Cellular Therapy Registry Update Marcelo C Pasquini MD





















- Slides: 47

Cellular Therapy Registry Update Marcelo C. Pasquini, MD Tiffany Hunt, CCRP February 19, 2019 The CIBMTR® (Center for International Blood and Marrow Transplant Research®) is a research collaboration between the National Marrow Donor Program® (NMDP)/Be The Match® and the Medical College of Wisconsin (MCW). TRAINING & DEVELOPMENT | .

CT Registry Outline • • • CT Registry Updates Commercial CAR T cells Reporting of Toxicity Forms and Reporting Other Projects in Cell Therapy TRAINING & DEVELOPMENT | 2 .

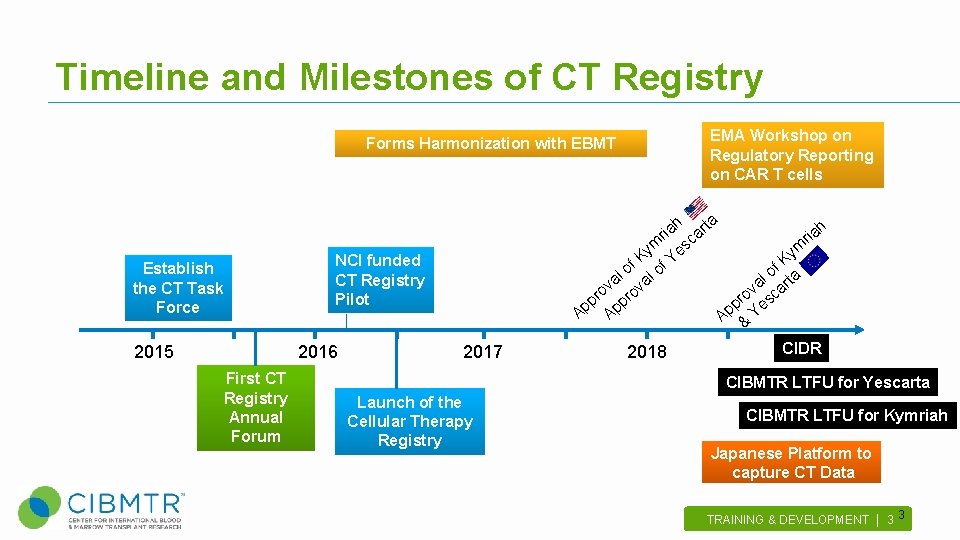
Timeline and Milestones of CT Registry Forms Harmonization with EBMT Establish the CT Task Force 2015 NCI funded CT Registry Pilot 2016 First CT Registry Annual Forum 2017 EMA Workshop on Regulatory Reporting on CAR T cells a h h ir a cart ia r ym es ym K Y f K o l of of ta l l va ova va car o o r r p pp pr es p p A A A & Y CIDR 2018 CIBMTR LTFU for Yescarta Launch of the Cellular Therapy Registry CIBMTR LTFU for Kymriah Japanese Platform to capture CT Data TRAINING & DEVELOPMENT | 3 . 3

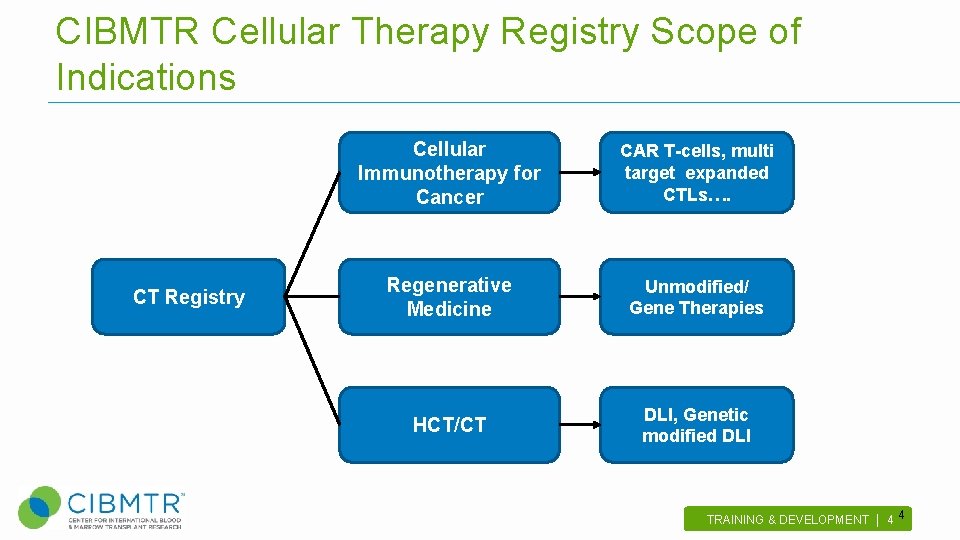
CIBMTR Cellular Therapy Registry Scope of Indications CT Registry Cellular Immunotherapy for Cancer CAR T-cells, multi target expanded CTLs…. Regenerative Medicine Unmodified/ Gene Therapies HCT/CT DLI, Genetic modified DLI TRAINING & DEVELOPMENT | 4 . 4

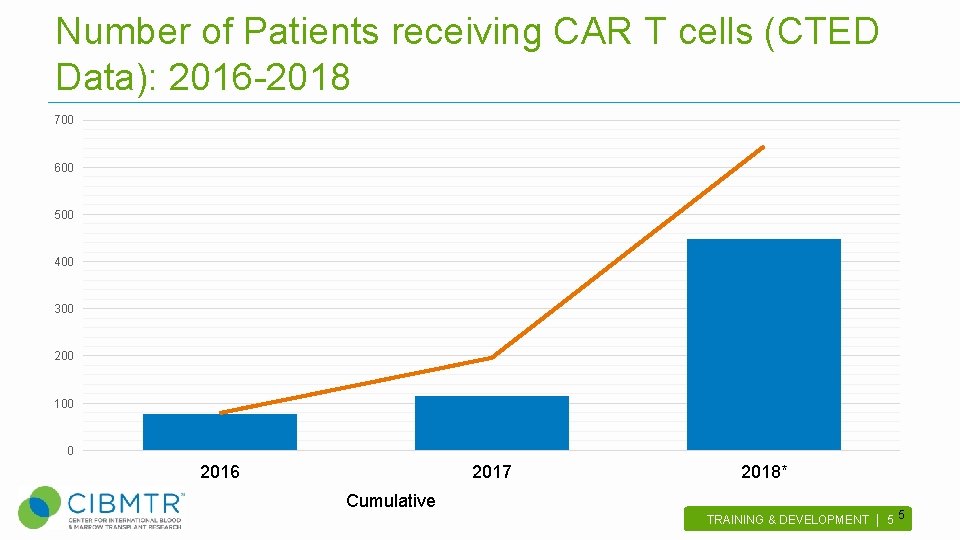
Number of Patients receiving CAR T cells (CTED Data): 2016 -2018 700 600 500 400 300 200 100 0 2016 2017 Cumulative 2018* TRAINING & DEVELOPMENT | 5 . 5

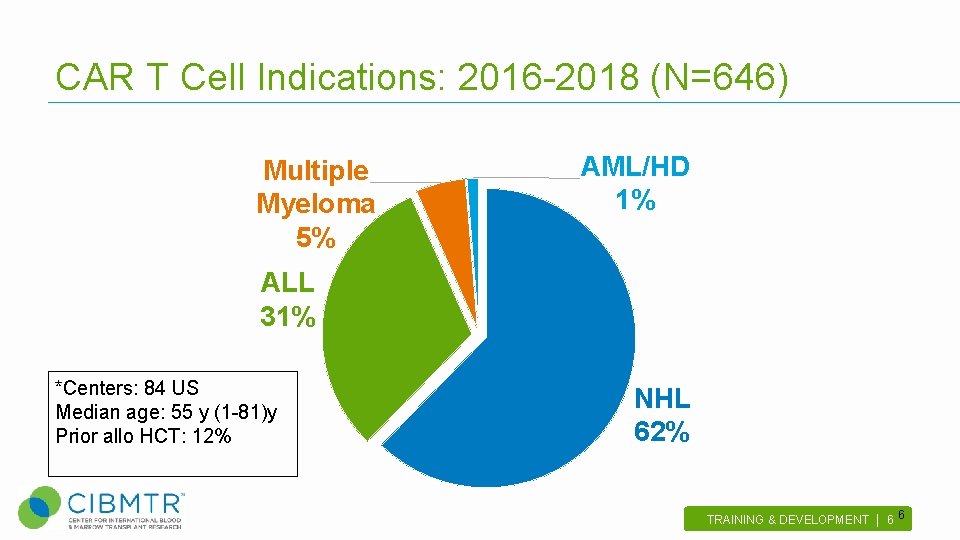
CAR T Cell Indications: 2016 -2018 (N=646) Multiple Myeloma 5% AML/HD 1% ALL 31% *Centers: 84 US Median age: 55 y (1 -81)y Prior allo HCT: 12% NHL 62% TRAINING & DEVELOPMENT | 6 . 6

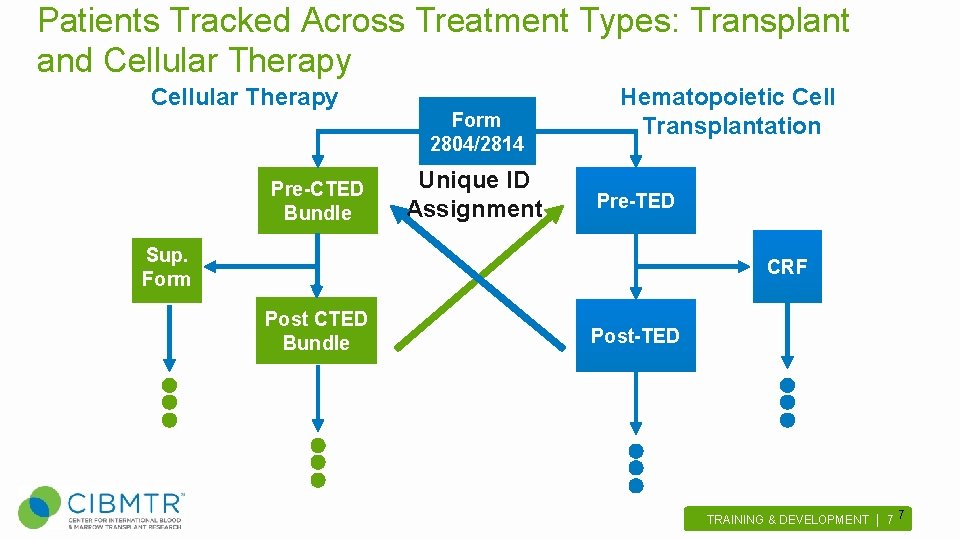
Patients Tracked Across Treatment Types: Transplant and Cellular Therapy Pre-CTED Bundle Form 2804/2814 Unique ID Assignment Hematopoietic Cell Transplantation Pre-TED Sup. Form CRF Post CTED Bundle Post-TED TRAINING & DEVELOPMENT | 7 . 7

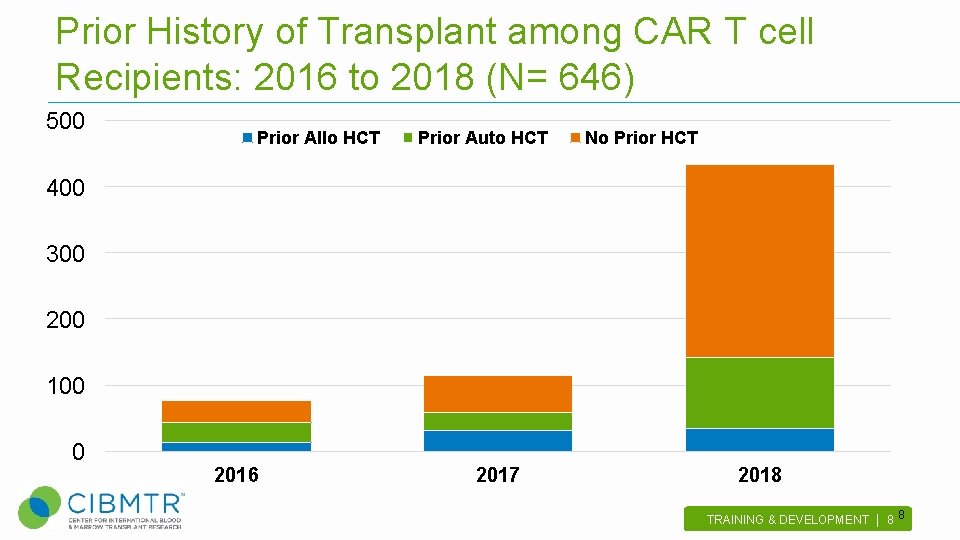
Prior History of Transplant among CAR T cell Recipients: 2016 to 2018 (N= 646) 500 Prior Allo HCT Prior Auto HCT No Prior HCT 400 300 200 100 0 2016 2017 2018 TRAINING & DEVELOPMENT | 8 . 8

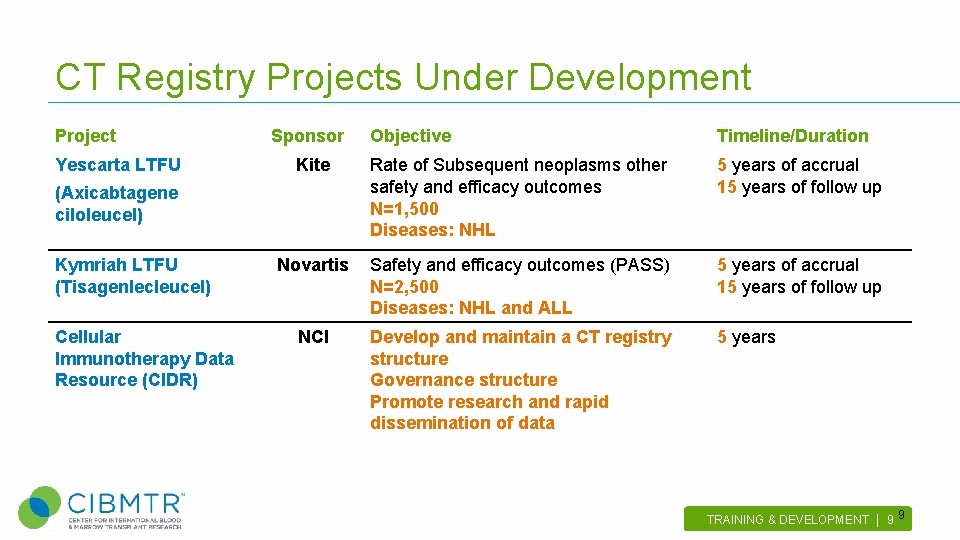
CT Registry Projects Under Development Project Yescarta LTFU Sponsor Objective Timeline/Duration Kite Rate of Subsequent neoplasms other safety and efficacy outcomes N=1, 500 Diseases: NHL 5 years of accrual 15 years of follow up Novartis Safety and efficacy outcomes (PASS) N=2, 500 Diseases: NHL and ALL 5 years of accrual 15 years of follow up NCI Develop and maintain a CT registry structure Governance structure Promote research and rapid dissemination of data 5 years (Axicabtagene ciloleucel) Kymriah LTFU (Tisagenlecleucel) Cellular Immunotherapy Data Resource (CIDR) TRAINING & DEVELOPMENT | 9 . 9

Mandatory vs. Required vs. Strongly Recommended • FDA requires companies to have a structure in place to follow up patients long term • Data collection by the center remains voluntary but strongly recommended for commercial CAR T-cell recipients • Patients have the right not to consent to share data for research 10 TRAINING & DEVELOPMENT | 10 .

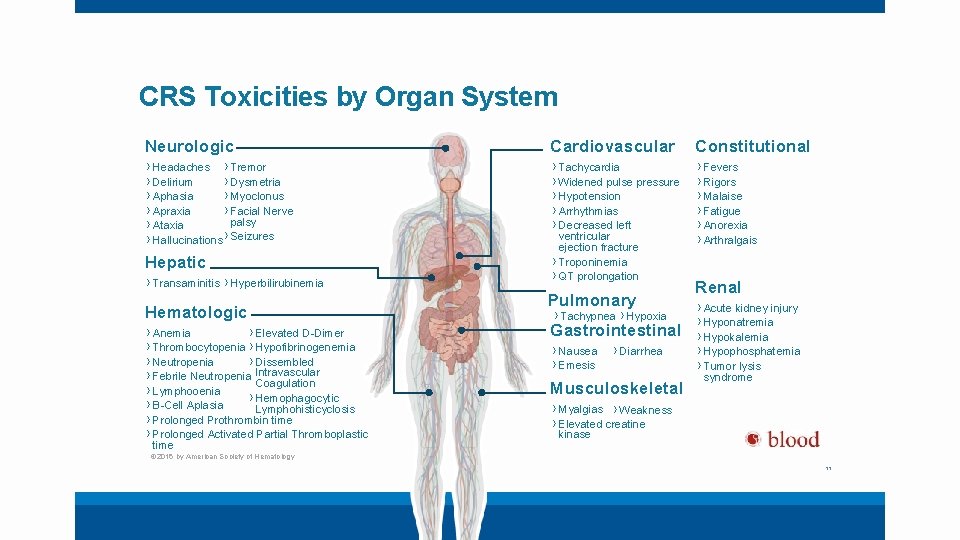
CRS Toxicities by Organ System Neurologic Cardiovascular Constitutional › Headaches › Tremor › Delirium › Dysmetria › Aphasia › Myoclonus › Apraxia › Facial Nerve palsy › Ataxia › Hallucinations › Seizures › Tachycardia › Widened pulse pressure › Hypotension › Arrhythmias › Decreased left › Fevers › Rigors › Malaise › Fatigue › Anorexia › Arthralgais Hepatic › Transaminitis › Hyperbilirubinemia Hematologic › Anemia › Elevated D-Dimer › Thrombocytopenia › Hypofibrinogenemia › Neutropenia › Dissembled › Febrile Neutropenia Intravascular Coagulation › Lymphooenia › Hemophagocytic › B-Cell Aplasia Lymphohisticyclosis › Prolonged Prothrombin time › Prolonged Activated Partial Thromboplastic time ventricular ejection fracture › Troponinemia › QT prolongation Pulmonary › Tachypnea › Hypoxia Gastrointestinal › Nausea › Emesis › Diarrhea Musculoskeletal Renal › Acute kidney injury › Hyponatremia › Hypokalemia › Hypophosphatemia › Tumor lysis syndrome › Myalgias › Weakness › Elevated creatine kinase © 2016 by American Society of Hematology 11

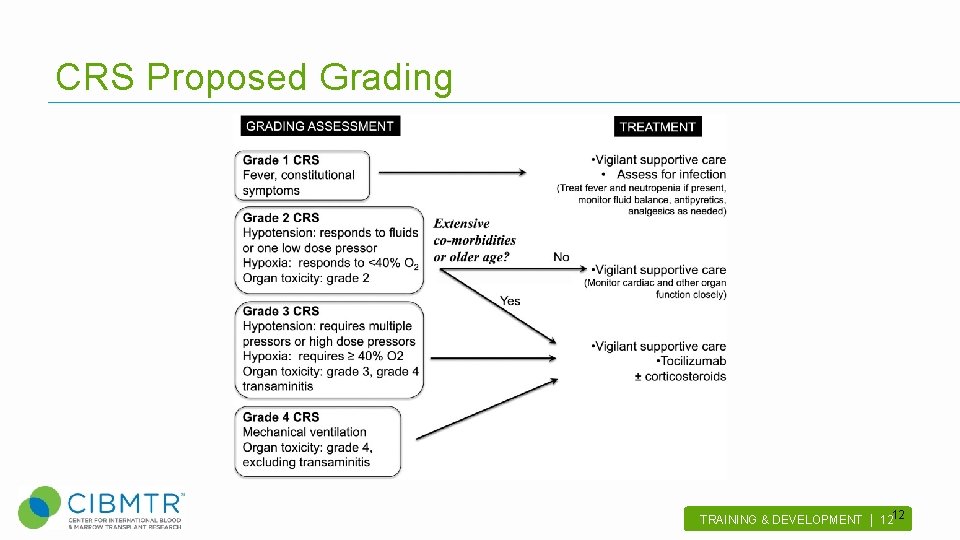
CRS Proposed Grading 12 TRAINING & DEVELOPMENT | 12 .

Consensus on Defining and Grading Toxicities after CAR T cells 13 TRAINING & DEVELOPMENT | 13 .

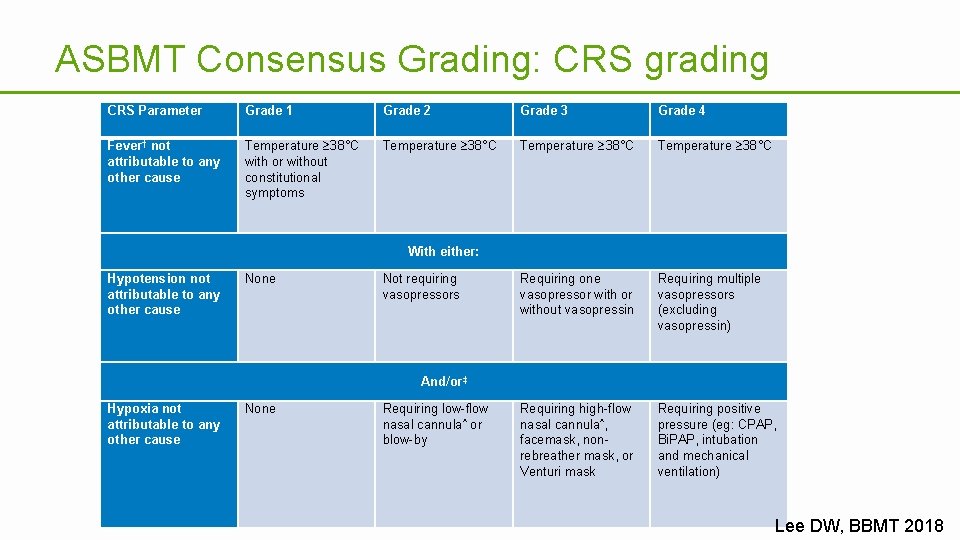
ASBMT Consensus Grading: CRS grading CRS Parameter Grade 1 Grade 2 Grade 3 Grade 4 Fever† not attributable to any other cause Temperature 38°C with or without constitutional symptoms Temperature 38°C Requiring one vasopressor without vasopressin Requiring multiple vasopressors (excluding vasopressin) Requiring high-flow nasal cannula^, facemask, nonrebreather mask, or Venturi mask Requiring positive pressure (eg: CPAP, Bi. PAP, intubation and mechanical ventilation) With either: Hypotension not attributable to any other cause None Not requiring vasopressors And/or‡ Hypoxia not attributable to any other cause None Requiring low-flow nasal cannula^ or blow-by Lee DW, BBMT 2018

Cytokine release syndrome • Key elements being collected – Fever – Hypotension • Vasopressor use – Hypoxia • Level of supplemental oxygen require or pressure ventilation – Date of diagnosis and resolution, if applicable – Treatment • Capture maximum severity within the reporting period • Most events will occur within the first reporting period 15 TRAINING & DEVELOPMENT | 15 .

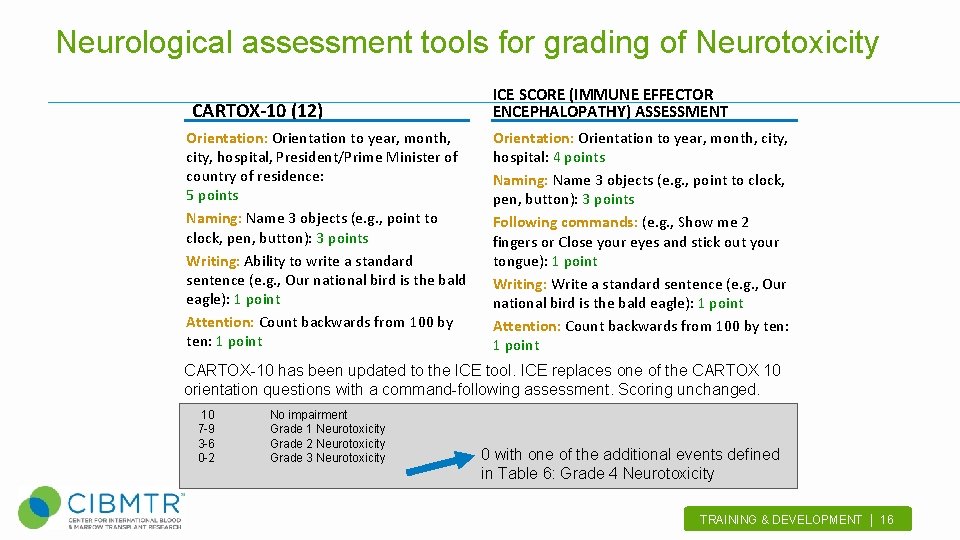
Neurological assessment tools for grading of Neurotoxicity CARTOX-10 (12) Orientation: Orientation to year, month, city, hospital, President/Prime Minister of country of residence: 5 points Naming: Name 3 objects (e. g. , point to clock, pen, button): 3 points Writing: Ability to write a standard sentence (e. g. , Our national bird is the bald eagle): 1 point Attention: Count backwards from 100 by ten: 1 point ICE SCORE (IMMUNE EFFECTOR ENCEPHALOPATHY) ASSESSMENT Orientation: Orientation to year, month, city, hospital: 4 points Naming: Name 3 objects (e. g. , point to clock, pen, button): 3 points Following commands: (e. g. , Show me 2 fingers or Close your eyes and stick out your tongue): 1 point Writing: Write a standard sentence (e. g. , Our national bird is the bald eagle): 1 point Attention: Count backwards from 100 by ten: 1 point CARTOX-10 has been updated to the ICE tool. ICE replaces one of the CARTOX 10 orientation questions with a command-following assessment. Scoring unchanged. 10 7 -9 3 -6 0 -2 No impairment Grade 1 Neurotoxicity Grade 2 Neurotoxicity Grade 3 Neurotoxicity 0 with one of the additional events defined in Table 6: Grade 4 Neurotoxicity TRAINING & DEVELOPMENT | 16 .

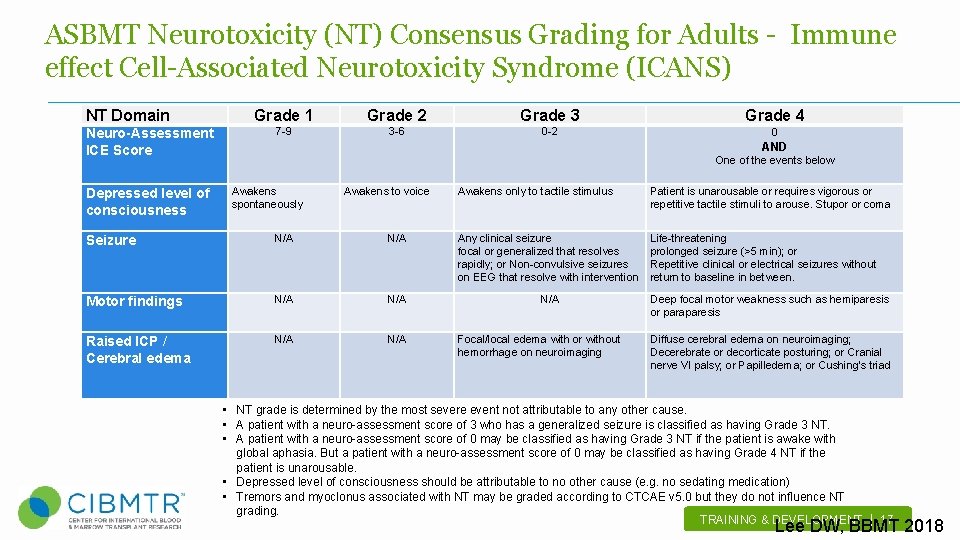
ASBMT Neurotoxicity (NT) Consensus Grading for Adults - Immune effect Cell-Associated Neurotoxicity Syndrome (ICANS) NT Domain Neuro-Assessment ICE Score Depressed level of consciousness Grade 1 Grade 2 Grade 3 Grade 4 7 -9 3 -6 0 -2 0 AND Awakens spontaneously One of the events below Awakens to voice Awakens only to tactile stimulus Patient is unarousable or requires vigorous or repetitive tactile stimuli to arouse. Stupor or coma Life-threatening prolonged seizure (>5 min); or Repetitive clinical or electrical seizures without return to baseline in between. Seizure N/A Any clinical seizure focal or generalized that resolves rapidly; or Non-convulsive seizures on EEG that resolve with intervention Motor findings N/A Raised ICP / Cerebral edema N/A Focal/local edema with or without hemorrhage on neuroimaging Deep focal motor weakness such as hemiparesis or paraparesis Diffuse cerebral edema on neuroimaging; Decerebrate or decorticate posturing; or Cranial nerve VI palsy; or Papilledema; or Cushing's triad • NT grade is determined by the most severe event not attributable to any other cause. • A patient with a neuro-assessment score of 3 who has a generalized seizure is classified as having Grade 3 NT. • A patient with a neuro-assessment score of 0 may be classified as having Grade 3 NT if the patient is awake with global aphasia. But a patient with a neuro-assessment score of 0 may be classified as having Grade 4 NT if the patient is unarousable. • Depressed level of consciousness should be attributable to no other cause (e. g. no sedating medication) • Tremors and myoclonus associated with NT may be graded according to CTCAE v 5. 0 but they do not influence NT grading. TRAINING & DEVELOPMENT | 17 . Lee DW, BBMT 2018

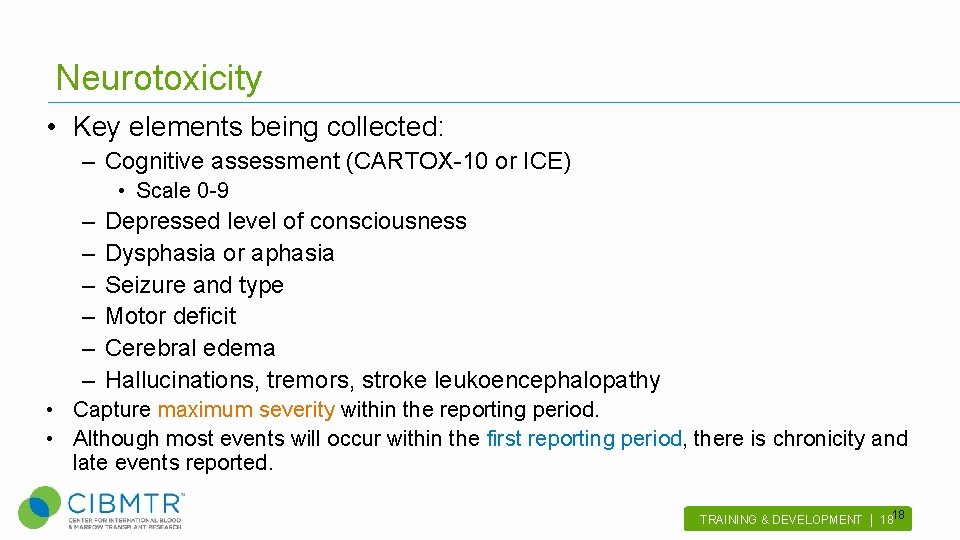
Neurotoxicity • Key elements being collected: – Cognitive assessment (CARTOX-10 or ICE) • Scale 0 -9 – – – Depressed level of consciousness Dysphasia or aphasia Seizure and type Motor deficit Cerebral edema Hallucinations, tremors, stroke leukoencephalopathy • Capture maximum severity within the reporting period. • Although most events will occur within the first reporting period, there is chronicity and late events reported. 18 TRAINING & DEVELOPMENT | 18 .

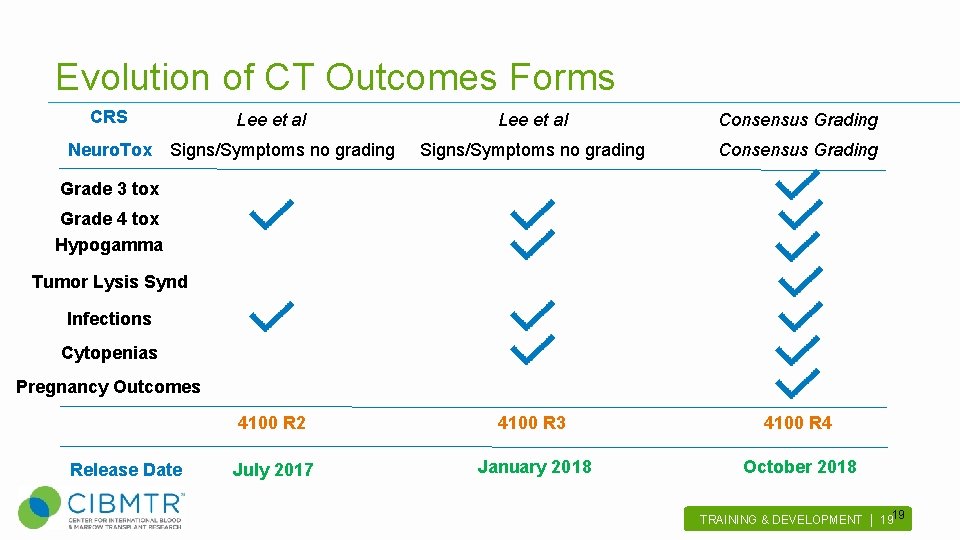
Evolution of CT Outcomes Forms CRS Lee et al Consensus Grading Signs/Symptoms no grading Consensus Grading 4100 R 2 4100 R 3 4100 R 4 July 2017 January 2018 October 2018 Neuro. Tox Signs/Symptoms no grading Grade 3 tox Grade 4 tox Hypogamma Tumor Lysis Synd Infections Cytopenias Pregnancy Outcomes Release Date 19 TRAINING & DEVELOPMENT | 19 .

Toxicity Reporting in Patient Registries • Different from clinical trials infrastructure – Limited event-driven report – No narrative, con-meds and other components of individual safety reports. • Excellent to capture aggregate data of expected toxicities – CRS/neurotoxicities – Subsequent neoplasm – Pregnancy • Current recommendations for CAR T-cells – LTFU infrastructure – REMS – Unexpected serious AE (Med. Watch) TRAINING & DEVELOPMENT | 20

Forms and reporting TRAINING & DEVELOPMENT | 21 .

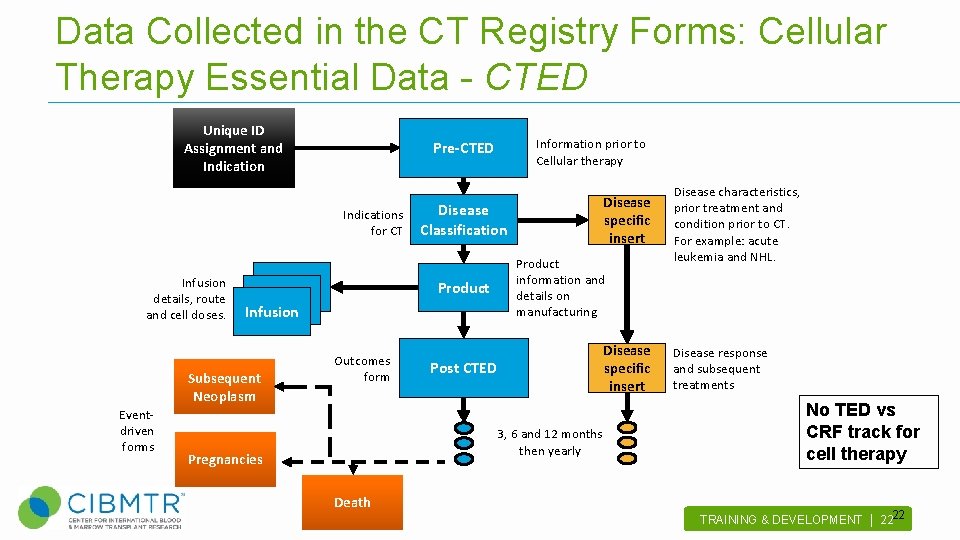
Data Collected in the CT Registry Forms: Cellular Therapy Essential Data - CTED Unique ID Assignment and Indications for CT Infusion details, route and cell doses. Infusion Eventdriven forms Outcomes form Disease specific insert Disease Classification Product Infusion Subsequent Neoplasm Information prior to Cellular therapy Pre-CTED Product information and details on manufacturing Disease specific insert Post CTED 3, 6 and 12 months then yearly Pregnancies Death Disease characteristics, prior treatment and condition prior to CT. For example: acute leukemia and NHL. Disease response and subsequent treatments No TED vs CRF track for cell therapy 22 TRAINING & DEVELOPMENT | 22 .

Subsequent Neoplasms Form 3500 R 1 This follow-up form collects: • Post therapy neoplasm type and diagnosis date • PTLD testing (when PTLD is selected as the subsequent neoplasm type) 23 TRAINING & DEVELOPMENT | 23 .

Two ways to report a subsequent neoplasm • F 4100 R 4 Q 33 “did a new malignancy occur that is different than the disease for which the cell therapy was performed? ” – If ‘yes’, complete a F 3500 • Create an unscheduled form 3500 – Can be completed on demand 24 TRAINING & DEVELOPMENT | 24 .

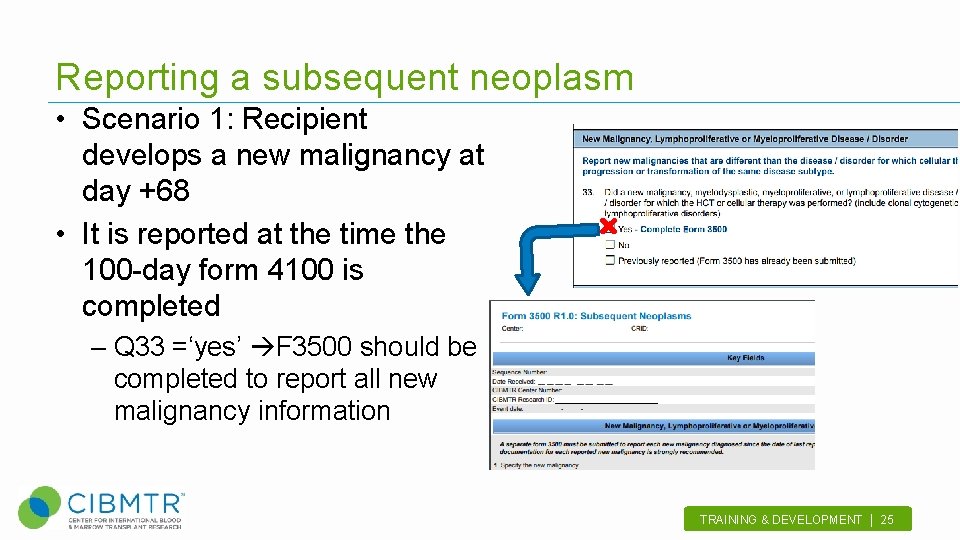
Reporting a subsequent neoplasm • Scenario 1: Recipient develops a new malignancy at day +68 • It is reported at the time the 100 -day form 4100 is completed – Q 33 =‘yes’ F 3500 should be completed to report all new malignancy information TRAINING & DEVELOPMENT | 25 .

Reporting a subsequent neoplasm • Scenario 2: Recipient develops a new malignancy at day +68 and had received a commercial CAR Tcell product – Per protocol, the new malignancy should be reported at the time of knowledge of the new malignancy • The F 3500 should be created as an unscheduled form in Forms. Net 3 and completed in a timely manner TRAINING & DEVELOPMENT | 26 .

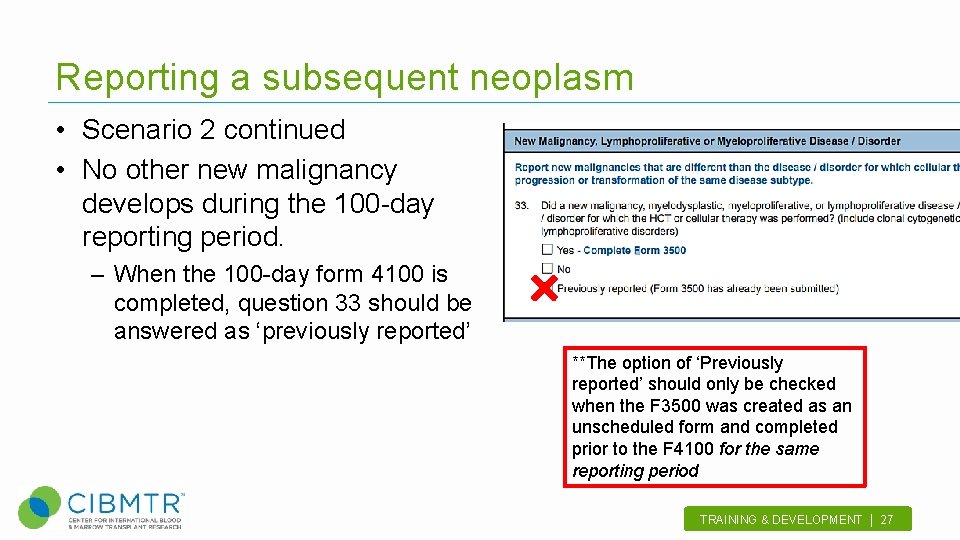
Reporting a subsequent neoplasm • Scenario 2 continued • No other new malignancy develops during the 100 -day reporting period. – When the 100 -day form 4100 is completed, question 33 should be answered as ‘previously reported’ **The option of ‘Previously reported’ should only be checked when the F 3500 was created as an unscheduled form and completed prior to the F 4100 for the same reporting period TRAINING & DEVELOPMENT | 27 .

Pregnancy form 3501 This follow form collects: • Pregnancy outcomes • Delivery date, if applicable TRAINING & DEVELOPMENT | 28 .

Two ways to report a pregnancy • F 4100 R 4 Q 193/194 “Was the recipient / recipient’s female partner pregnant at any time in this reporting period? ” – If ‘yes’, complete a F 3501 • Create an unscheduled form 3501 – Can be completed on demand 29 TRAINING & DEVELOPMENT | 29 .

Reporting a pregnancy • Just like reporting a new malignancy • The option of ‘Previously reported’ should only be checked when the F 3501 was created as an unscheduled form and completed prior to the F 4100 for the same reporting period 0 0 TRAINING & DEVELOPMENT | 30 .

4100 Post CTED • Updated validation for date of contact • Now using F 2900 Recipient death form 0 TRAINING & DEVELOPMENT | 31 .

Reporting Clinical trials on F 4000 • If a recipient is treated as part of a clinical trial and the center decides to report clinicaltrials. gov number to the CIBMTR: – Include (NCT#) – Needs to be the correct number, please check to make sure that the number is related to the cellular therapy. • Genetic modified products – If it is not an FDA approved commercial cellular therapy, it must have an NCT#. • Commercial products – If commercial products are used in clinical trials, please include the NCT# and the product name. TRAINING & DEVELOPMENT | 32 .

4003 form disabling • When a commercial product is reported in question 1, several fields will disable on the form – collection – manipulation TRAINING & DEVELOPMENT | 33 .

F 4006 • Batch and Lot Number – Batch number: Kmyriah – Lot number: Yescarta • These can be found on the infusion bag, as well as on the Certificate of Analysis – May have to contact the lab to get the information – Very important to report TRAINING & DEVELOPMENT | 34 .

Cell Therapy and HCT reporting tracks • For cases where both a cell therapy and HCT are received, if a cell therapy product is genetically modified, FDA requires 15 years of follow-up – Both CT and HCT forms will be completed simultaneously – Finding ways to reduce duplicate reporting • Exceptions: – Recipient receives a non-genetically modified CT product then goes on to HCT or another non-genetically modified CT product, future F 4100 s will be removed – Non-genetically modified product received post-HCT, requires single 4100 TRAINING & DEVELOPMENT | 35 .

Other projects in cell therapy TRAINING & DEVELOPMENT | 36 .

Cell therapy forms and CPI • Cell therapy forms will be phased into CPI this year • Includes all cell therapy forms (based on form ID) – January 2019 - monitor only – May 2019 - partial implementation at 75% – September 2019 - fully implemented at 90% TRAINING & DEVELOPMENT | 37 .

Cell therapy forms and CPI What does this mean? • Reporting cell therapy infusions is still voluntary – For those infusions that have been reported, form completion will now be tracked via CPI – Includes all cell therapy forms based on form ID, if it’s in FN 3 it will be counted – Have one year to reach 90% – Will include study forms, which are tracked by due date first TRAINING & DEVELOPMENT | 38 .

Forms due lists for studies • Have been receiving a separate forms due list for any Kymriah or Yescarta recipients – Looking at form due date – Asking to complete forms in a timely manner – Will count towards CPI TRAINING & DEVELOPMENT | 39 .

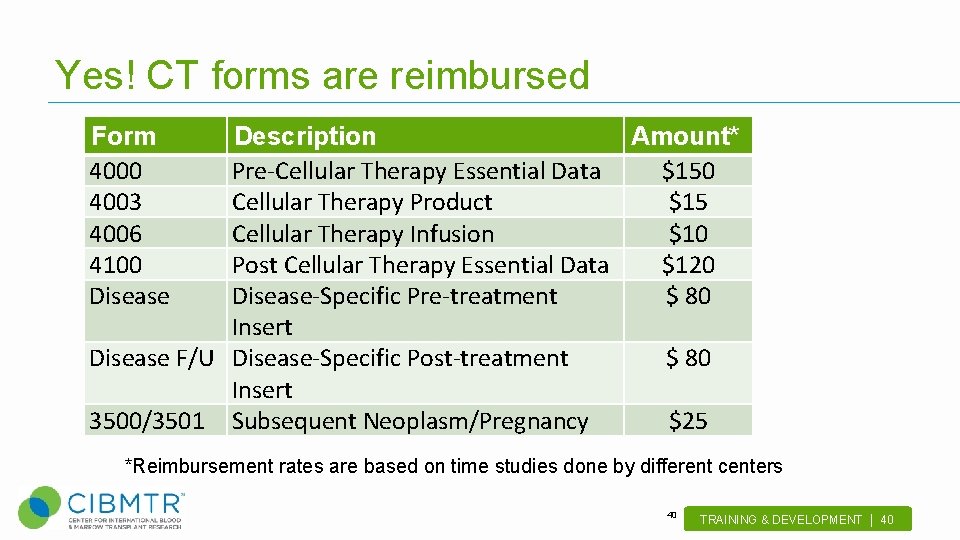
Yes! CT forms are reimbursed Form 4000 4003 4006 4100 Disease Description Amount* Pre-Cellular Therapy Essential Data $150 Cellular Therapy Product $15 Cellular Therapy Infusion $10 Post Cellular Therapy Essential Data $120 Disease-Specific Pre-treatment $ 80 Insert Disease F/U Disease-Specific Post-treatment $ 80 Insert 3500/3501 Subsequent Neoplasm/Pregnancy $25 *Reimbursement rates are based on time studies done by different centers 40 TRAINING & DEVELOPMENT | 40 .

Transfer scenarios • HCT at prior CCN, comes to your center for Cell Therapy (CT) infusion – Search patient details in FN 3 to find existing CRID – If existing CRID found, request a transfer • HCT at your center, goes to another center for CT infusion – Other CCN won’t accept transfer – Can report on CT infusion if records are available TRAINING & DEVELOPMENT | 41 .

Concurrent reporting • What if a transfer isn’t possible? – Other CCN does not accept – Recipient only receiving CT infusion at your center, no follow up care • Want to allow multiple CCNs to report on one CRID at the same time – CCN X is responsible for reporting HCT forms – CCN Y is responsible for reporting Cellular Therapy forms • Currently a manual process – Contact cibmtr-celltherapy@mcw. edu TRAINING & DEVELOPMENT | 42 .

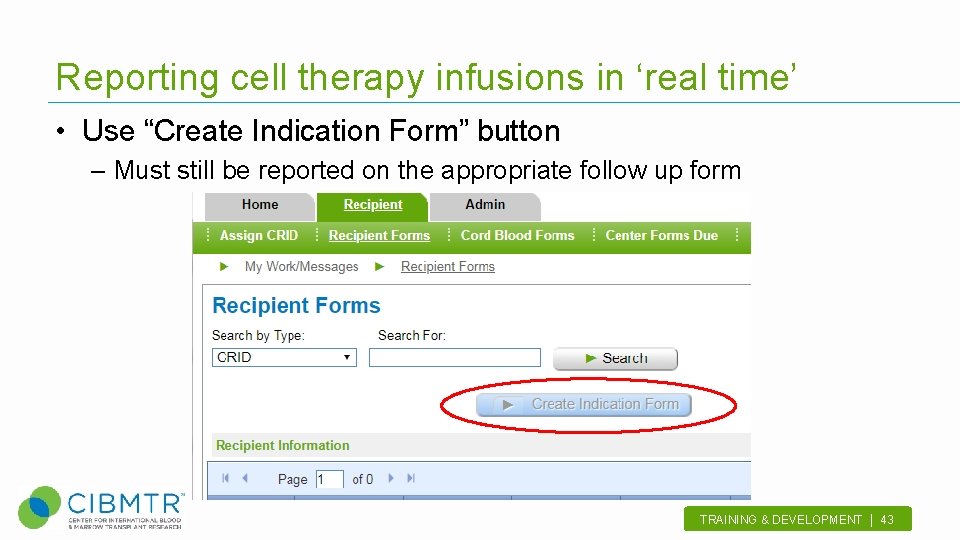
Reporting cell therapy infusions in ‘real time’ • Use “Create Indication Form” button – Must still be reported on the appropriate follow up form TRAINING & DEVELOPMENT | 43 .

Consent • CIBMTR research database protocol was updated in 2012 to include cellular therapy language • Cases where research database consent is “no” – Voluntary to report, all forms will come due – Data will not be used in research or reporting • If ‘not approached’ is reported and the product is a commercial CAR-T, will probably get a query – Expect all recipients to be approached for consent TRAINING & DEVELOPMENT | 44 .

Cell therapy manuals • The manuals can be updated on demand – Use the question received to constantly clarify sections • If you think a question could be better clarified, send us your feedback! TRAINING & DEVELOPMENT | 45 .

Resources • Form instruction manuals: https: //www. cibmtr. org/manuals/fim/1/en/topic/cellular-therapy • Cellular Therapy e. Learning module: http: //www. cibmtr. org/Data. Management/Training. Reference/e Learning/Pages/index. aspx • Cell Therapy email: cibmtr-celltherapy@mcw. edu TRAINING & DEVELOPMENT | 46 .

Questions? TRAINING & DEVELOPMENT | 47 .