Chapter 9 Cellular Respiration Harvesting Chemical Energy Power




![A closer look at the energy investment phase: [Step 1] Energy input required Terminal A closer look at the energy investment phase: [Step 1] Energy input required Terminal](https://slidetodoc.com/presentation_image_h/0ab6b6c041589a0224ffaeb0155ad151/image-22.jpg)
![A closer look at the energy payoff phase [Step 6] 2 Glyceraldehyde-3 -phosphate molecules A closer look at the energy payoff phase [Step 6] 2 Glyceraldehyde-3 -phosphate molecules](https://slidetodoc.com/presentation_image_h/0ab6b6c041589a0224ffaeb0155ad151/image-23.jpg)

- Slides: 48

Chapter 9 Cellular Respiration: Harvesting Chemical Energy Power. Point Lectures for Biology, Seventh Edition Neil Campbell and Jane Reece Lectures by Chris Romero Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings

Overview: Life Is Work • Living cells – Require transfusions of energy from outside sources to perform their many tasks Ex: The giant panda – Obtains (chemical, potential) energy for its cells by eating plants Figure 9. 1 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings

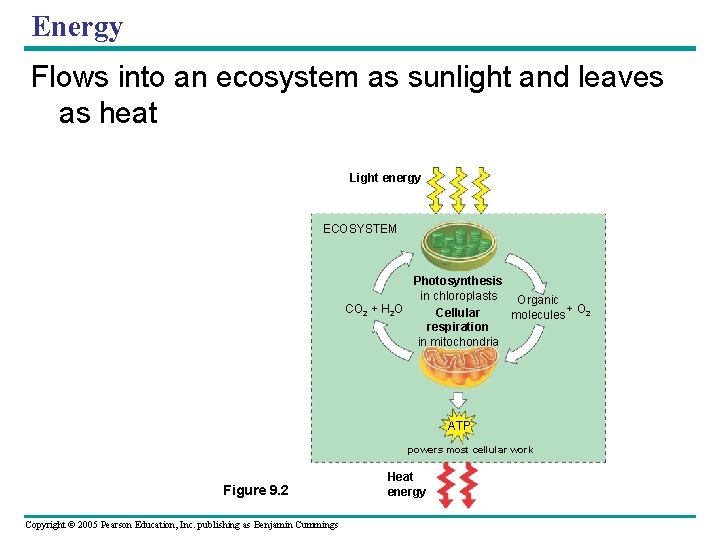
Energy Flows into an ecosystem as sunlight and leaves as heat Light energy ECOSYSTEM Photosynthesis in chloroplasts Organic CO 2 + H 2 O + O 2 Cellular molecules respiration in mitochondria ATP powers most cellular work Figure 9. 2 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Heat energy

Review: • 1 st law- Energy cannot be created or destroyed. Energy can be converted from one form to another. The sum of the energy before the conversion is equal to the sum of the energy after the conversion. • 2 nd law- Some usable energy dissipates during transformations and is lost. During changes from one form of energy to another, some usable energy dissipates, usually as heat. The amount of usable energy therefore decreases. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings

Cellular Respiration • Glucose + O 2 energy (ATP) + CO 2 + H 2 O • This is exergonic –DG and converts one form of chemical energy (glucose) into another (ATP), with heat dissipates – Consumes oxygen and organic molecules (such as glucose) – Glucose Oxidation is the most prevalent and efficient catabolic pathway – Yields lots of ATP • An alternate catabolic process, fermentation – Is a partial degradation of sugars that occurs without oxygen (in anaerobic conditions) Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings

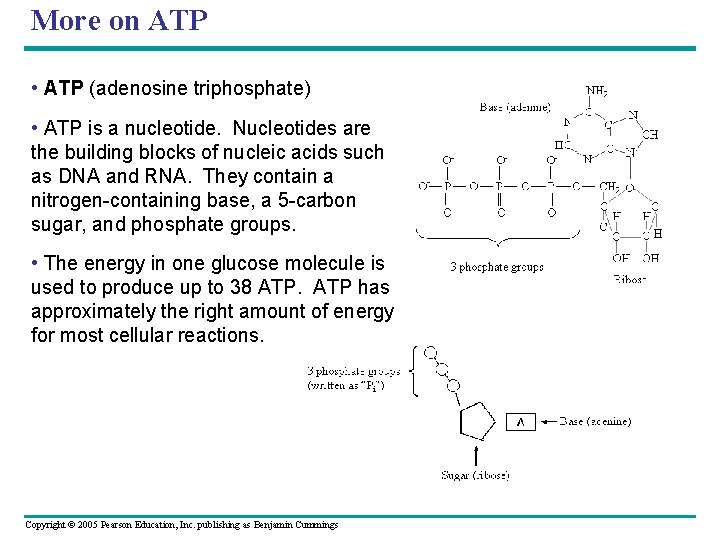
More on ATP • ATP (adenosine triphosphate) • ATP is a nucleotide. Nucleotides are the building blocks of nucleic acids such as DNA and RNA. They contain a nitrogen-containing base, a 5 -carbon sugar, and phosphate groups. • The energy in one glucose molecule is used to produce up to 38 ATP has approximately the right amount of energy for most cellular reactions. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings

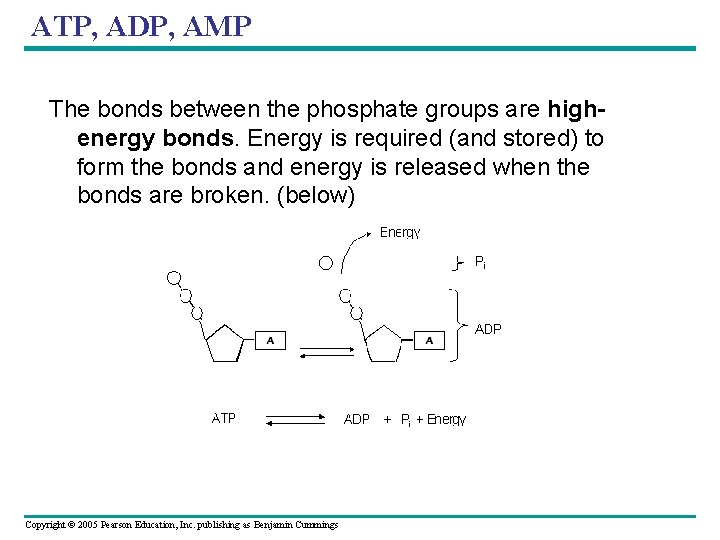
ATP, ADP, AMP The bonds between the phosphate groups are highenergy bonds. Energy is required (and stored) to form the bonds and energy is released when the bonds are broken. (below) Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings

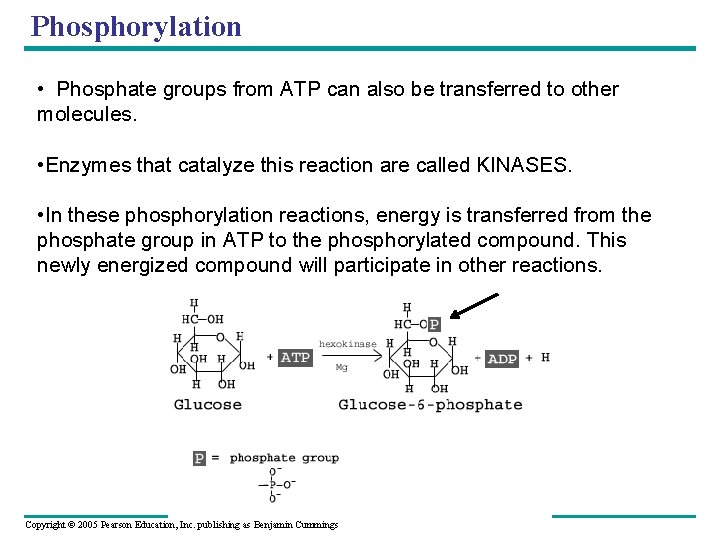
Phosphorylation • ATP is produced and used continuously. The entire amount of ATP in an organism is recycled once per minute. Most cells maintain only a few seconds supply of ATP. • To keep working, cells must regenerate ATP • When a phosphate group is transferred to another molecule, it is called PHOSPHORYLATION. ADP + Pi + energy ----> ATP • It takes about 7. 3 kcals of energy to phosphorylate ADP into ATP • Enzyme that catalyzes this reaction is ATP synthase Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings

Phosphorylation • Phosphate groups from ATP can also be transferred to other molecules. • Enzymes that catalyze this reaction are called KINASES. • In these phosphorylation reactions, energy is transferred from the phosphate group in ATP to the phosphorylated compound. This newly energized compound will participate in other reactions. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings

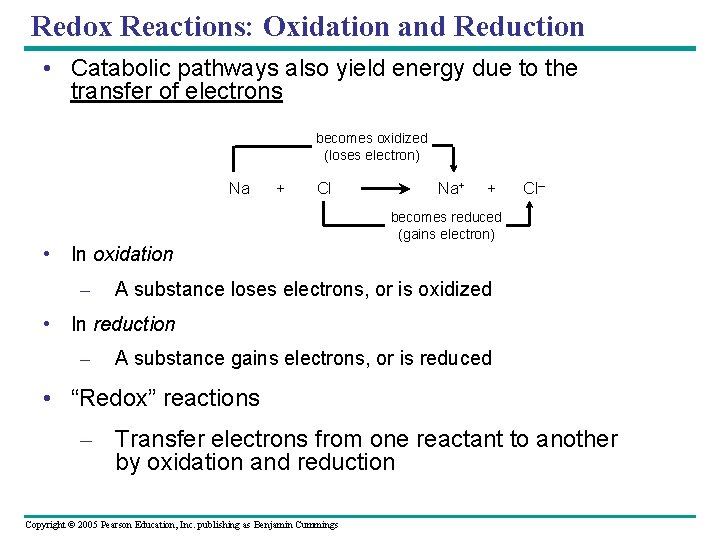
Redox Reactions: Oxidation and Reduction • Catabolic pathways also yield energy due to the transfer of electrons becomes oxidized (loses electron) Na + Cl Na + + Cl– becomes reduced (gains electron) • In oxidation – A substance loses electrons, or is oxidized • In reduction – A substance gains electrons, or is reduced • “Redox” reactions – Transfer electrons from one reactant to another by oxidation and reduction Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings

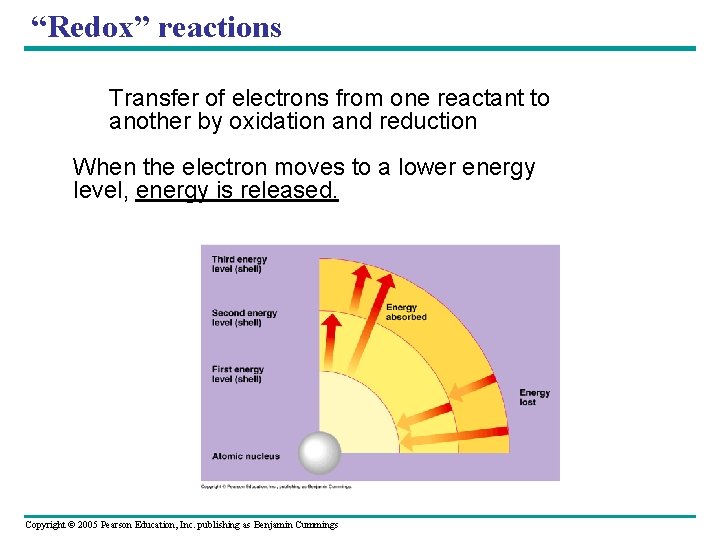
“Redox” reactions Transfer of electrons from one reactant to another by oxidation and reduction When the electron moves to a lower energy level, energy is released. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings

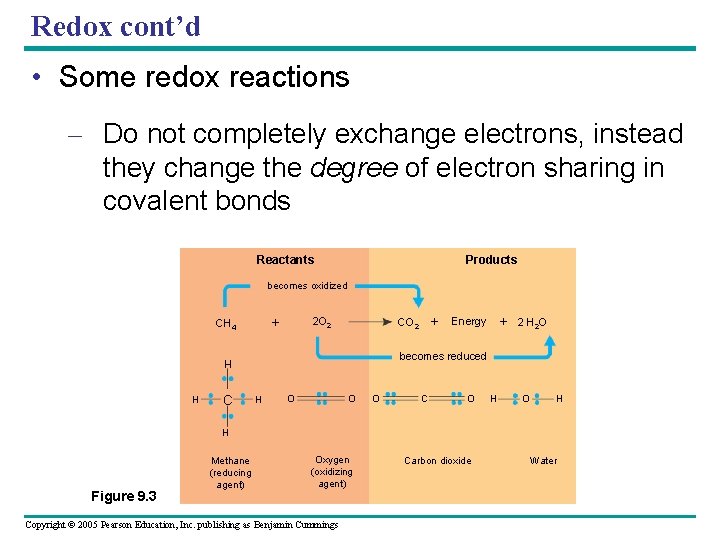
Redox cont’d • Some redox reactions – Do not completely exchange electrons, instead they change the degree of electron sharing in covalent bonds Products Reactants becomes oxidized + CH 4 2 O 2 + Energy 2 H 2 O becomes reduced O O C O H O O H H H C + CO 2 H H Figure 9. 3 Methane (reducing agent) Oxygen (oxidizing agent) Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Carbon dioxide Water

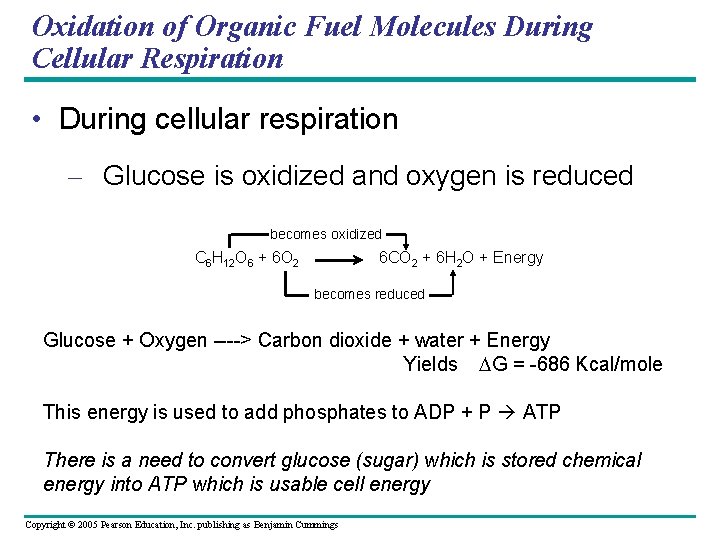
Oxidation of Organic Fuel Molecules During Cellular Respiration • During cellular respiration – Glucose is oxidized and oxygen is reduced becomes oxidized C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O + Energy becomes reduced Glucose + Oxygen ----> Carbon dioxide + water + Energy Yields DG = -686 Kcal/mole This energy is used to add phosphates to ADP + P ATP There is a need to convert glucose (sugar) which is stored chemical energy into ATP which is usable cell energy Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings

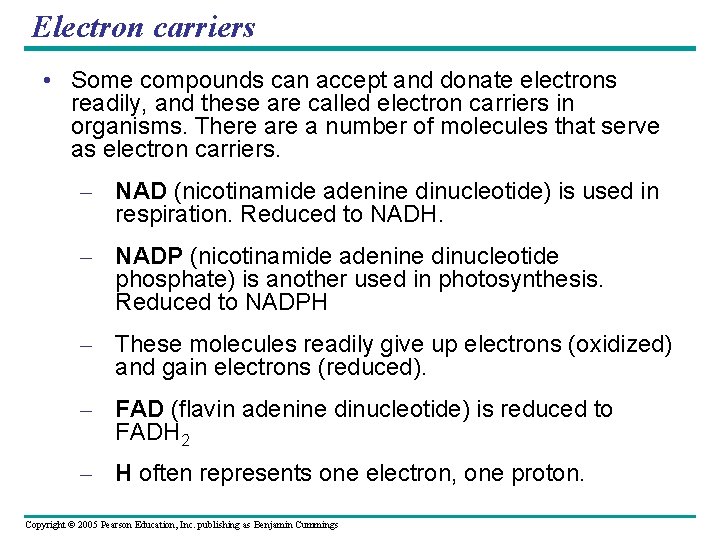
Electron carriers • Some compounds can accept and donate electrons readily, and these are called electron carriers in organisms. There a number of molecules that serve as electron carriers. – NAD (nicotinamide adenine dinucleotide) is used in respiration. Reduced to NADH. – NADP (nicotinamide adenine dinucleotide phosphate) is another used in photosynthesis. Reduced to NADPH – These molecules readily give up electrons (oxidized) and gain electrons (reduced). – FAD (flavin adenine dinucleotide) is reduced to FADH 2 – H often represents one electron, one proton. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings

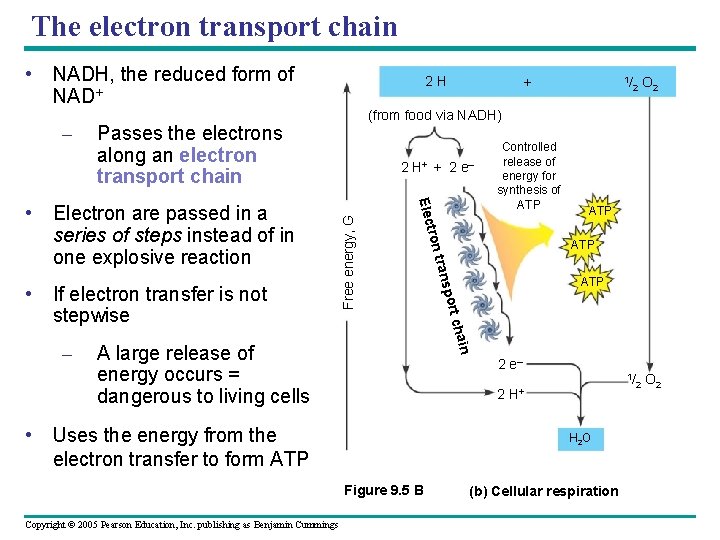
The electron transport chain • NADH, the reduced form of NAD+ Passes the electrons along an electron transport chain 2 O 2 ATP cha port in A large release of energy occurs = dangerous to living cells 2 e– 1/ 2 H+ • Uses the energy from the electron transfer to form ATP H 2 O Figure 9. 5 B Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings 1/ ATP s tran – Controlled release of energy for synthesis of ATP tron • If electron transfer is not stepwise 2 H+ + 2 e– Elec • Electron are passed in a series of steps instead of in one explosive reaction + (from food via NADH) Free energy, G – 2 H (b) Cellular respiration 2 O 2

ATP originates in cellular respiration • What happens is that sugar is broken down into smaller molecules and energy is released. • This energy is used to generate ATP from ADP and P. • Sugar ------> smaller molecules----->ADP + Pi ------> ATP Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings

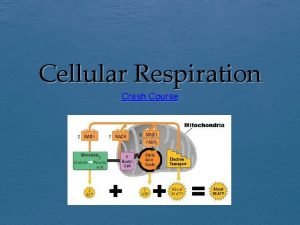
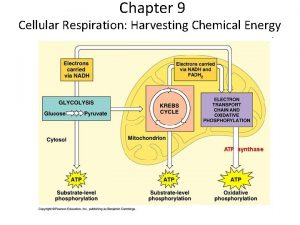
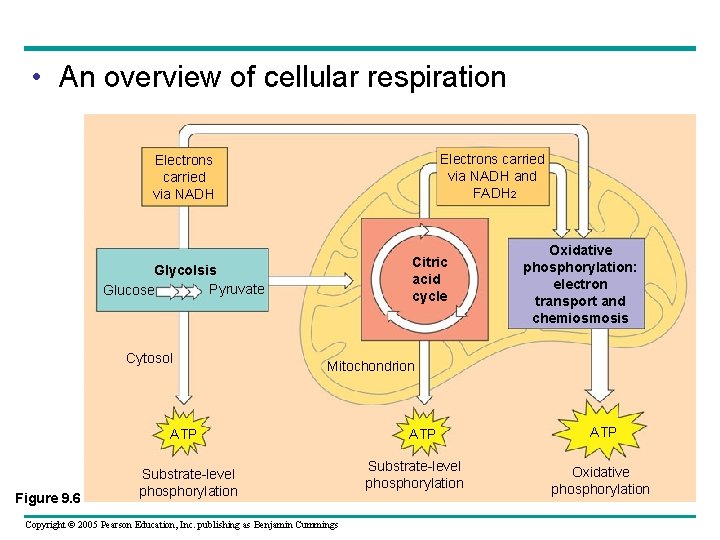
The Stages of Cellular Respiration: A Preview Respiration is a cumulative function of three metabolic stages • Glycolysis – Breaks down glucose into two molecules of pyruvate • The citric acid (Krebs) cycle – Completes the breakdown of glucose • Oxidative phosphorylation – Is driven by the electron transport chain (ETC) – Generates ATP Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings

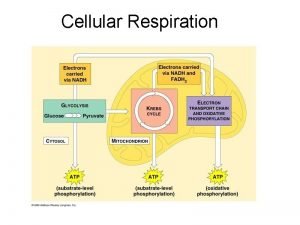
• An overview of cellular respiration Electrons carried via NADH and FADH 2 Electrons carried via NADH Citric acid cycle Glycolsis Pyruvate Glucose Cytosol Mitochondrion ATP Figure 9. 6 Oxidative phosphorylation: electron transport and chemiosmosis Substrate-level phosphorylation Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings ATP Substrate-level phosphorylation ATP Oxidative phosphorylation

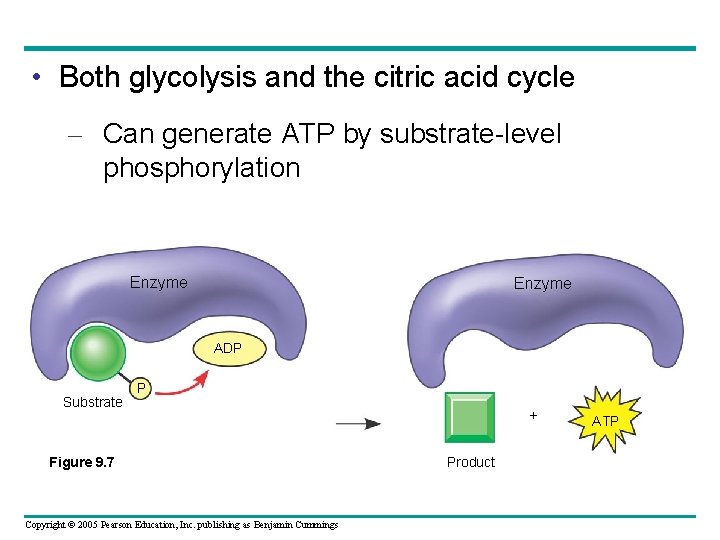
• Both glycolysis and the citric acid cycle – Can generate ATP by substrate-level phosphorylation Enzyme ADP Substrate P Figure 9. 7 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings + Product ATP

Concept 9. 2: Glycolysis harvests energy by oxidizing glucose to pyruvate Glycolysis – Means “splitting of sugar” – Breaks down glucose into pyruvate – Occurs in the cytoplasm of the cell Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings

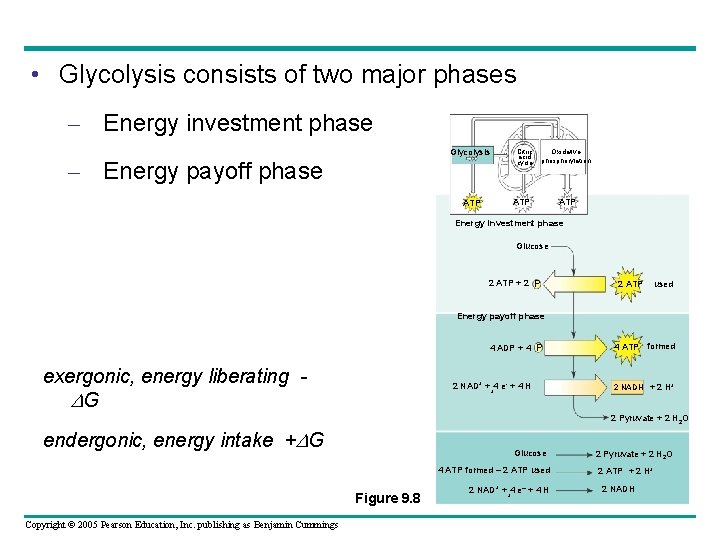
• Glycolysis consists of two major phases – Energy investment phase Glycolysis – Energy payoff phase Citric acid cycle Oxidative phosphorylation ATP ATP Energy investment phase Glucose 2 ATP + 2 P 2 ATP used Energy payoff phase 4 ADP + 4 P exergonic, energy liberating DG 2 NAD+ + 4 e- + 4 H + 4 ATP formed 2 NADH + 2 H+ 2 Pyruvate + 2 H 2 O endergonic, energy intake +DG Glucose 4 ATP formed – 2 ATP used Figure 9. 8 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings 2 NAD+ + 4 e– + 4 H + 2 Pyruvate + 2 H 2 O 2 ATP + 2 H+ 2 NADH
![A closer look at the energy investment phase Step 1 Energy input required Terminal A closer look at the energy investment phase: [Step 1] Energy input required Terminal](https://slidetodoc.com/presentation_image_h/0ab6b6c041589a0224ffaeb0155ad151/image-22.jpg)
A closer look at the energy investment phase: [Step 1] Energy input required Terminal P from an ATP (-->ADP) is bonded to the C 6 of a glucose DG = - 3. 3 (enzyme: hexokinase) [Step 2] glucose - 6 - phosphate------> fructose-6 -phosphate • atoms rearranged DG = + 0. 4 (enzyme: phosphoglucoisomerase) CH 2 OH HH H HO OH H OH Glucose ATP ADP [Step 3] fructose-6 -phosphate------> fructose 1, 6 biphosphate • Phosphate added to C 1 DG = - 3. 4 (enzyme: phosphofructokinase) • splits into two products [Step 4] fructose -1, 6 - biphosphate---> dihydroxyacetone phosphate AND glyceraldehyde phosphate* DG = + 5. 7 ( enzyme: aldolase) • Our 6 -carbon sugar is split into TWO 3 -carbon products [Step 5] *Ultimately, all of the dihydroxyacetone phosphate will be converted into glyceraldehyde-3 -phosphate, so each step is actually x 2 from here (because each of the two molecules will proceed through Steps 5 - 9 ) dihydroxyacetone phosphate-------> glyceraldehyde-3 -phosphate (enzyme: isomerase) Glycolysis 1 Hexokinase CH 2 OH P HH OH OH H HO H OH Glucose-6 -phosphate 2 Phosphoglucoisomerase CH 2 O P O CH 2 OH H HO HO H Fructose-6 -phosphate ATP 3 Phosphofructokinase ADP P O CH 2 O P HO H OH HO H Fructose 1, 6 -bisphosphate Aldolase 4 5 H P O CH 2 Isomerase C O CHOH CH 2 O P Figure 9. 9 A Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Dihydroxyacetone phosphate Glyceraldehyde 3 -phosphate Citric Oxidative acid cycle phosphorylation
![A closer look at the energy payoff phase Step 6 2 Glyceraldehyde3 phosphate molecules A closer look at the energy payoff phase [Step 6] 2 Glyceraldehyde-3 -phosphate molecules](https://slidetodoc.com/presentation_image_h/0ab6b6c041589a0224ffaeb0155ad151/image-23.jpg)
A closer look at the energy payoff phase [Step 6] 2 Glyceraldehyde-3 -phosphate molecules are then oxidized • 2 hydrogens (with e-) are removed, and NAD+ is reduced to NADH and H+ • Also, a free phosphate attaches to each of the glyceraldehyde-3 -phosphates 2 Glyceraldehyde-3 -phosphate--------> 1, 3 biphosphoglycerate (x 2) NAD+ is reduced to NADH and H+ • Pi = free phosphates, not taken from another molecule such as ATP • NAD = nicotinamide adenine dinucleotide (niacin derivative) DG = + 1. 5 (enzyme: triose phosphate dehydrogenase) [Step 7] a P is released from each 1, 3 biphosphoglycerate molecule and is used to "recharge" 2 ADPs----> 2 ATPs thus, converting the 1, 3 biphosphoglycerate to 3 -phosphoglycerate ADP---> ATP DG = -4. 5 (enzyme: phosphoglycerokinase) 2 P i 2 NADH + 2 H+ 2 P O CHOH CH 2 O P 1, 3 -Bisphoglycerate 2 ADP 7 Phosphoglycerokinase 2 ATP O– 2 C CHOH (highly exergonic= large DG to pull preceding reactions forward) (x 2) 1, 3 Diphosphoglycerate---------------> 3 -phosphoglycerate 6 Triose phosphate dehydrogenase 2 NAD+ CH 2 O P 3 -Phosphoglycerate 8 Phosphoglyceromutase 2 O– C (x 2) [Step 8] The remaining P group is enzymatically transferred from the 3 C to the 2 C 3 - phosphoglycerate------------> 2 - phosphoglycerate DG = + 1. 0 (enzyme: phosphoglyceromutase) (x 2) [Step 9] A molecule of H 2 O removed 2 - phosphoglycerate--------> phosphoenolpyruvate - H 2 O DG = + 0. 4 (enzyme: enolase) ( highly exergonic, pulls 2 preceding rxns!!!) phosphoenolpyruvate----------> pyruvate (pyruvic acid) DG = - 7. 5 ( enzyme: pyruvate kinase) ADP---> ATP Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings P CH 2 OH 2 -Phosphoglycerate 9 Enolase 2 H O 2 2 O– C O P CH 2 Phosphoenolpyruvate 2 ADP 10 Pyruvate kinase 2 ATP 2 (x 2) [Step 10] Another phosphate P is transferred to ADP O H C O O– C O CH 3 Pyruvate

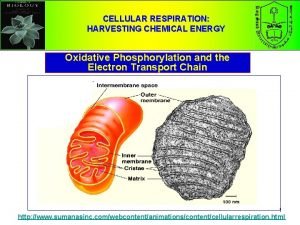
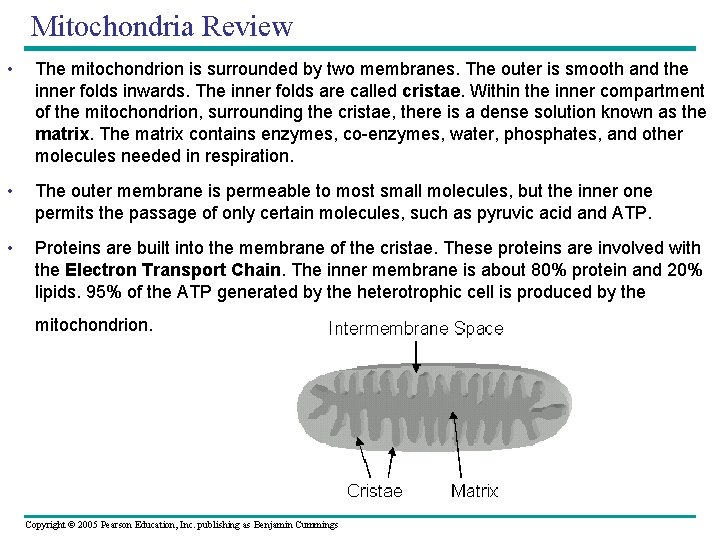
Mitochondria Review • The mitochondrion is surrounded by two membranes. The outer is smooth and the inner folds inwards. The inner folds are called cristae. Within the inner compartment of the mitochondrion, surrounding the cristae, there is a dense solution known as the matrix. The matrix contains enzymes, co-enzymes, water, phosphates, and other molecules needed in respiration. • The outer membrane is permeable to most small molecules, but the inner one permits the passage of only certain molecules, such as pyruvic acid and ATP. • Proteins are built into the membrane of the cristae. These proteins are involved with the Electron Transport Chain. The inner membrane is about 80% protein and 20% lipids. 95% of the ATP generated by the heterotrophic cell is produced by the mitochondrion. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings

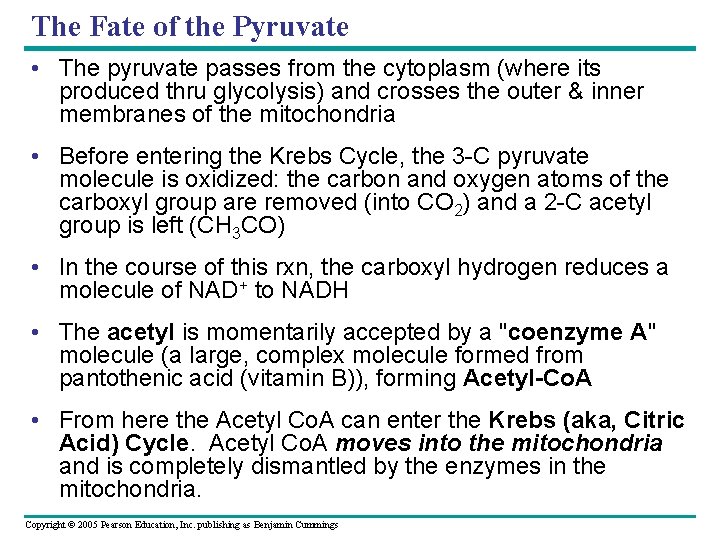
The Fate of the Pyruvate • The pyruvate passes from the cytoplasm (where its produced thru glycolysis) and crosses the outer & inner membranes of the mitochondria • Before entering the Krebs Cycle, the 3 -C pyruvate molecule is oxidized: the carbon and oxygen atoms of the carboxyl group are removed (into CO 2) and a 2 -C acetyl group is left (CH 3 CO) • In the course of this rxn, the carboxyl hydrogen reduces a molecule of NAD+ to NADH • The acetyl is momentarily accepted by a "coenzyme A" molecule (a large, complex molecule formed from pantothenic acid (vitamin B)), forming Acetyl-Co. A • From here the Acetyl Co. A can enter the Krebs (aka, Citric Acid) Cycle. Acetyl Co. A moves into the mitochondria and is completely dismantled by the enzymes in the mitochondria. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings

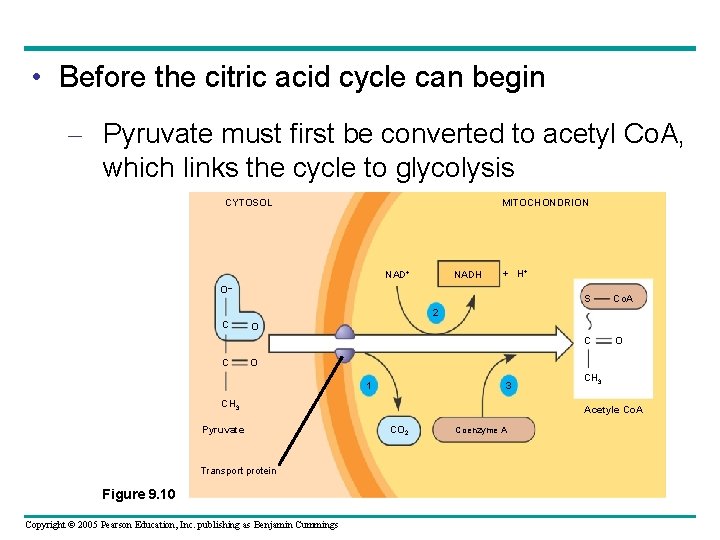
• Before the citric acid cycle can begin – Pyruvate must first be converted to acetyl Co. A, which links the cycle to glycolysis CYTOSOL MITOCHONDRION NAD+ NADH + H+ O– S Co. A C O 2 C C O O 1 3 CH 3 Pyruvate Transport protein Figure 9. 10 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings CH 3 Acetyle Co. A CO 2 Coenzyme A

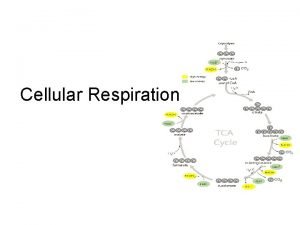
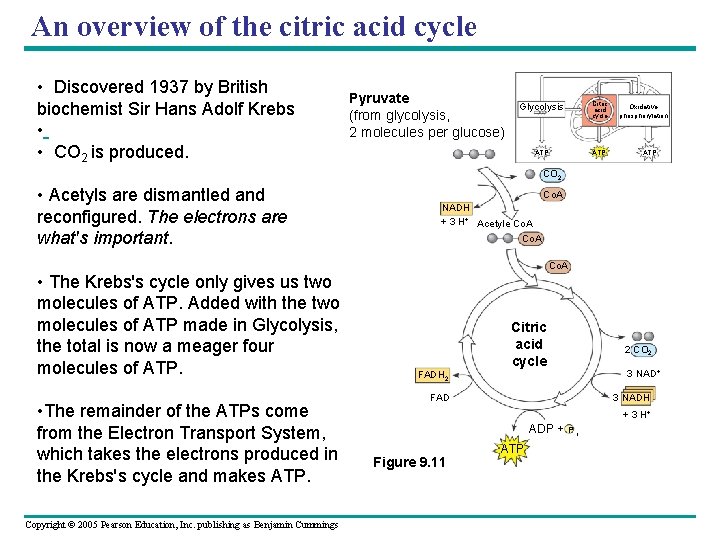
An overview of the citric acid cycle • Discovered 1937 by British biochemist Sir Hans Adolf Krebs • • CO 2 is produced. Pyruvate (from glycolysis, 2 molecules per glucose) Glycolysis Citric acid cycle ATP Oxidative phosphorylation ATP CO 2 • Acetyls are dismantled and reconfigured. The electrons are what's important. • The Krebs's cycle only gives us two molecules of ATP. Added with the two molecules of ATP made in Glycolysis, the total is now a meager four molecules of ATP. • The remainder of the ATPs come from the Electron Transport System, which takes the electrons produced in the Krebs's cycle and makes ATP. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Co. A NADH + 3 H+ Acetyle Co. A Citric acid cycle FADH 2 FAD 2 CO 2 3 NAD+ 3 NADH + 3 H+ ADP + P i Figure 9. 11 ATP

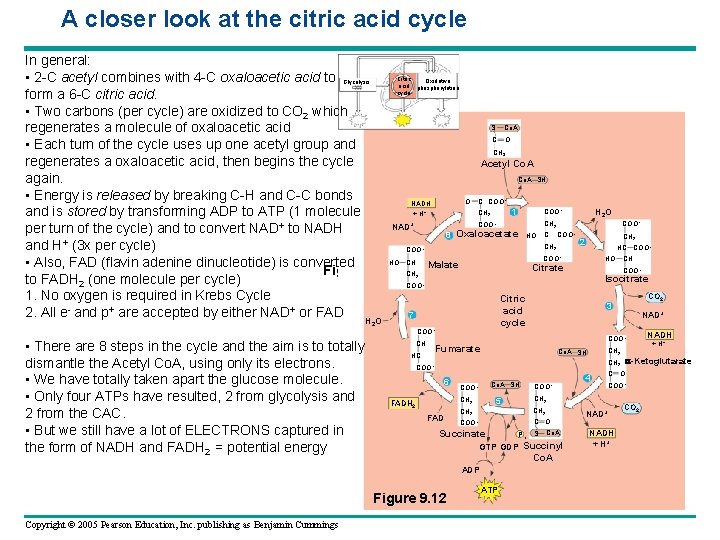
A closer look at the citric acid cycle In general: Citric • 2 -C acetyl combines with 4 -C oxaloacetic acid to Glycolysis Oxidative acid phosphorylation cycle form a 6 -C citric acid. • Two carbons (per cycle) are oxidized to CO 2 which S Co. A regenerates a molecule of oxaloacetic acid C O • Each turn of the cycle uses up one acetyl group and CH regenerates a oxaloacetic acid, then begins the cycle Acetyl Co. A SH again. • Energy is released by breaking C-H and C-C bonds O C COO NADH COO CH 1 + H and is stored by transforming ADP to ATP (1 molecule CH COO + NAD per turn of the cycle) and to convert NAD+ to NADH 8 Oxaloacetate HO C COO CH and H+ (3 x per cycle) COO HO CH • Also, FAD (flavin adenine dinucleotide) is converted Citrate Figure 9. 12 CH Malate to FADH 2 (one molecule per cycle) COO 1. No oxygen is required in Krebs Cycle Citric - + + acid 2. All e and p are accepted by either NAD or FAD 7 HO 3 – + H 2 O – 2 – COO– 2 – CH 2 2 HC COO– – HO Isocitrate 2 – 2 • There are 8 steps in the cycle and the aim is to totally dismantle the Acetyl Co. A, using only its electrons. • We have totally taken apart the glucose molecule. • Only four ATPs have resulted, 2 from glycolysis and 2 from the CAC. • But we still have a lot of ELECTRONS captured in the form of NADH and FADH 2 = potential energy CH 2 Co. A SH 6 Co. A SH COO– FAD CH 2 C O COO– Succinate P i S Co. A GTP GDP Succinyl Co. A Figure 9. 12 ATP 4 C O COO– CH 2 5 CH 2 FADH 2 COO– NAD+ NADH + H+ a-Ketoglutarate CH 2 COO– ADP Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings NAD+ COO– Fumarate HC CO 2 3 cycle COO– CH CH COO– CO 2

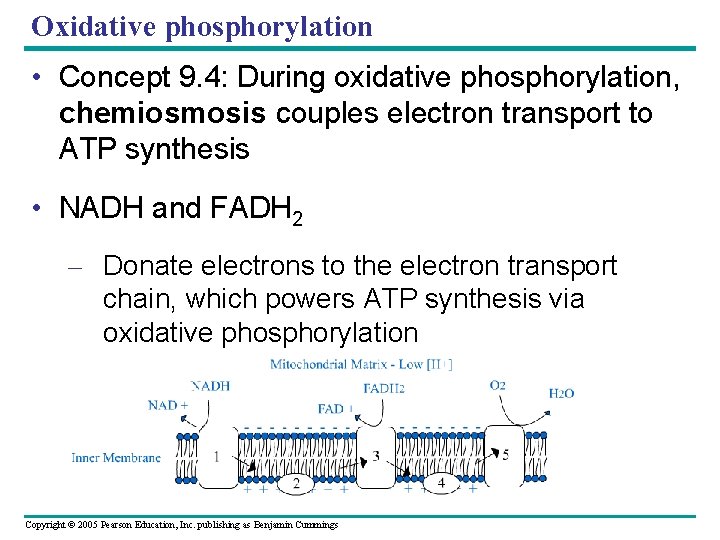
Oxidative phosphorylation • Concept 9. 4: During oxidative phosphorylation, chemiosmosis couples electron transport to ATP synthesis • NADH and FADH 2 – Donate electrons to the electron transport chain, which powers ATP synthesis via oxidative phosphorylation Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings

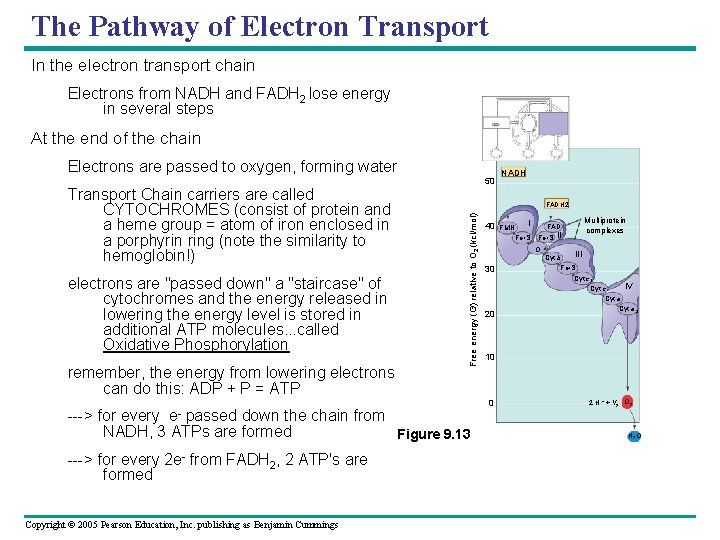
The Pathway of Electron Transport In the electron transport chain Electrons from NADH and FADH 2 lose energy in several steps At the end of the chain Electrons are passed to oxygen, forming water electrons are "passed down" a "staircase" of cytochromes and the energy released in lowering the energy level is stored in additional ATP molecules. . . called Oxidative Phosphorylation remember, the energy from lowering electrons can do this: ADP + P = ATP ---> for every e- passed down the chain from NADH, 3 ATPs are formed Figure 9. 13 ---> for every 2 e- from FADH 2, 2 ATP's are formed Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings NADH FADH 2 Free energy (G) relative to O 2 (kcl/mol) Transport Chain carriers are called CYTOCHROMES (consist of protein and a heme group = atom of iron enclosed in a porphyrin ring (note the similarity to hemoglobin!) 50 40 FMN I Fe • S II O 30 20 Multiprotein complexes FAD III Cyt b Fe • S Cyt c 1 IV Cyt c Cyt a 3 10 0 2 H + + 1 2 O 2 H 2 O

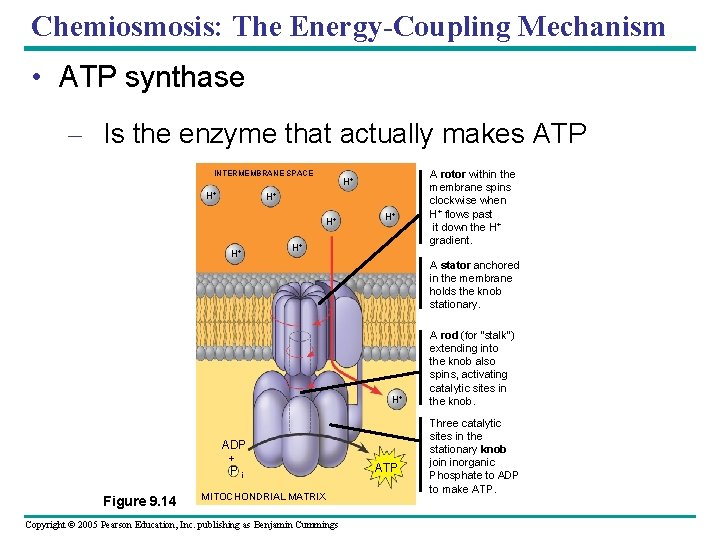
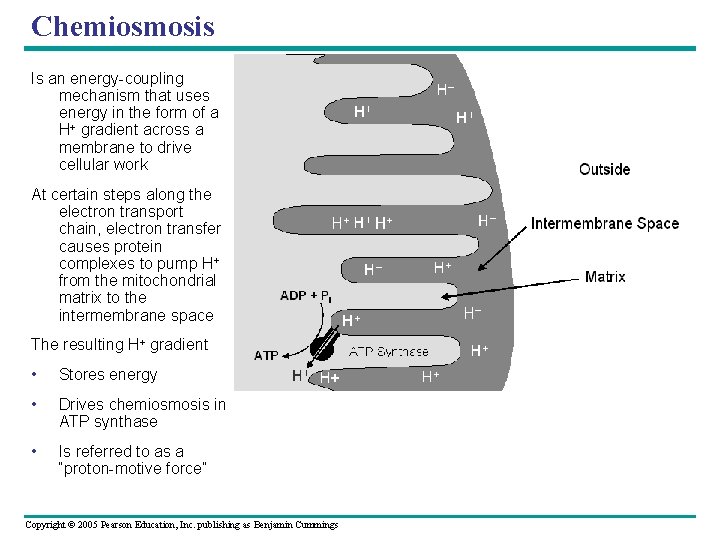
Chemiosmosis: The Energy-Coupling Mechanism • ATP synthase – Is the enzyme that actually makes ATP INTERMEMBRANE SPACE H+ H+ A stator anchored in the membrane holds the knob stationary. H+ ADP + P i Figure 9. 14 MITOCHONDRIAL MATRIX Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings A rotor within the membrane spins clockwise when H+ flows past it down the H+ gradient. ATP A rod (for “stalk”) extending into the knob also spins, activating catalytic sites in the knob. Three catalytic sites in the stationary knob join inorganic Phosphate to ADP to make ATP.

Chemiosmosis Is an energy-coupling mechanism that uses energy in the form of a H+ gradient across a membrane to drive cellular work At certain steps along the electron transport chain, electron transfer causes protein complexes to pump H+ from the mitochondrial matrix to the intermembrane space The resulting H+ gradient • Stores energy • Drives chemiosmosis in ATP synthase • Is referred to as a “proton-motive force” Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings

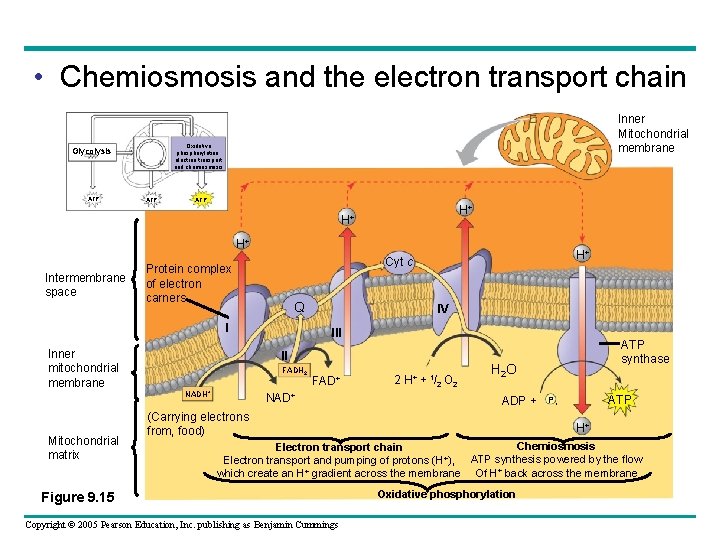
• Chemiosmosis and the electron transport chain Oxidative phosphorylation. electron transport and chemiosmosis Glycolysis ATP Inner Mitochondrial membrane ATP H+ H+ H+ Intermembrane space Protein complex of electron carners Q I Inner mitochondrial membrane IV III II FADH 2 NADH+ Mitochondrial matrix H+ Cyt c FAD+ NAD+ 2 H+ + 1/2 O 2 ATP synthase H 2 O ADP + (Carrying electrons from, food) ATP P i H+ Chemiosmosis Electron transport chain + ATP synthesis powered by the flow Electron transport and pumping of protons (H ), which create an H+ gradient across the membrane Of H+ back across the membrane Figure 9. 15 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Oxidative phosphorylation

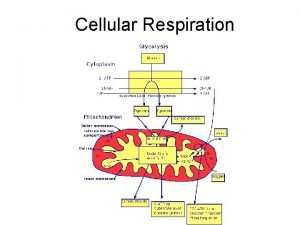
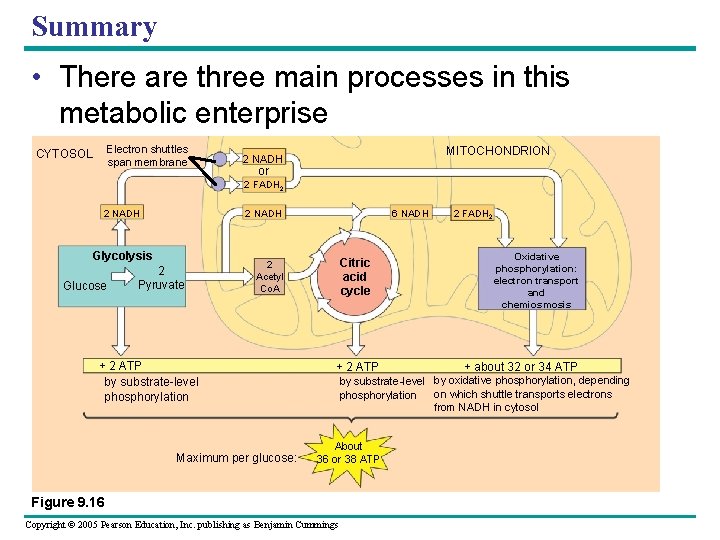
Summary • There are three main processes in this metabolic enterprise Electron shuttles span membrane CYTOSOL MITOCHONDRION 2 NADH or 2 FADH 2 2 NADH Glycolysis Glucose 2 Pyruvate 6 NADH Citric acid cycle 2 Acetyl Co. A + 2 ATP by substrate-level phosphorylation Maximum per glucose: + 2 ATP 2 FADH 2 Oxidative phosphorylation: electron transport and chemiosmosis + about 32 or 34 ATP by substrate-level by oxidative phosphorylation, depending on which shuttle transports electrons phosphorylation from NADH in cytosol About 36 or 38 ATP Figure 9. 16 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings

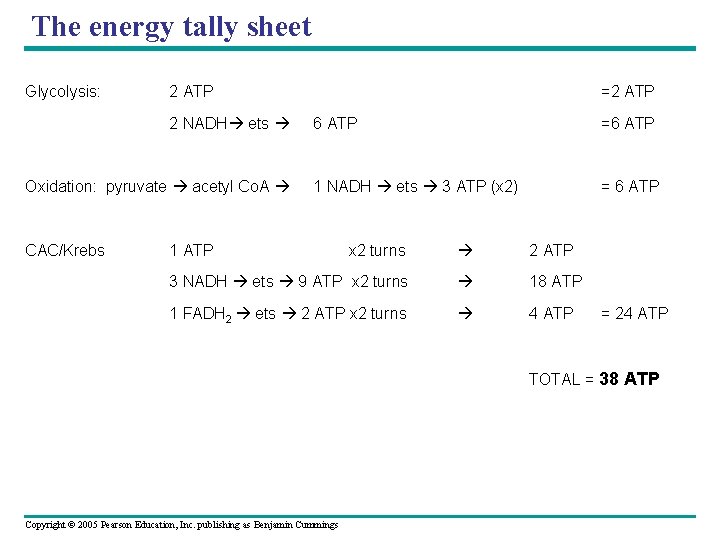
The energy tally sheet Glycolysis: 2 ATP 2 NADH ets =2 ATP 6 ATP =6 ATP Oxidation: pyruvate acetyl Co. A 1 NADH ets 3 ATP (x 2) = 6 ATP CAC/Krebs x 2 turns 2 ATP 3 NADH ets 9 ATP x 2 turns 18 ATP 1 FADH 2 ets 2 ATP x 2 turns 4 ATP 1 ATP = 24 ATP TOTAL = 38 ATP Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings

efficiency • Less than 40% of the energy in a glucose molecule is transferred to ATP during cellular respiration, making approximately 38 ATP DG = ADP + Pi --> ATP = about 7 kcal/mole X 38 = 266 kcal/mole (captured in P bonds) DG = -686 kcal/mole (free energy change during glycolysis and oxidation for glucose) = about 39% efficiency Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings

FERMENTATION • Concept 9. 5: Fermentation enables some cells to produce ATP without the use of oxygen • Oxidative cellular respiration – Relies on oxygen to produce ATP – Involves Krebs cycle and ETS • In the absence of oxygen – Cells can still produce a little bit of ATP through fermentation Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings

Without oxygen: • Glycolysis – Can produce ATP with or without oxygen, in either aerobic or anaerobic conditions – Pyruvate is produced, some ATP is made In anaerobic respiration (fermentation) pyruvate must be processed in the absence of oxygen • The breakdown of the sugar still takes place through a series of chemical reactions, but with a different outcome. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings

The Evolutionary Significance of Glycolysis • Glycolysis – Occurs in the cytoplasm of nearly all organisms – Probably evolved in ancient prokaryotes before there was oxygen in the atmosphere Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings

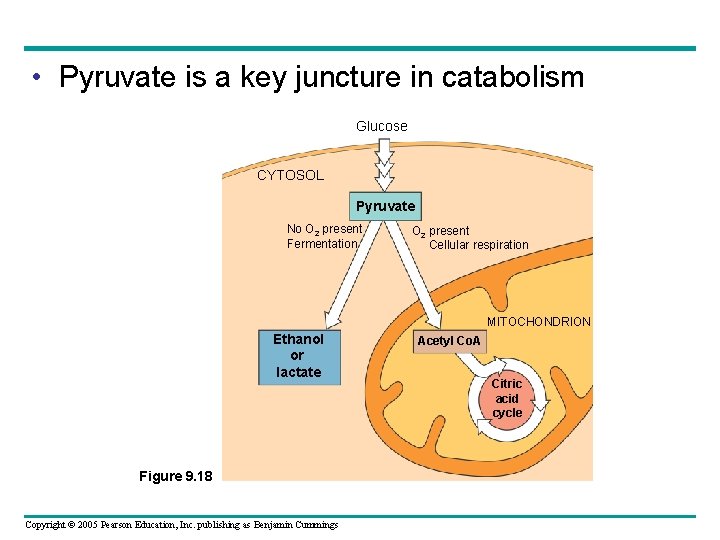
• Pyruvate is a key juncture in catabolism Glucose CYTOSOL Pyruvate No O 2 present Fermentation O 2 present Cellular respiration MITOCHONDRION Ethanol or lactate Figure 9. 18 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Acetyl Co. A Citric acid cycle

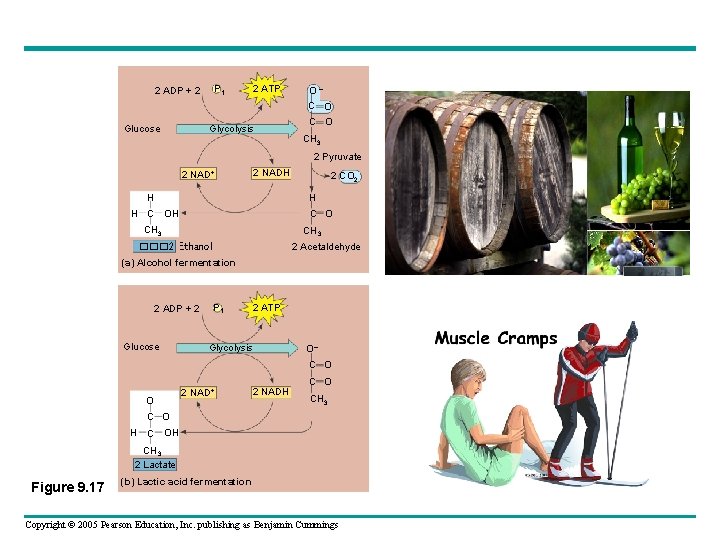
Fermentation • Living organisms have developed numerous and different fermentation pathways; however, most organisms use the following Embden-Meyerhoff pathway, named for the two discoverers. • The pyruvate can take two pathways in anaerobic respiration: – a. Pyruvate will be converted to ethyl alcohol (ethanol) and carbon dioxide. This is called alcoholic fermentation and is the basis of our wine, beer and liquor industry. – b. The pyruvate will be converted to lactic acid. This is called lactic acid fermentation. Lactic acid is what makes your muscles burn during prolonged exercise, this process is also used to make yogurt. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings

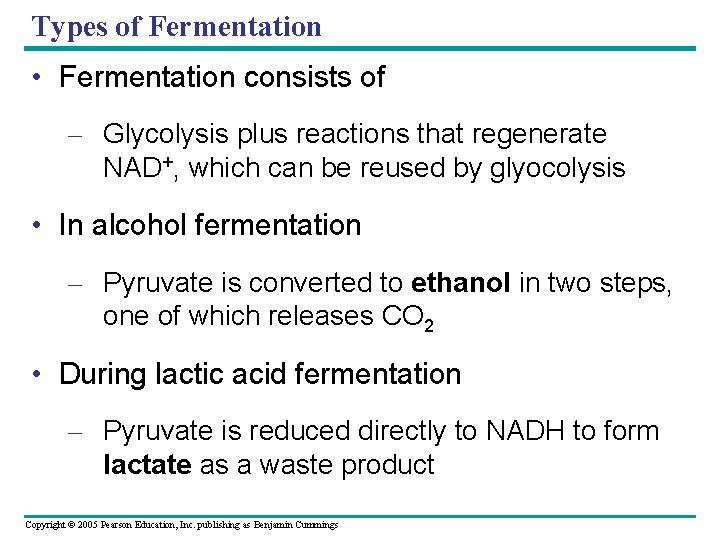
Types of Fermentation • Fermentation consists of – Glycolysis plus reactions that regenerate NAD+, which can be reused by glyocolysis • In alcohol fermentation – Pyruvate is converted to ethanol in two steps, one of which releases CO 2 • During lactic acid fermentation – Pyruvate is reduced directly to NADH to form lactate as a waste product Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings

P 1 2 ADP + 2 2 ATP O – C O Glucose Glycolysis C O CH 3 2 Pyruvate 2 NAD+ 2 NADH H 2 CO 2 H H C O CH 3 2 Acetaldehyde ��� 2 Ethanol (a) Alcohol fermentation 2 ADP + 2 Glucose P 1 2 ATP Glycolysis O– C O O 2 NAD+ 2 NADH C O CH 3 C O H C OH CH 3 2 Lactate Figure 9. 17 (b) Lactic acid fermentation Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings

Fermentation and Cellular Respiration Compared • Both fermentation and cellular respiration – Use glycolysis to oxidize glucose and other organic fuels to pyruvate • Fermentation and cellular respiration – Differ in their final electron acceptor • Oxidative cellular respiration – Produces more ATP Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings

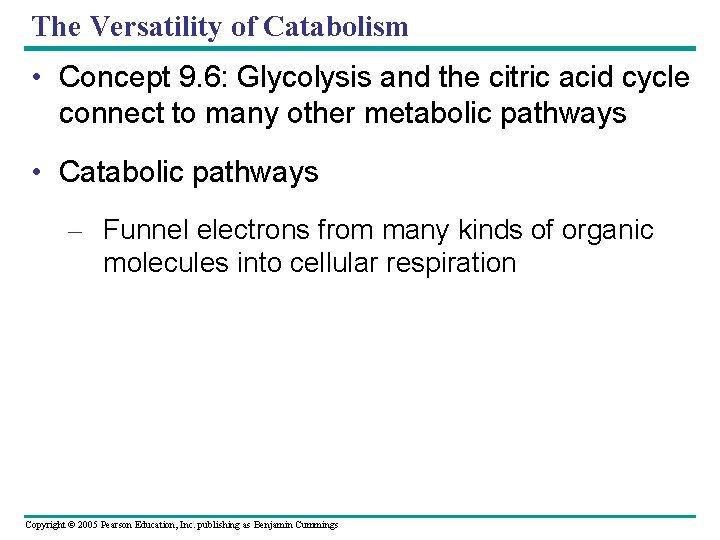
The Versatility of Catabolism • Concept 9. 6: Glycolysis and the citric acid cycle connect to many other metabolic pathways • Catabolic pathways – Funnel electrons from many kinds of organic molecules into cellular respiration Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings

• The catabolism of various molecules from food Proteins Carbohydrates Amino acids Sugars Fats Glycerol Glycolysis Glucose Glyceraldehyde-3 - P NH 3 Pyruvate Acetyl Co. A Citric acid cycle Figure 9. 19 Oxidative phosphorylation Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Fatty acids

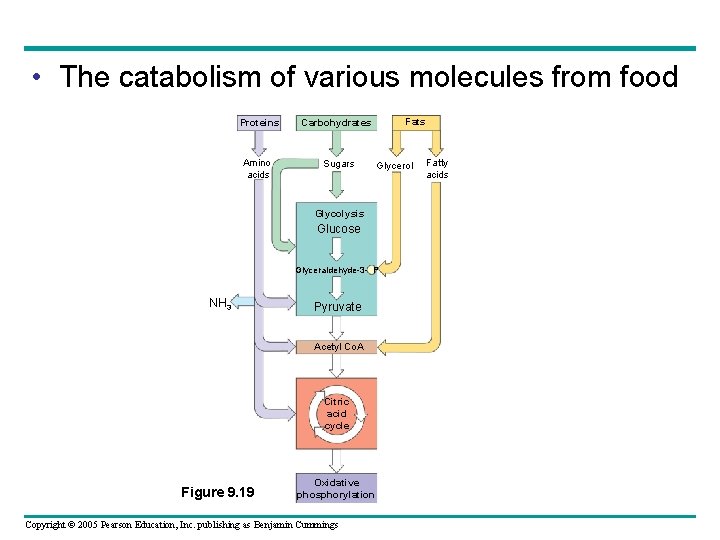
Regulation of Cellular Respiration via Feedback Mechanisms • Cellular respiration – Is controlled by allosteric enzymes at key points in glycolysis and the citric acid cycle Enzyme 1 A Enzyme 2 D C B Reaction 1 Enzyme 3 Reaction 2 Starting molecule Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Reaction 3 Product

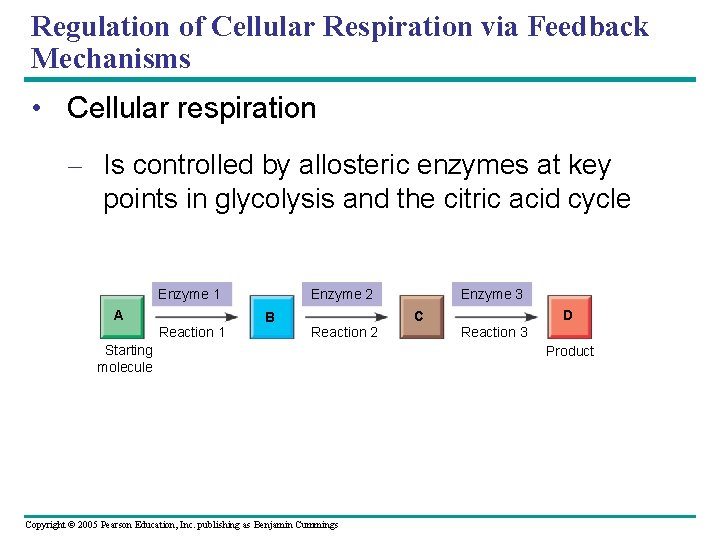
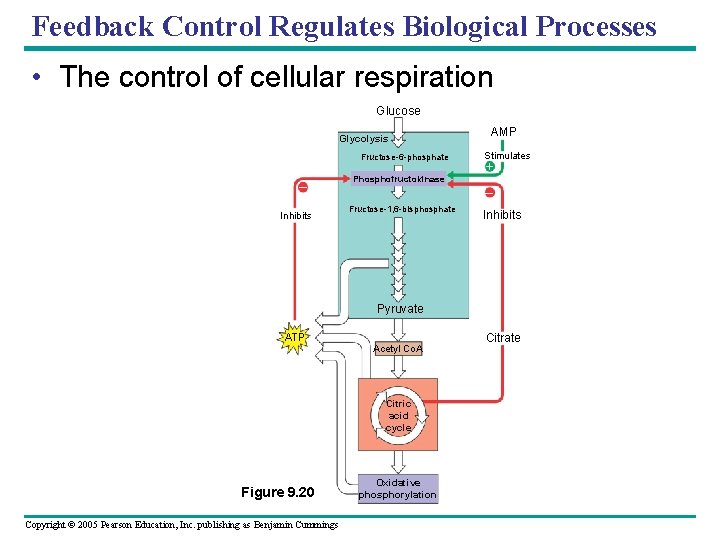
Feedback Control Regulates Biological Processes • The control of cellular respiration Glucose Glycolysis Fructose-6 -phosphate – Inhibits Phosphofructokinase AMP Stimulates + – Fructose-1, 6 -bisphosphate Inhibits Pyruvate ATP Acetyl Co. A Citric acid cycle Figure 9. 20 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Oxidative phosphorylation Citrate
 The stages of cellular respiration
The stages of cellular respiration Explain how amp stimulates cellular respiration
Explain how amp stimulates cellular respiration Chapter 9: cellular respiration: harvesting chemical energy
Chapter 9: cellular respiration: harvesting chemical energy Electron transport chain summary
Electron transport chain summary What's the equation for cellular respiration
What's the equation for cellular respiration Formula for cellular respiration
Formula for cellular respiration Cellular respiration chemical equation
Cellular respiration chemical equation Inputs of glycolysis
Inputs of glycolysis Consumer
Consumer Cellular respiration obtaining energy from food
Cellular respiration obtaining energy from food Cellular respiration obtaining energy from food
Cellular respiration obtaining energy from food Chapter 8 section 3 cellular respiration
Chapter 8 section 3 cellular respiration Chapter 8 section 1 how organisms obtain energy
Chapter 8 section 1 how organisms obtain energy Redox reaction in cellular respiration
Redox reaction in cellular respiration The gray-brown haze often found over large cities is called
The gray-brown haze often found over large cities is called Chemiosmosis steps
Chemiosmosis steps Cellular respiration product
Cellular respiration product Fermentation diagram
Fermentation diagram Why is cellular respiration important
Why is cellular respiration important Complimentary processes
Complimentary processes Amloplast
Amloplast Cellular respiration equation
Cellular respiration equation Starting materials for cellular respiration
Starting materials for cellular respiration What happens during glycolysis
What happens during glycolysis Overview of cellular respiration
Overview of cellular respiration Overview of cellular respiration
Overview of cellular respiration Cellular respiration
Cellular respiration Cellular respiration
Cellular respiration Lab bench cellular respiration
Lab bench cellular respiration What is the word equation for cellular respiration
What is the word equation for cellular respiration Higher human biology cellular respiration
Higher human biology cellular respiration Function of cellular respiration
Function of cellular respiration Cellular respiration releases
Cellular respiration releases Starting materials for cellular respiration
Starting materials for cellular respiration Redox reaction in cellular respiration
Redox reaction in cellular respiration Where in the cell does cellular respiration occur
Where in the cell does cellular respiration occur Role of cellular respiration
Role of cellular respiration Cellular respiration releases
Cellular respiration releases Cellular respiration redox
Cellular respiration redox Respiration crash course
Respiration crash course Aerobic cellular respiration equation
Aerobic cellular respiration equation Process of cellular respiration
Process of cellular respiration Overall reaction of cellular respiration
Overall reaction of cellular respiration Krebs cycle occurs in
Krebs cycle occurs in Electron transport chain cellular respiration
Electron transport chain cellular respiration Where does cellular respiration take place
Where does cellular respiration take place Function of cellular respiration
Function of cellular respiration Cellular respiration endothermic or exothermic
Cellular respiration endothermic or exothermic Does fermentation occur in the mitochondria
Does fermentation occur in the mitochondria