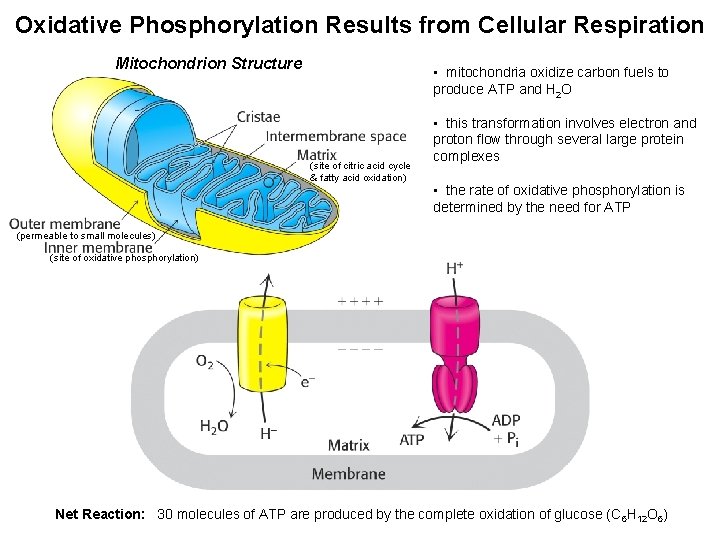
Oxidative Phosphorylation Results from Cellular Respiration Mitochondrion Structure
- Slides: 7

Oxidative Phosphorylation Results from Cellular Respiration Mitochondrion Structure • mitochondria oxidize carbon fuels to produce ATP and H 2 O (site of citric acid cycle & fatty acid oxidation) • this transformation involves electron and proton flow through several large protein complexes • the rate of oxidative phosphorylation is determined by the need for ATP (permeable to small molecules) (site of oxidative phosphorylation) Net Reaction: 30 molecules of ATP are produced by the complete oxidation of glucose (C 6 H 12 O 6)

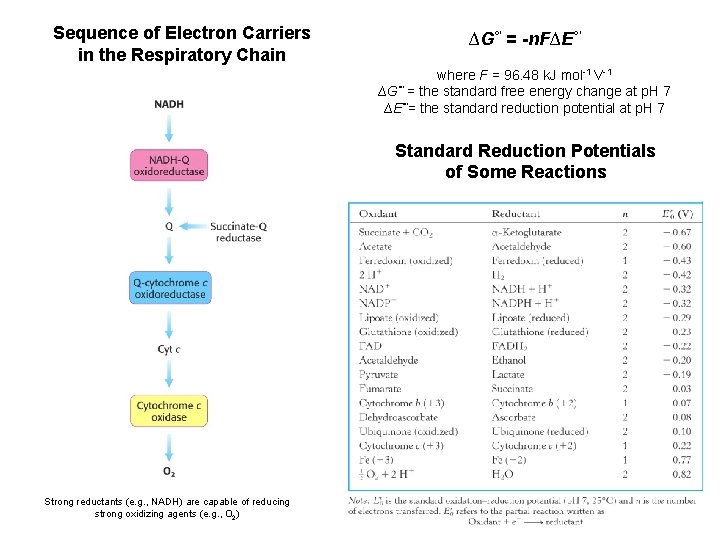
Sequence of Electron Carriers in the Respiratory Chain ∆G°' = -n. F∆E°' where F = 96. 48 k. J mol-1 V-1 ∆G°' = the standard free energy change at p. H 7 ∆E°'= the standard reduction potential at p. H 7 Standard Reduction Potentials of Some Reactions Strong reductants (e. g. , NADH) are capable of reducing strong oxidizing agents (e. g. , O 2)

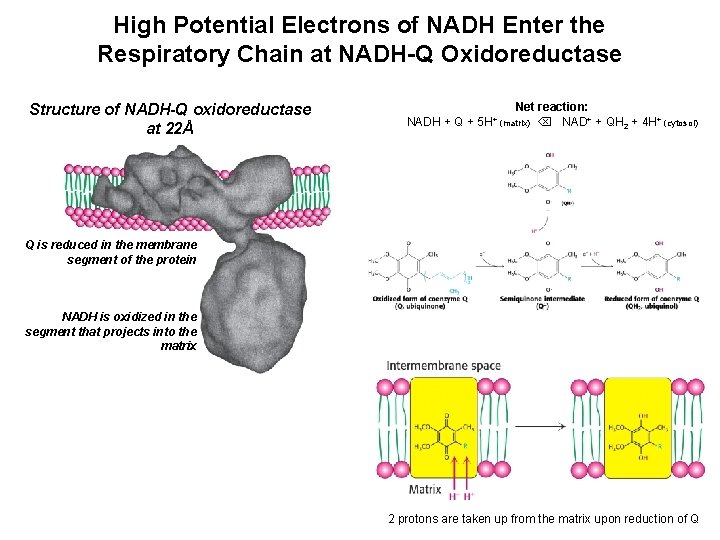
High Potential Electrons of NADH Enter the Respiratory Chain at NADH-Q Oxidoreductase Structure of NADH-Q oxidoreductase at 22Å NADH + Q + Net reaction: NAD+ + QH 2 + 4 H+ (cytosol) 5 H+ (matrix) Q is reduced in the membrane segment of the protein NADH is oxidized in the segment that projects into the matrix 2 protons are taken up from the matrix upon reduction of Q

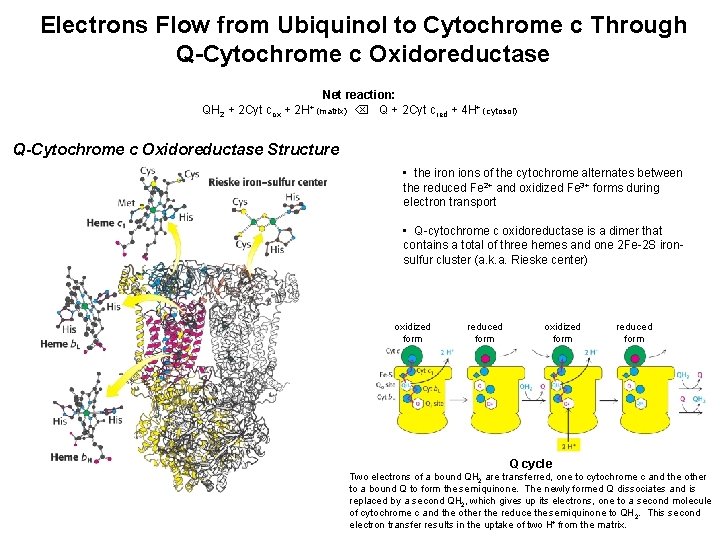
Electrons Flow from Ubiquinol to Cytochrome c Through Q-Cytochrome c Oxidoreductase QH 2 + 2 Cyt cox + Net reaction: Q + 2 Cyt cred + 4 H+ (cytosol) 2 H+ (matrix) Q-Cytochrome c Oxidoreductase Structure • the iron ions of the cytochrome alternates between the reduced Fe 2+ and oxidized Fe 3+ forms during electron transport • Q-cytochrome c oxidoreductase is a dimer that contains a total of three hemes and one 2 Fe-2 S ironsulfur cluster (a. k. a. Rieske center) oxidized form reduced form Q cycle Two electrons of a bound QH 2 are transferred, one to cytochrome c and the other to a bound Q to form the semiquinone. The newly formed Q dissociates and is replaced by a second QH 2, which gives up its electrons, one to a second molecule of cytochrome c and the other the reduce the semiquinone to QH 2. This second electron transfer results in the uptake of two H+ from the matrix.

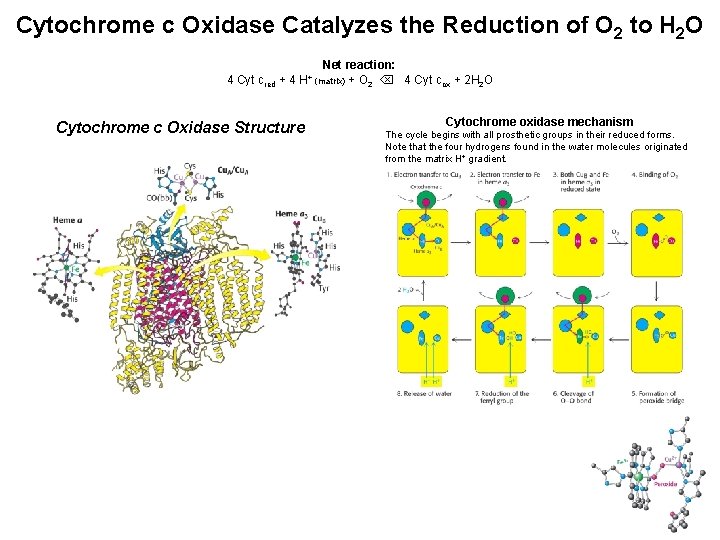
Cytochrome c Oxidase Catalyzes the Reduction of O 2 to H 2 O Net reaction: 4 Cyt cred + 4 H+ (matrix) + O 2 4 Cyt cox + 2 H 2 O Cytochrome c Oxidase Structure Cytochrome oxidase mechanism The cycle begins with all prosthetic groups in their reduced forms. Note that the four hydrogens found in the water molecules originated from the matrix H+ gradient.

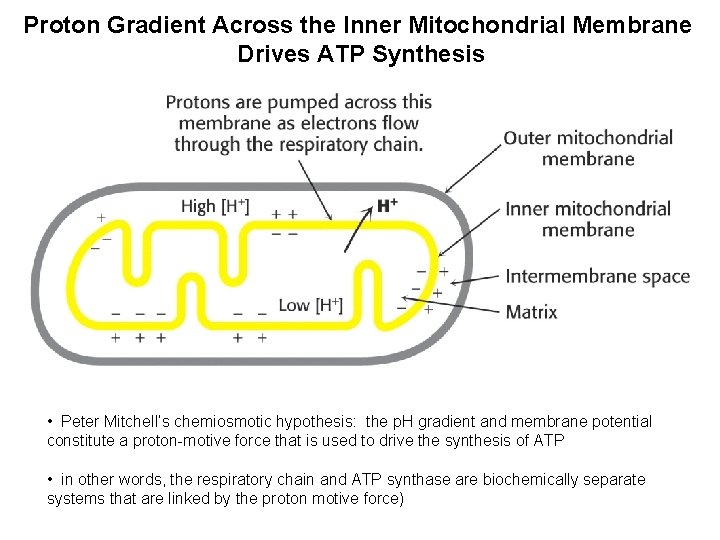
Proton Gradient Across the Inner Mitochondrial Membrane Drives ATP Synthesis • Peter Mitchell’s chemiosmotic hypothesis: the p. H gradient and membrane potential constitute a proton-motive force that is used to drive the synthesis of ATP • in other words, the respiratory chain and ATP synthase are biochemically separate systems that are linked by the proton motive force)

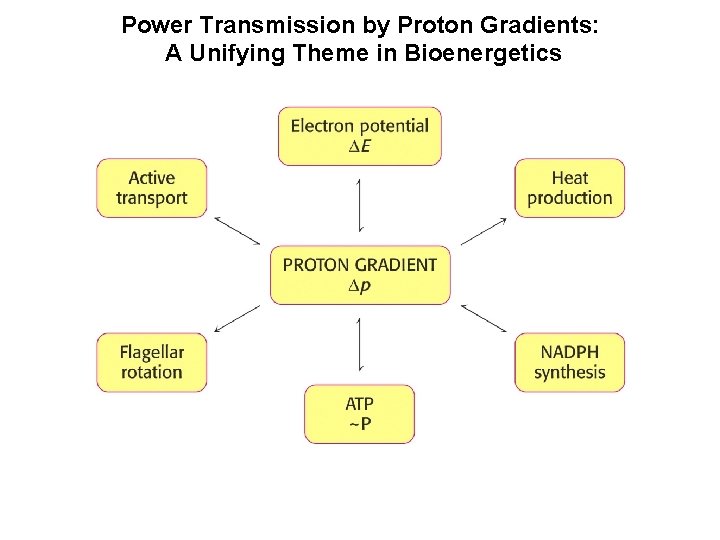
Power Transmission by Proton Gradients: A Unifying Theme in Bioenergetics