Cellular Respiration Cellular Respiration l l Conversion of









- Slides: 52

Cellular Respiration

Cellular Respiration l l Conversion of stored energy in biological molecules into ATP for cellular work - produces carbon dioxide and usually requires oxygen HOW: Gradual release of energy through a series of steps – slower release allows for a more efficient capture of the energy

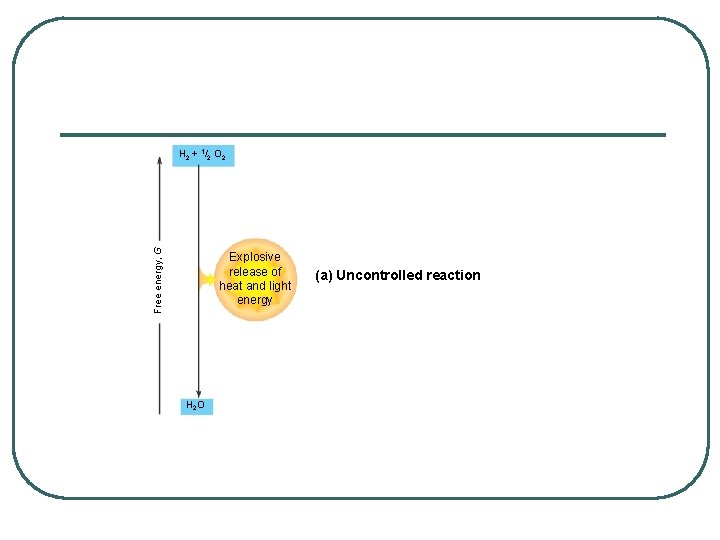
Free energy, G H 2 + 1/2 O 2 Explosive release of heat and light energy H 2 O (a) Uncontrolled reaction

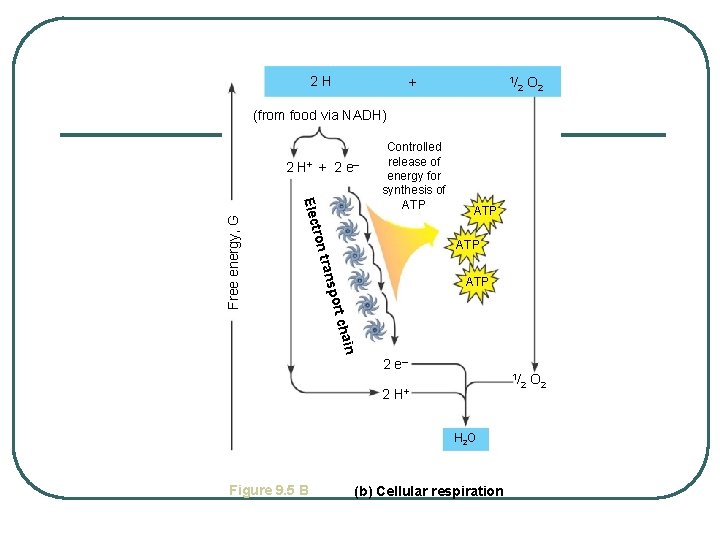
2 H + 1/ 2 O 2 1/ O 2 (from food via NADH) tron Elec ATP trans ATP port Free energy, G 2 H+ + 2 e – Controlled release of energy for synthesis of ATP n chai 2 e– 2 H+ H 2 O Figure 9. 5 B (b) Cellular respiration 2

Overall Reaction C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O

Mechanisms of Cellular Respiration Transfer of electrons from a higher energy state (glucose) to a lower energy state (Water) through Oxidation-reduction reactions (REDOX) l REDOX reactions: l - transfer of electrons from one substance (reducing agent) to another (oxidzing agent) l - result: reducing agent becomes more positive (Oxidized) and the oxidizing agent becomes more negative (REDUCED in charge)

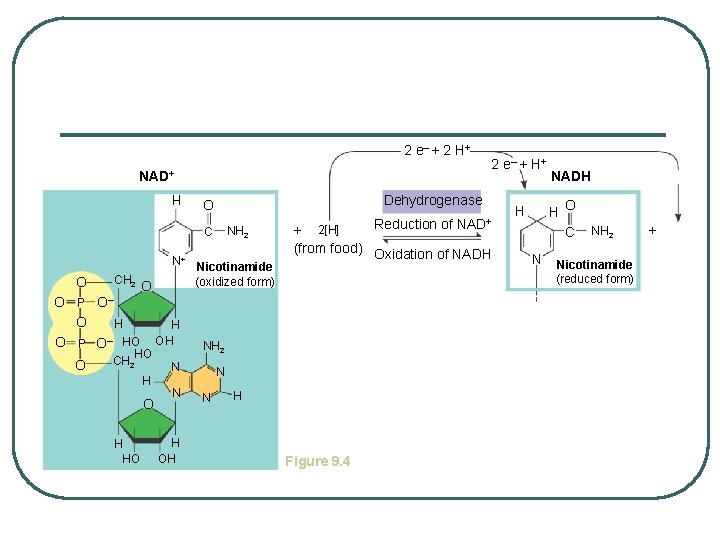
IMPORTANCE OF HYDROGEN ACCEPTORS chemicals that accept hydrogens from molecules and transfer them for other reactions l l NAD+ - nicotinamide adenine dinucleotide FAD - flavin adenine dinucleotide - allow for the gradual controlled release of energy

2 e– + 2 H + NAD+ Dehydrogenase O NH 2 H C N+ CH 2 O O– O P O HO O O HO CH 2 H O H HO H OH Nicotinamide (oxidized form) N H OH Reduction of NAD+ + 2[H] (from food) Oxidation of NADH NH 2 N N N 2 e– + H Figure 9. 4 NADH H O C H N NH 2 Nicotinamide (reduced form) +


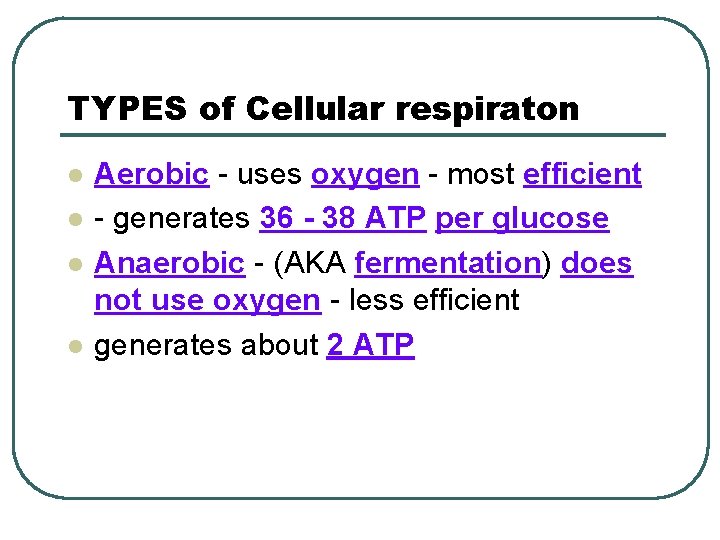
TYPES of Cellular respiraton l l Aerobic - uses oxygen - most efficient - generates 36 - 38 ATP per glucose Anaerobic - (AKA fermentation) does not use oxygen - less efficient generates about 2 ATP

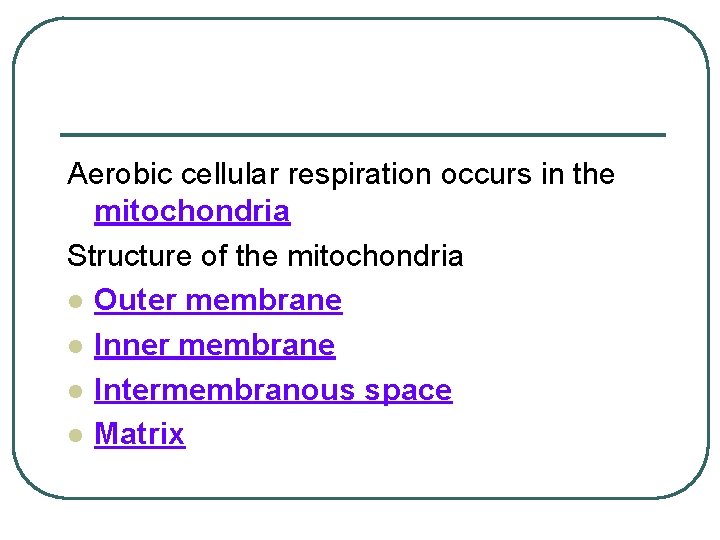
Aerobic cellular respiration occurs in the mitochondria Structure of the mitochondria l Outer membrane l Inner membrane l Intermembranous space l Matrix

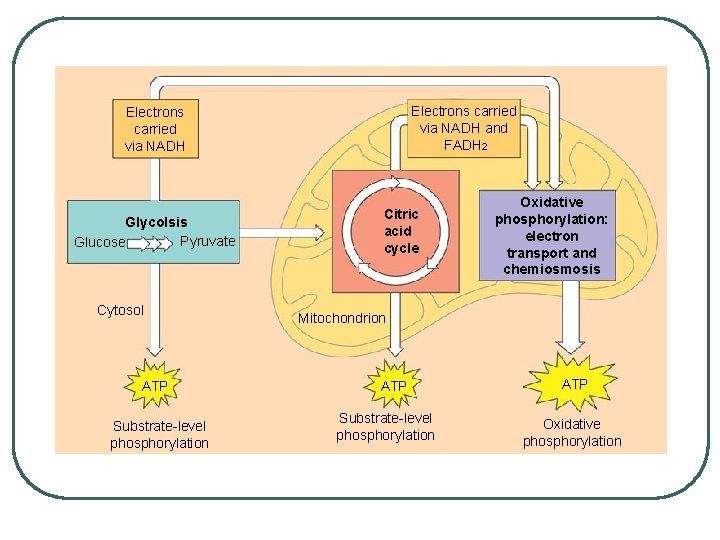
Electrons carried via NADH and FADH 2 Electrons carried via NADH Glycolsis Pyruvate Glucose Cytosol ATP Substrate-level phosphorylation Citric acid cycle Oxidative phosphorylation: electron transport and chemiosmosis Mitochondrion ATP Substrate-level phosphorylation ATP Oxidative phosphorylation

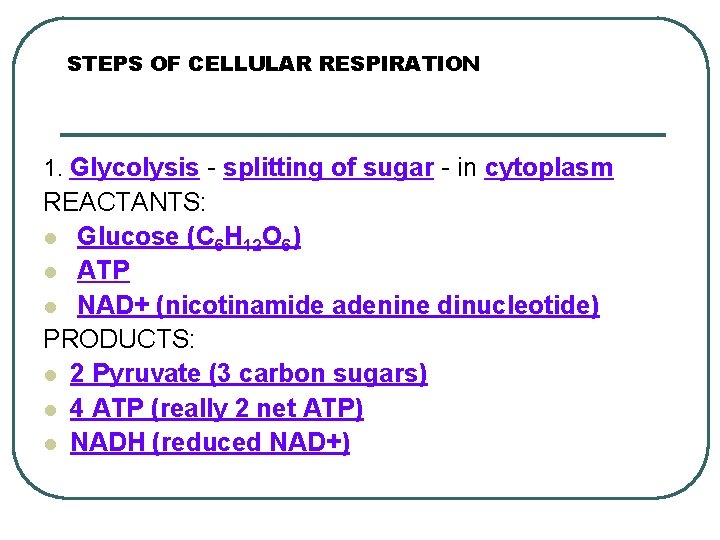
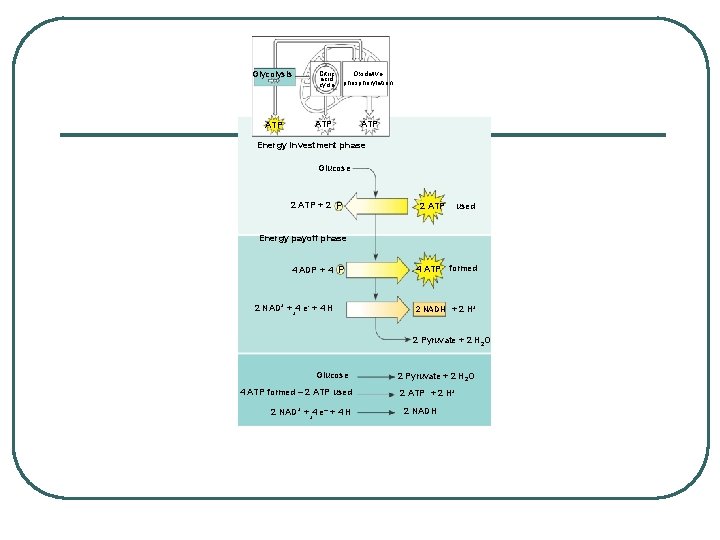
STEPS OF CELLULAR RESPIRATION 1. Glycolysis - splitting of sugar - in cytoplasm REACTANTS: l Glucose (C 6 H 12 O 6) l ATP l NAD+ (nicotinamide adenine dinucleotide) PRODUCTS: l 2 Pyruvate (3 carbon sugars) l 4 ATP (really 2 net ATP) l NADH (reduced NAD+)

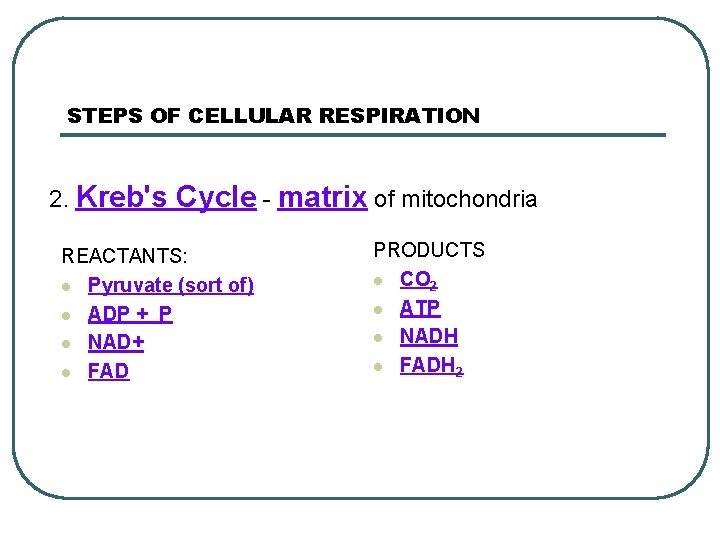
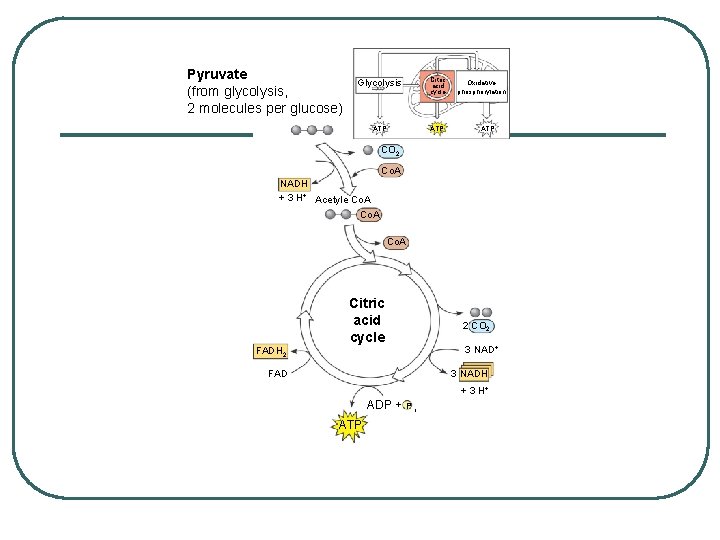
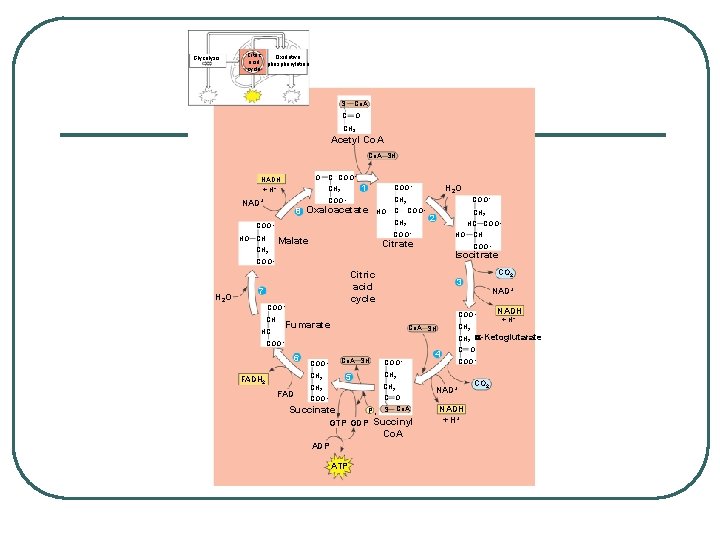
STEPS OF CELLULAR RESPIRATION 2. Kreb's Cycle - matrix of mitochondria REACTANTS: l Pyruvate (sort of) l ADP + P l NAD+ l FAD PRODUCTS l CO 2 l ATP l NADH l FADH 2

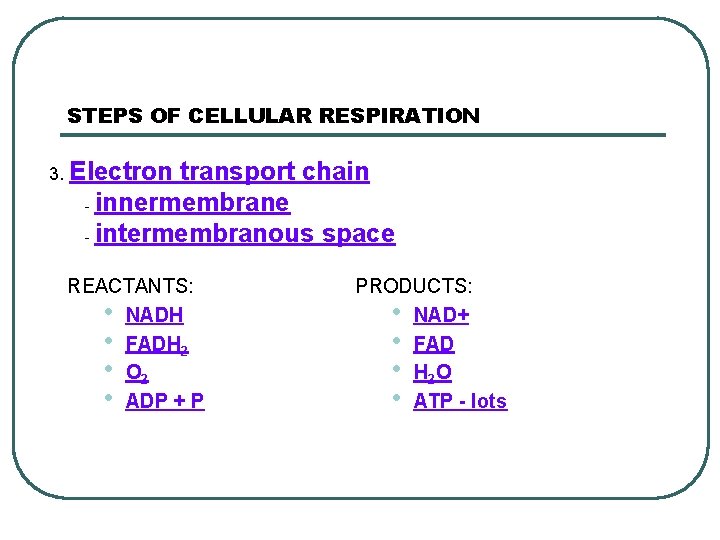
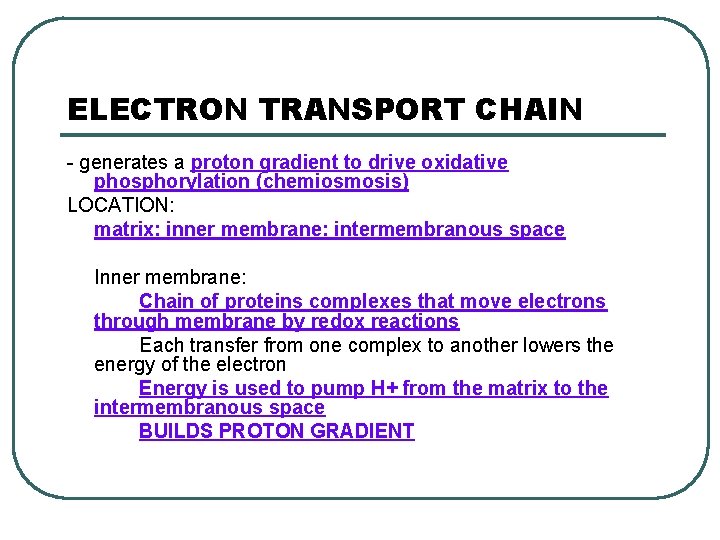
STEPS OF CELLULAR RESPIRATION 3. Electron transport chain - innermembrane - intermembranous space REACTANTS: • NADH • FADH 2 • O 2 • ADP + P PRODUCTS: • NAD+ • FAD • H 2 O • ATP - lots

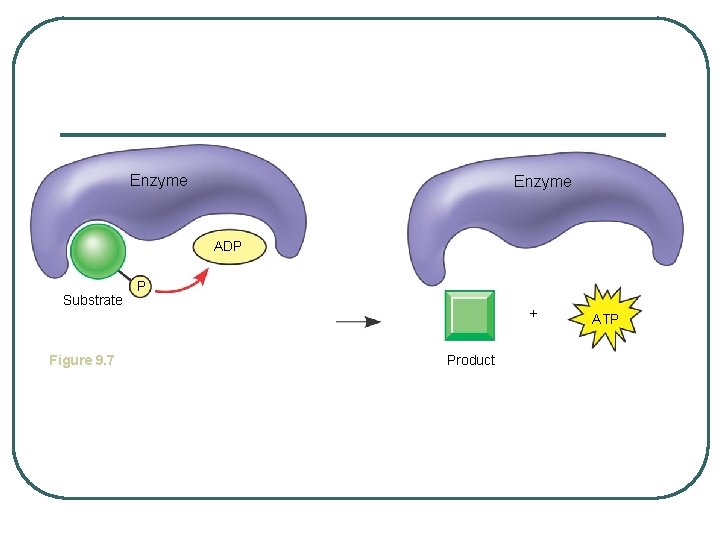
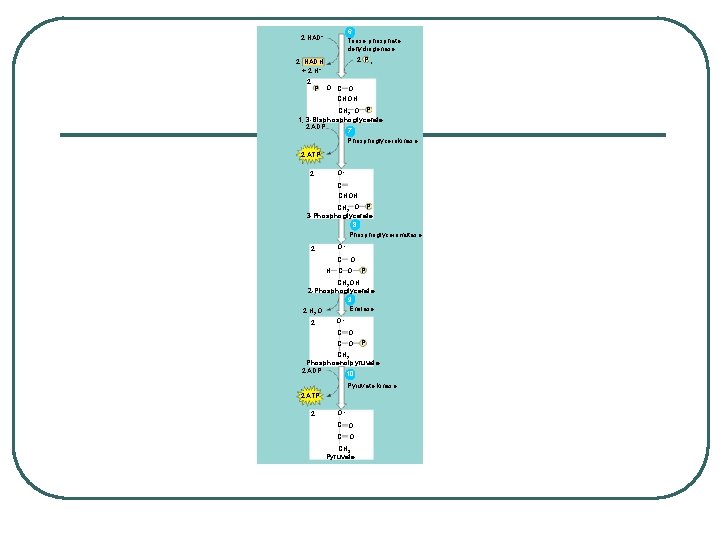
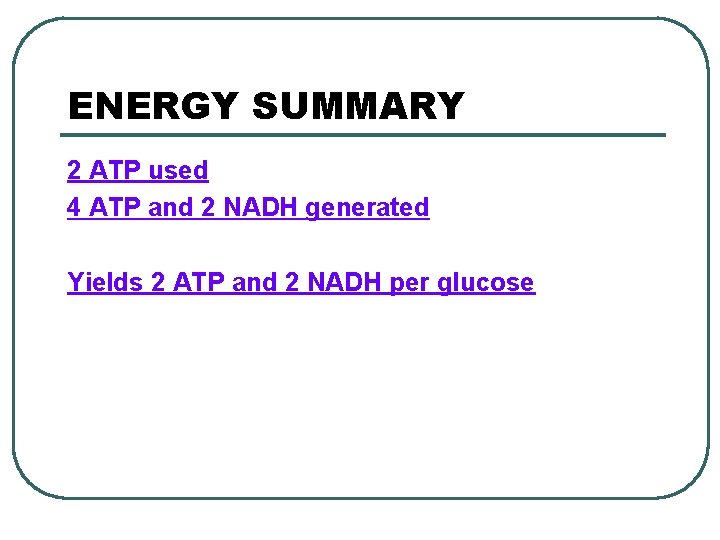
GLYCOLYSIS - splitting of sugar l 6 carbon sugar split into 2, 3 carbon compounds to be made into PYRUVATE l 10 steps in the cytosol of the cell broken into 2 parts: 1. Energy-Investment Phase: 2 ATP USED 2. Energy-Payoff Phase: 4 ATP and 2 NADH generated l SUBSTRATE LEVEL PHOSPHORYLATION – transfer of phosphates from a molecule to ADP using an enzyme

Enzyme ADP Substrate Figure 9. 7 P + Product ATP

Glycolysis Citric acid cycle Oxidative phosphorylation ATP ATP Energy investment phase Glucose 2 ATP + 2 P 2 ATP used Energy payoff phase 4 ADP + 4 P 2 NAD+ + 4 e- + 4 H + 4 ATP formed 2 NADH + 2 H+ 2 Pyruvate + 2 H 2 O Glucose 4 ATP formed – 2 ATP used 2 NAD+ + 4 e– + 4 H + 2 Pyruvate + 2 H 2 O 2 ATP + 2 H+ 2 NADH

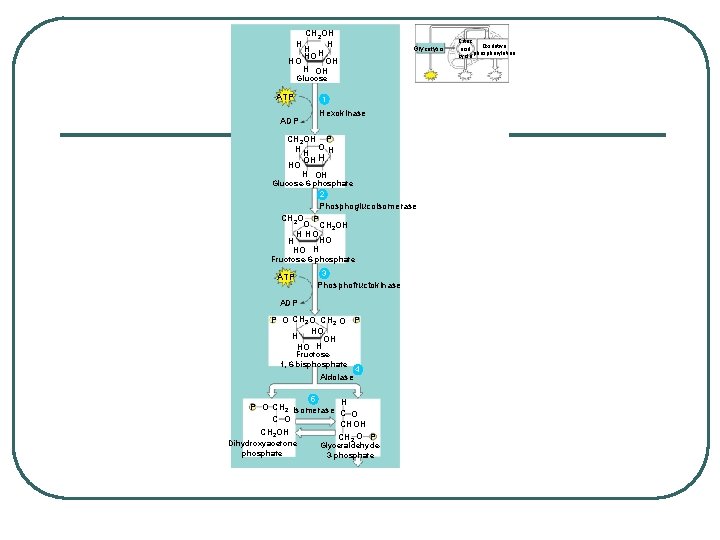
CH 2 OH HH H HO OH H OH Glycolysis Glucose ATP ADP 1 Hexokinase CH 2 OH P HH OH OH H HO H OH Glucose-6 -phosphate 2 Phosphoglucoisomerase CH 2 O P O CH 2 OH H HO HO H Fructose-6 -phosphate ATP 3 Phosphofructokinase ADP P O CH 2 O P HO H OH HO H Fructose 1, 6 -bisphosphate Aldolase 4 5 H P O CH 2 Isomerase C O CHOH CH 2 O P Dihydroxyacetone phosphate Glyceraldehyde 3 -phosphate Citric Oxidative acid cycle phosphorylation

6 Triose phosphate dehydrogenase 2 NAD+ 2 Pi 2 NADH + 2 H+ 2 P O CHOH CH 2 O P 1, 3 -Bisphoglycerate 2 ADP 7 Phosphoglycerokinase 2 ATP O– 2 C CHOH CH 2 O P 3 -Phosphoglycerate 8 Phosphoglyceromutase 2 O– C O H C O P CH 2 OH 2 -Phosphoglycerate 9 Enolase 2 H O 2 2 O– C O P CH 2 Phosphoenolpyruvate 2 ADP 10 Pyruvate kinase 2 ATP 2 O– C O CH 3 Pyruvate

ENERGY SUMMARY 2 ATP used 4 ATP and 2 NADH generated Yields 2 ATP and 2 NADH per glucose

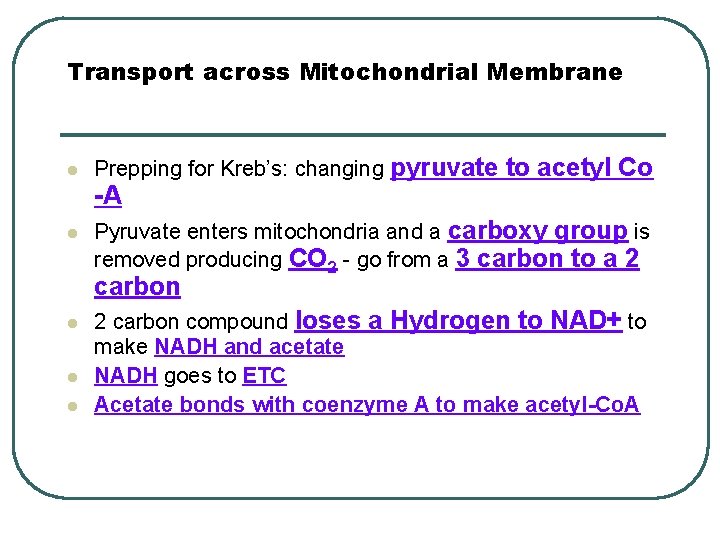
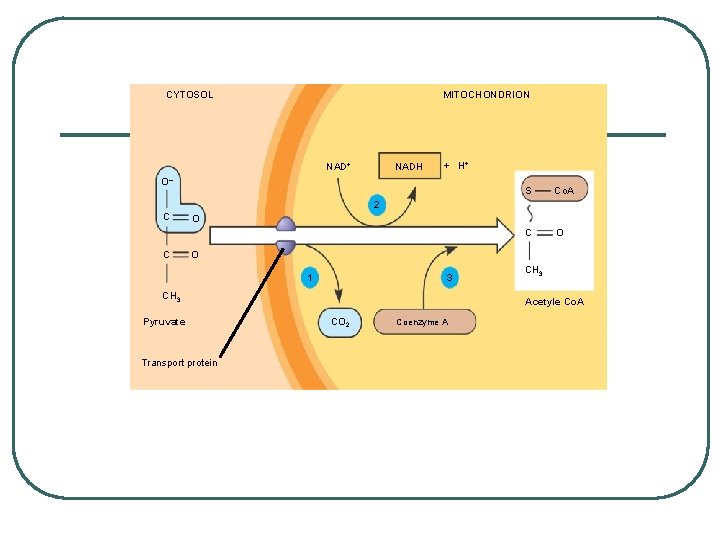
Transport across Mitochondrial Membrane l Prepping for Kreb’s: changing pyruvate to acetyl Co -A l Pyruvate enters mitochondria and a carboxy group is removed producing CO 2 - go from a 3 carbon to a 2 carbon l l l 2 carbon compound loses a Hydrogen to NAD+ to make NADH and acetate NADH goes to ETC Acetate bonds with coenzyme A to make acetyl-Co. A

CYTOSOL MITOCHONDRION NAD+ NADH + H+ O– S Co. A C O 2 C C O O 1 3 CH 3 Pyruvate Transport protein CH 3 Acetyle Co. A CO 2 Coenzyme A

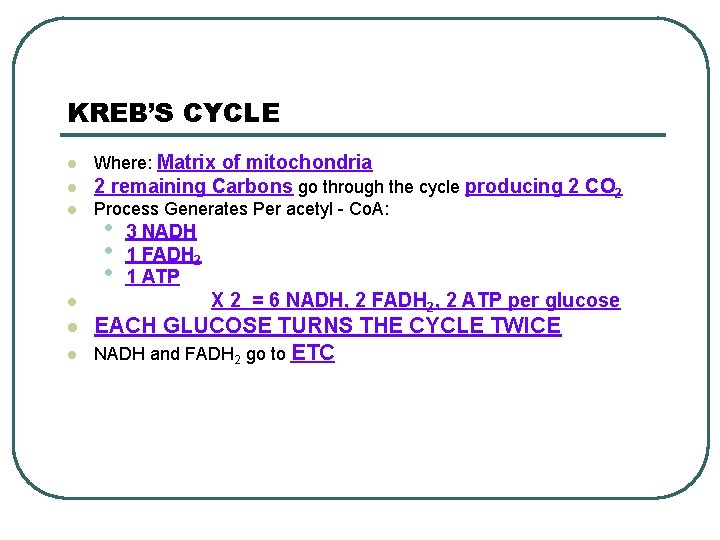
KREB’S CYCLE Where: Matrix of mitochondria l 2 remaining Carbons go through the cycle producing 2 CO 2 l Process Generates Per acetyl - Co. A: l • • • l l l 3 NADH 1 FADH 2 1 ATP X 2 = 6 NADH, 2 FADH 2, 2 ATP per glucose EACH GLUCOSE TURNS THE CYCLE TWICE NADH and FADH 2 go to ETC

Pyruvate (from glycolysis, 2 molecules per glucose) Glycolysis Citric acid cycle ATP Oxidative phosphorylation ATP CO 2 Co. A NADH + 3 H+ Acetyle Co. A Citric acid cycle FADH 2 FAD 2 CO 2 3 NAD+ 3 NADH + 3 H+ ADP + P i ATP

Glycolysis Citric Oxidative acid phosphorylation cycle S Co. A C O CH 3 Acetyl Co. A SH O NADH + H+ C COO– 1 CH 2 8 Oxaloacetate HO C CH 2 COO– HO CH COO– CH 2 COO– NAD+ H 2 O COO– CH 2 2 HC COO– Malate HO Citrate Isocitrate COO– H 2 O Citric acid cycle 7 COO– CH CO 2 3 NAD+ COO– Fumarate HC CH COO– CH 2 Co. A SH 6 Co. A SH COO– FAD CH 2 C O COO– Succinate Pi S Co. A GTP GDP Succinyl Co. A ADP ATP 4 C O COO– CH 2 5 CH 2 FADH 2 COO– NAD+ NADH + H+ a-Ketoglutarate CH 2 COO– NADH CO 2


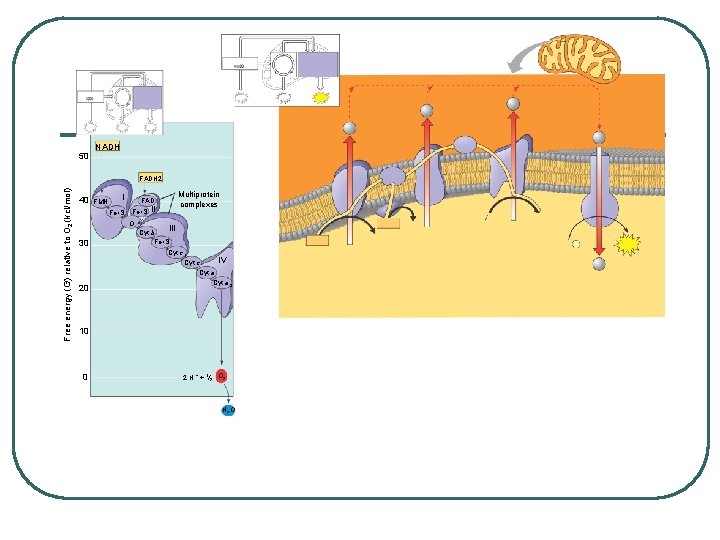
ELECTRON TRANSPORT CHAIN - generates a proton gradient to drive oxidative phosphorylation (chemiosmosis) LOCATION: matrix: inner membrane: intermembranous space Inner membrane: Chain of proteins complexes that move electrons through membrane by redox reactions Each transfer from one complex to another lowers the energy of the electron Energy is used to pump H+ from the matrix to the intermembranous space BUILDS PROTON GRADIENT

50 NADH Free energy (G) relative to O 2 (kcl/mol) FADH 2 40 FMN I Fe • S II O 30 20 Multiprotein complexes FAD III Cyt b Fe • S Cyt c 1 IV Cyt c Cyt a 3 10 0 2 H + + 1 2 O 2 H 2 O

l l l NADH breaks into NAD+ and H+ and electrons go into electron transport chain – FADH 2 deposits electrons further down chain At specific points H+ are pumped across the membrane - NAD+ is reused Each NADH gives enough energy for the production of 3 ATP Each FADH 2 gives enough energy for the production of 2 ATP

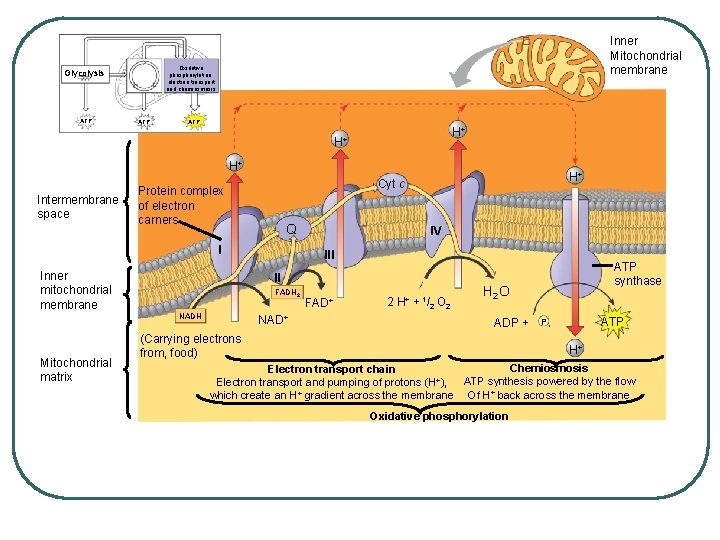
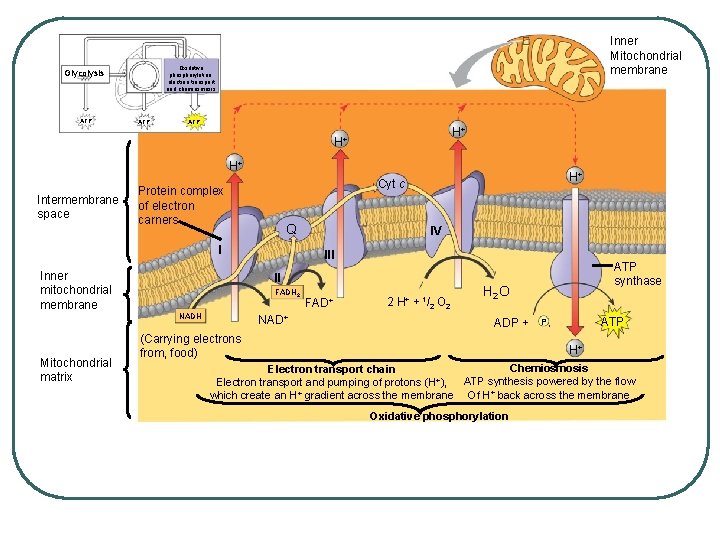
Oxidative phosphorylation. electron transport and chemiosmosis Glycolysis ATP Inner Mitochondrial membrane ATP H+ H+ H+ Intermembrane space Protein complex of electron carners Q I Inner mitochondrial membrane IV III II FADH 2 NADH Mitochondrial matrix H+ Cyt c NAD+ FAD+ 2 H+ + 1/2 O 2 ATP synthase H 2 O ADP + (Carrying electrons from, food) ATP Pi H+ Chemiosmosis Electron transport chain Electron transport and pumping of protons (H+), ATP synthesis powered by the flow which create an H+ gradient across the membrane Of H+ back across the membrane Oxidative phosphorylation

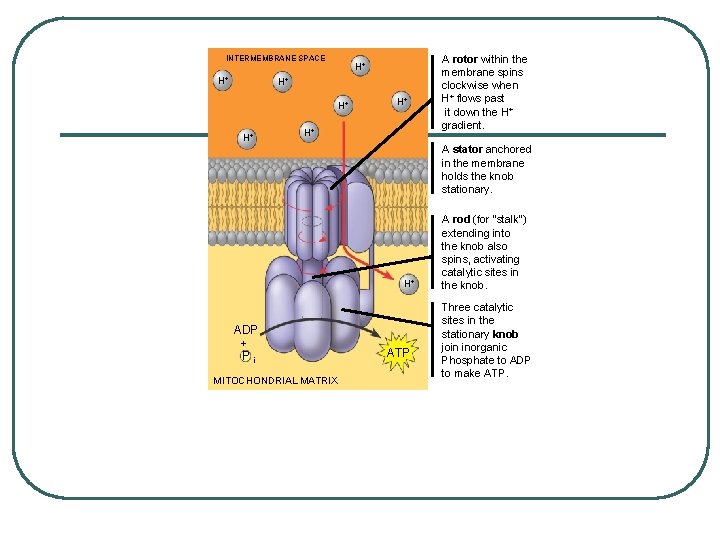
l l H+s pumping builds PROTON-MOTIVE FORCE or CHEMIOSMOTIC GRADIENT protons diffuse back across to other side through a protein (facilitated diffusion) ATP synthase • • cylidrical rotor knob with a rod in the rotor

Oxidative phosphorylation. electron transport and chemiosmosis Glycolysis ATP Inner Mitochondrial membrane ATP H+ H+ H+ Intermembrane space Protein complex of electron carners Q I Inner mitochondrial membrane IV III II FADH 2 NADH Mitochondrial matrix H+ Cyt c NAD+ FAD+ 2 H+ + 1/2 O 2 ATP synthase H 2 O ADP + (Carrying electrons from, food) ATP Pi H+ Chemiosmosis Electron transport chain Electron transport and pumping of protons (H+), ATP synthesis powered by the flow which create an H+ gradient across the membrane Of H+ back across the membrane Oxidative phosphorylation

as H+ pass through, the rotor turns the rod and the movement of the rod causes conformational changes in the knob which then phosphorulates ADP into ATP CHEMIOSMOTIC PHOSPHORYLATION or OXIDATIVE PHOSPHORYLATION l

INTERMEMBRANE SPACE H+ H+ A stator anchored in the membrane holds the knob stationary. H+ ADP + Pi MITOCHONDRIAL MATRIX A rotor within the membrane spins clockwise when H+ flows past it down the H+ gradient. ATP A rod (for “stalk”) extending into the knob also spins, activating catalytic sites in the knob. Three catalytic sites in the stationary knob join inorganic Phosphate to ADP to make ATP.

END OF ELECTRON TRANSPORT CHAIN Final electron acceptor is O 2 l for every 2 NADH or 1 FADH 2 one O 2 is reduced and forms water Electron Transport Chain Animation

CELLULAR RESPIRATION: AN OVERVIEW Overall Energy Yield = 36 – 38 ATP Glycolysis: 2 ATP, 2 NADH Kreb’s Cycle: 2 ATP, 8 NADH, 2 FADH 2 ETC: ATP based on #’s of NADH and FADH 2 # of NADH = 8 (Krebs) = 24 ATP # of FADH 2 = 4 ATP Total ATP = 2 (Glyc) + 2 (Kreb’s) + 28 ATP = 32 ATP Remaining 4 – 6 from the 2 NADH from Glycolysis

Glycolysis NADH l l l transfers Hydrogen across membrane of mitochondria membrane not permeable to NAD+ Transfers H’s to either NADH or FADH 2 • if to NADH = 3 ATP • if to FADH 2 = 2 ATP

l ATP TOTALS: l TOTAL Yield: about 38 ATP per glucose • Glycolysis: 2 • Kreb’s: 2 • ETC: 32 - 34 • efficiency of 40% • compared to a car which is at best 25% • Remaining energy lost as heat

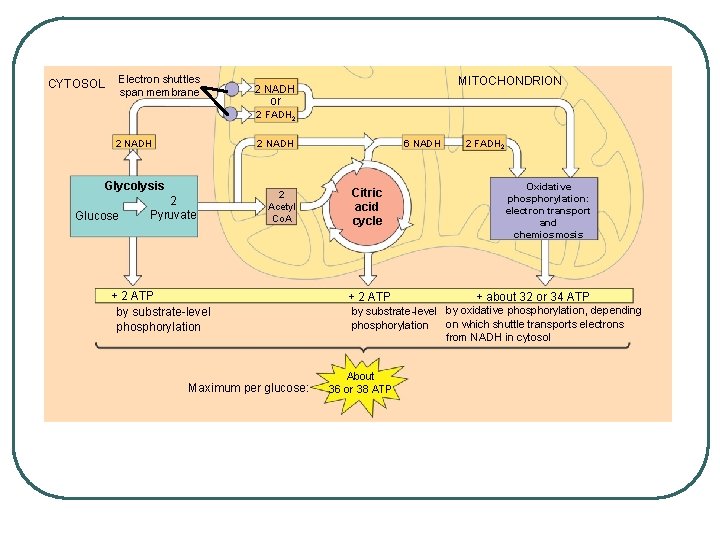
Electron shuttles span membrane CYTOSOL MITOCHONDRION 2 NADH or 2 FADH 2 2 NADH Glycolysis Glucose 2 Pyruvate 2 Acetyl Co. A + 2 ATP by substrate-level phosphorylation Maximum per glucose: 6 NADH Citric acid cycle + 2 ATP 2 FADH 2 Oxidative phosphorylation: electron transport and chemiosmosis + about 32 or 34 ATP by substrate-level by oxidative phosphorylation, depending on which shuttle transports electrons phosphorylation from NADH in cytosol About 36 or 38 ATP

RELATED METABOLIC PROPERTIES Glycolysis uses NAD+ to Oxidize Glucose l for glucose to continue to be used for the generation of energy the cell must recycle the NADH to NAD+ l NEED A FINAL ELECTRON ACCEPTOR - usually Oxygen WHAT HAPPENS WHEN THERE IS NO OXYGEN? l NADH has no place to put its hydrogens - glucose can’t be broken down l NEED ANOTHER ELECTRON ACCEPTOR: • with oxygen aerobic l • without Oxygen anaerobic

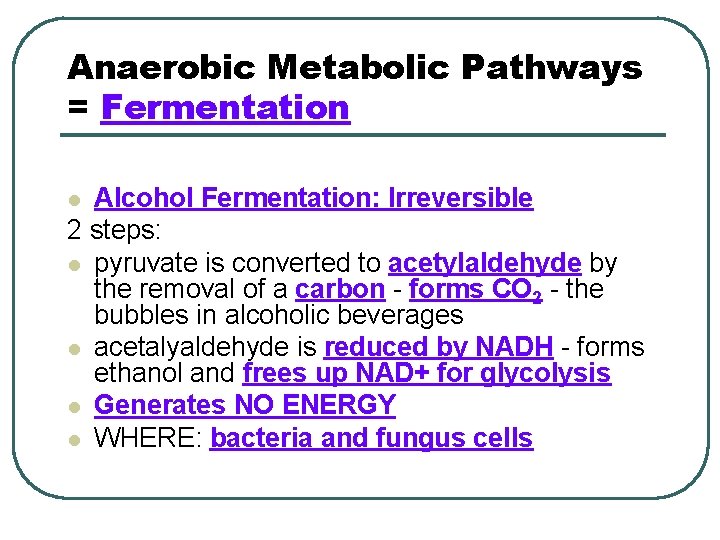
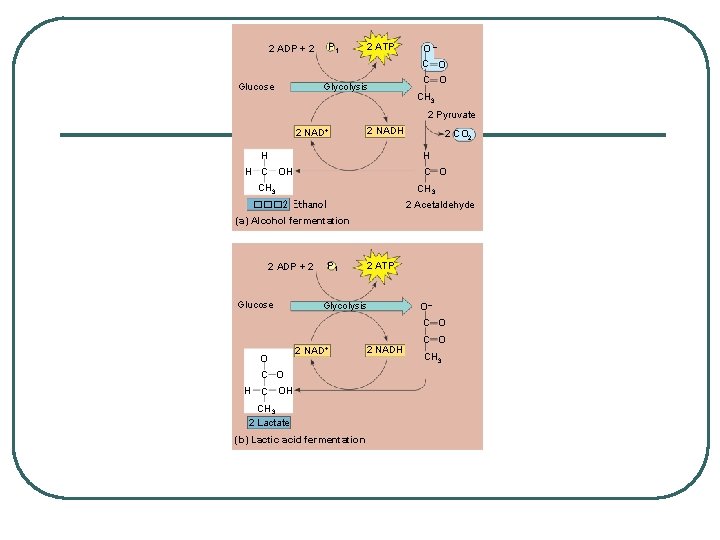
Anaerobic Metabolic Pathways = Fermentation Alcohol Fermentation: Irreversible 2 steps: l pyruvate is converted to acetylaldehyde by the removal of a carbon - forms CO 2 - the bubbles in alcoholic beverages l acetalyaldehyde is reduced by NADH - forms ethanol and frees up NAD+ for glycolysis l Generates NO ENERGY l WHERE: bacteria and fungus cells l

Lactic Acid Fermentation: Reversible l Pyruvate is reduced to lactate - frees up NAD+ for glycolysis l PRODUCES NO ENERGY and NO CO 2 - burning and pain in cells l Lactate can be converted back to pyruvate when removed from cells and processed in liver

P 1 2 ADP + 2 Glucose 2 ATP Glycolysis O– C O CH 3 2 Pyruvate 2 NAD+ 2 NADH H 2 CO 2 H H C O CH 3 2 Acetaldehyde ��� 2 Ethanol (a) Alcohol fermentation 2 ADP + 2 Glucose P 1 2 ATP Glycolysis O– C O O 2 NAD+ C O H C OH CH 3 2 Lactate (b) Lactic acid fermentation 2 NADH C O CH 3

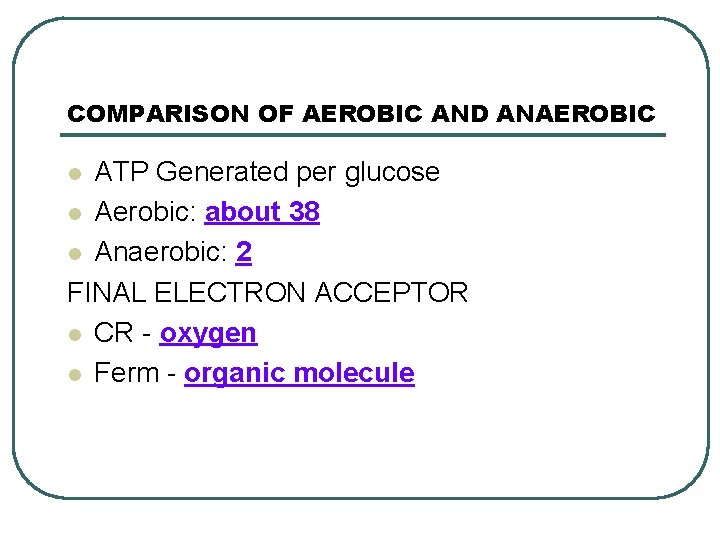
COMPARISON OF AEROBIC AND ANAEROBIC ATP Generated per glucose l Aerobic: about 38 l Anaerobic: 2 FINAL ELECTRON ACCEPTOR l CR - oxygen l Ferm - organic molecule l

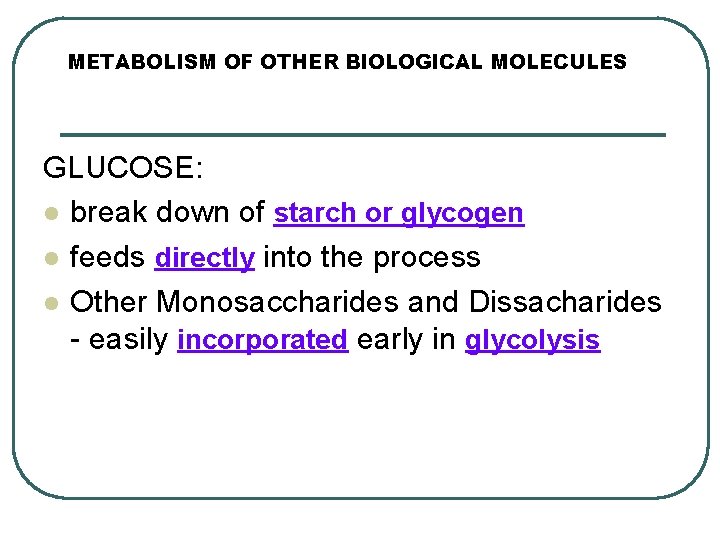
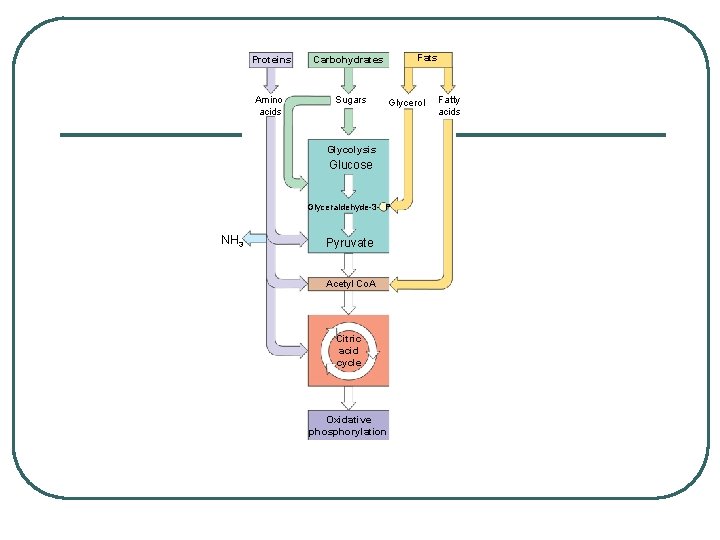
METABOLISM OF OTHER BIOLOGICAL MOLECULES GLUCOSE: l break down of starch or glycogen l feeds directly into the process l Other Monosaccharides and Dissacharides - easily incorporated early in glycolysis

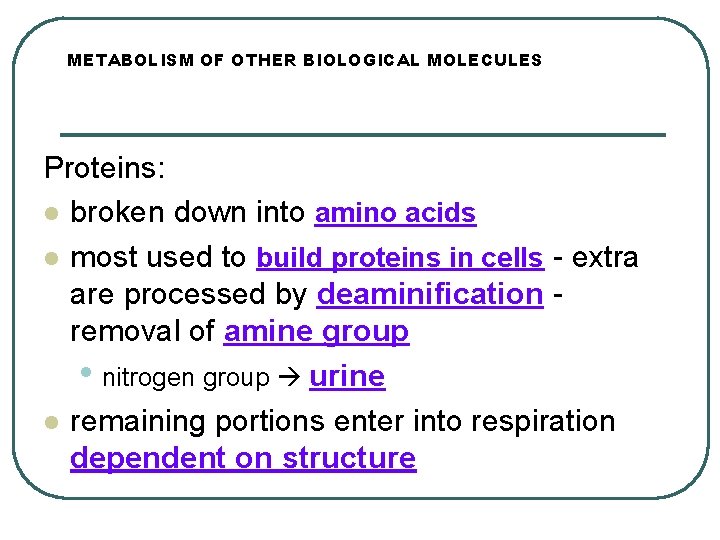
METABOLISM OF OTHER BIOLOGICAL MOLECULES Proteins: l broken down into amino acids l most used to build proteins in cells - extra are processed by deaminification removal of amine group • nitrogen group urine l remaining portions enter into respiration dependent on structure

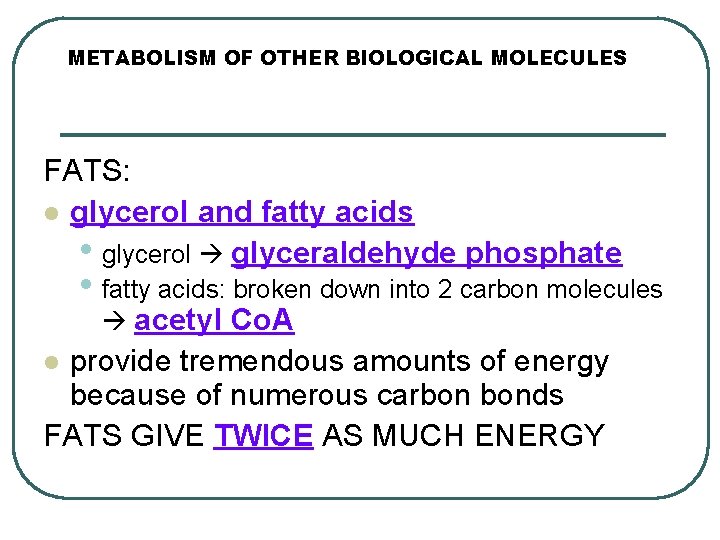
METABOLISM OF OTHER BIOLOGICAL MOLECULES FATS: l glycerol and fatty acids • glycerol glyceraldehyde phosphate • fatty acids: broken down into 2 carbon molecules acetyl Co. A provide tremendous amounts of energy because of numerous carbon bonds FATS GIVE TWICE AS MUCH ENERGY l

METABOLISM OF OTHER BIOLOGICAL MOLECULES HOWEVER: NOT ALL BIOMOLECULES OR INTERMEDIATES OF CELLULAR RESPIRATION ARE USED FOR PRODUCTION OF ENERGY l some are used to build other molecules

Proteins Carbohydrates Amino acids Sugars Fats Glycerol Glycolysis Glucose Glyceraldehyde-3 - P NH 3 Pyruvate Acetyl Co. A Citric acid cycle Oxidative phosphorylation Fatty acids

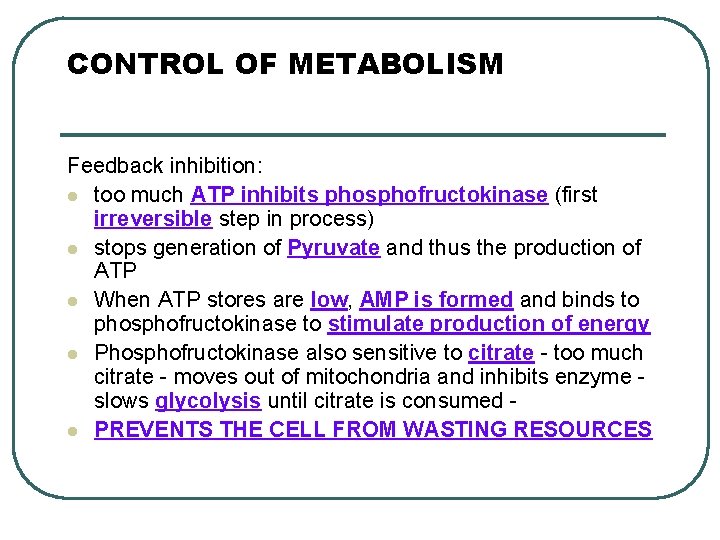
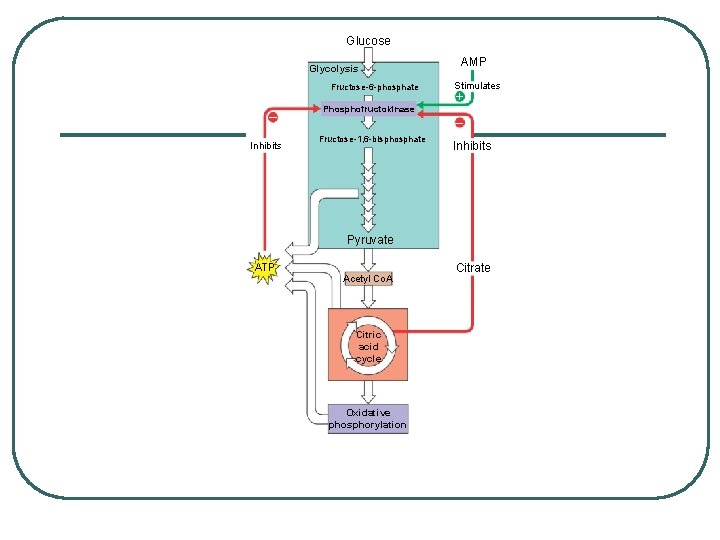
CONTROL OF METABOLISM Feedback inhibition: l too much ATP inhibits phosphofructokinase (first irreversible step in process) l stops generation of Pyruvate and thus the production of ATP l When ATP stores are low, AMP is formed and binds to phosphofructokinase to stimulate production of energy l Phosphofructokinase also sensitive to citrate - too much citrate - moves out of mitochondria and inhibits enzyme slows glycolysis until citrate is consumed l PREVENTS THE CELL FROM WASTING RESOURCES

Glucose Glycolysis Fructose-6 -phosphate – Inhibits Phosphofructokinase AMP Stimulates + – Fructose-1, 6 -bisphosphate Inhibits Pyruvate ATP Acetyl Co. A Citric acid cycle Oxidative phosphorylation Citrate