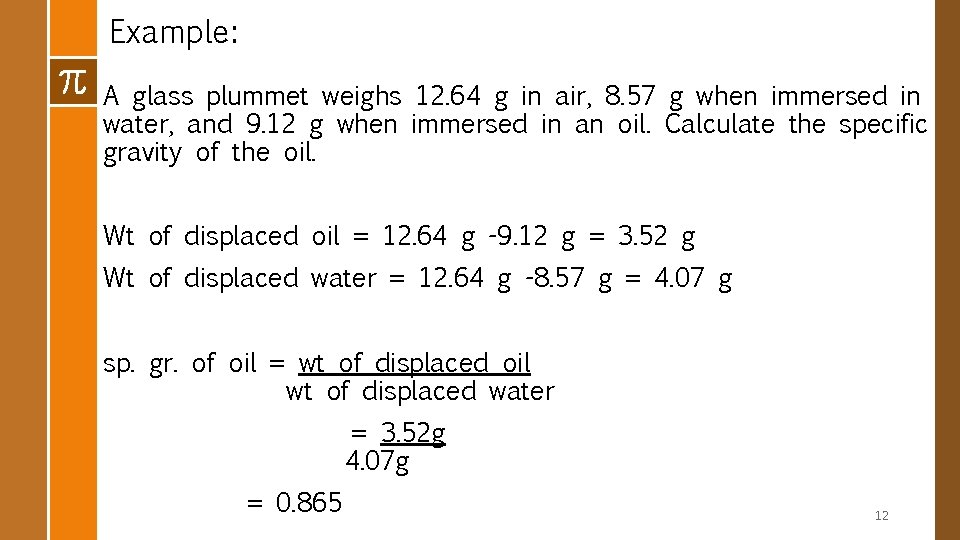
v Method 2: Liquid Displacement or Plummet Method § This is based on Archimedes principle § A body immersed in a liquid displaces an amount of the liquid equal to its own volume and suffers an apparent loss in weight equal to the weight of the displaced liquid STEPS: 1. Weigh a plummet in air. Sp gr of liquid = weight of displaced liquid weight of displaced water 2. Weigh the plummet suspended in water 3. Weigh the plummet again suspended in liquid. 4. Weight of displaced Liq. = wt of the plummet in air – wt of the plummet immersed in oil. 5. Weight of displaced water = wt of plummet in air –wt of the plummet immersed in water. 11

Example: A glass plummet weighs 12. 64 g in air, 8. 57 g when immersed in water, and 9. 12 g when immersed in an oil. Calculate the specific gravity of the oil. Wt of displaced oil = 12. 64 g -9. 12 g = 3. 52 g Wt of displaced water = 12. 64 g -8. 57 g = 4. 07 g sp. gr. of oil = wt of displaced oil wt of displaced water = 3. 52 g 4. 07 g = 0. 865 12

v Method 3: Pycnometer or Specific Gravity Bottle § A pycnometer is a special glass bottle used to determine specific gravity. § are generally available for laboratory use in volumes ranging from 1 m. L to 50 m. L. § Steps 1. Weigh empty pycnometer 2. Weigh pycnometer + water 3. Weigh pycnometer + liquid 4. Weight of water = (weight of pycnometer + water) – weight of pycnometer 5. Weight of liq = (weight of pycnometer + liq) – weight of pycnometer 13
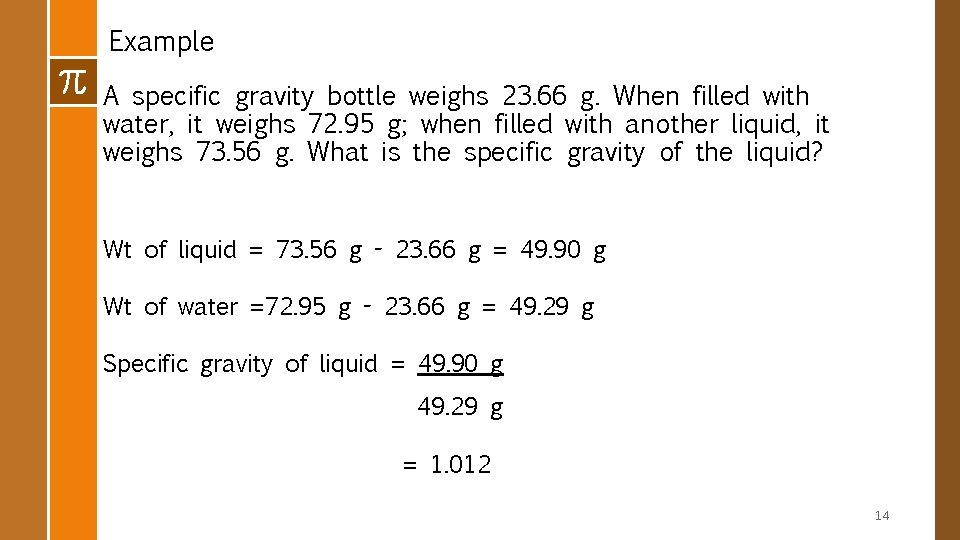
Example A specific gravity bottle weighs 23. 66 g. When filled with water, it weighs 72. 95 g; when filled with another liquid, it weighs 73. 56 g. What is the specific gravity of the liquid? Wt of liquid = 73. 56 g - 23. 66 g = 49. 90 g Wt of water =72. 95 g - 23. 66 g = 49. 29 g Specific gravity of liquid = 49. 90 g 49. 29 g = 1. 012 14

SPECIFIC VOLUME 15

§ Definition § Ratio, expressed decimally, of the volume of a substance to the volume of an equal weight of another substance taken as a standard. § Simply stated: Tells us how much greater (or smaller) in volume a weight is when compared to the same weight of water. § Specific gravity is a comparison of weights of equal volumes and specific volume is a comparison of volumes of equal weights. § Specific gravity and specific volume are reciprocals. 16

Specific volume = Volume of substance Volume of equal weight of water Example: Calculate the specific volume of a syrup, 91. 0 m. L which weighs 107. 16 g. Weight of syrup = 107. 16 g Volume of equal weight of water = 107. 16 m. L Specific volume = 91/107. 16 = 0. 849 17

PRACTICE PROBLEMS 18

1. What is the specific volume of phosphoric acid having a specific gravity of 1. 71? 2. If a liquid has specific volume of 1. 396, what is its specific gravity? 19
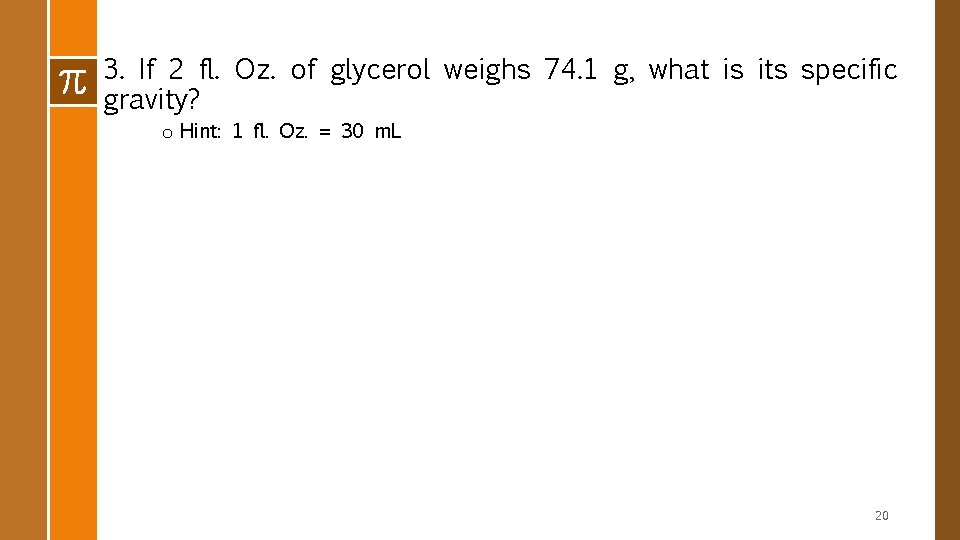
3. If 2 fl. Oz. of glycerol weighs 74. 1 g, what is its specific gravity? o Hint: 1 fl. Oz. = 30 m. L 20

4. 870 grams of sucrose are dissolved in 470 m. L of water, and the resulting volume is 1010 m. L. Calculate the specific gravity of the solution. 21

5. dissolved in enough water to make 8 liters. Assuming the specific gravity of the syrup to be 1. 313, how many milliliters of water were used? 22

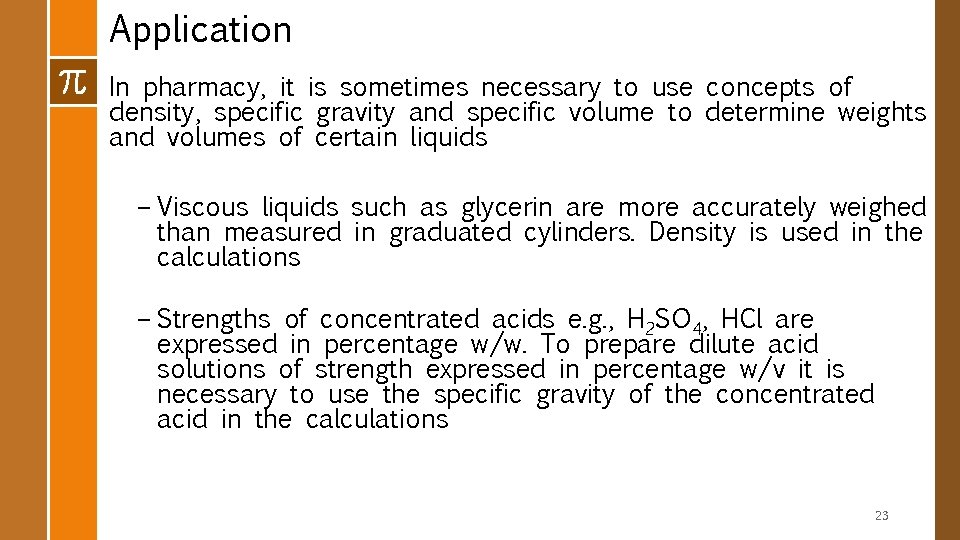
Application In pharmacy, it is sometimes necessary to use concepts of density, specific gravity and specific volume to determine weights and volumes of certain liquids – Viscous liquids such as glycerin are more accurately weighed than measured in graduated cylinders. Density is used in the calculations – Strengths of concentrated acids e. g. , H 2 SO 4, HCl are expressed in percentage w/w. To prepare dilute acid solutions of strength expressed in percentage w/v it is necessary to use the specific gravity of the concentrated acid in the calculations 23

Thanks ! Reference Ansel, H. C. (2009) Phamaceutical. Calculations (13 th Ed. ). Philadelphia: Lippincott. Williams & Wilkins, and Wolters. Kluwer Publishers 24