CCM presentation The forgotten area in ICU Fertility













- Slides: 71

CCM presentation The forgotten area in ICU …………. . Fertility? Dr. HK Tsang TMH ICU Resident

Case presentation n n 55/F Housewife Exsmoker and non drinker Lives with family, ADLI

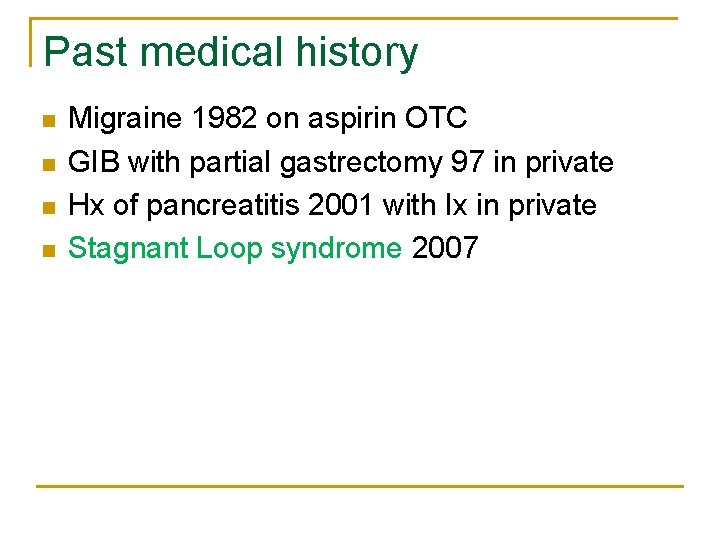
Past medical history n n Migraine 1982 on aspirin OTC GIB with partial gastrectomy 97 in private Hx of pancreatitis 2001 with Ix in private Stagnant Loop syndrome 2007

Past medical history n Stagnant loop syndrome 2007 q Presented with diarrhoea x 3/12 n BO 3 -5 x/day n No Mucus/PR bleeding/tenesmus n Subjective weight loss for few lbs n Abd distension n Ankle edema

Past medical history q Initial Ix n n Albumin 17, ALP 200, ALT 53, normal bilirubin 24 hr urine TP 0. 3 g/d Stool q WCC/RBC/Ova and cyst/C/ST/fat globulin/FOB neg Blood q Hepatitis serology, CMV pp 65 neg q Autoimmune markers/Ig pattern/Anit. SM/AMA neg q Tumour markers normal q TSH normal

Past medical history n Colonoscopy 11/07: colitis from transverse colon downward q Histology: lymphocytic infiltrate, no cryptitis/crypt abscess/viral inclusion/malignancy n CT abd: gross ascites and thickened colonic wall suggestive of colitis n OGD: Food residue+Previous BII with clear base GU q q n Imp q q q n Bx: active chronic inflammation, no villus atrophy Duodenal aspiration: AFB smear neg, heavy growth of GB/GN bacilli (Aeromonas caviae, E. Coli, Enterococcus, Bacteroides) Bacterial overgrowth Aspirin induced lymphocytic colitis d. LFT secondary to poor nutrition and starvation or PSC secondary to IBD Given ciproxin and flagyl symptoms improved

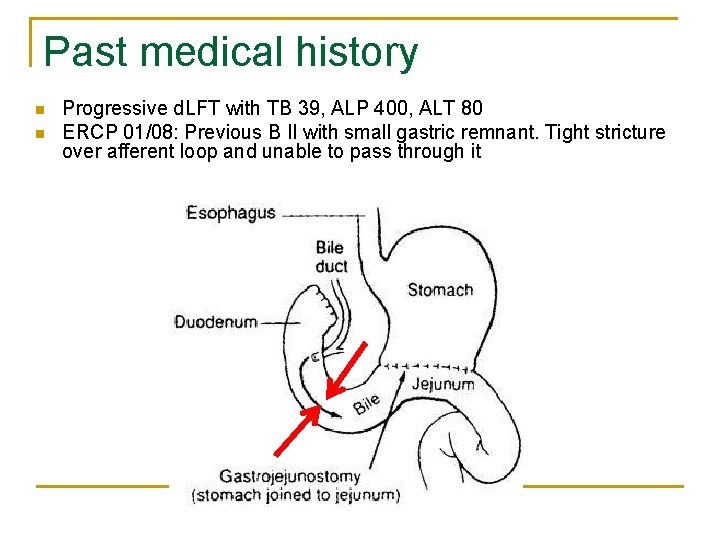
Past medical history n n Progressive d. LFT with TB 39, ALP 400, ALT 80 ERCP 01/08: Previous B II with small gastric remnant. Tight stricture over afferent loop and unable to pass through it

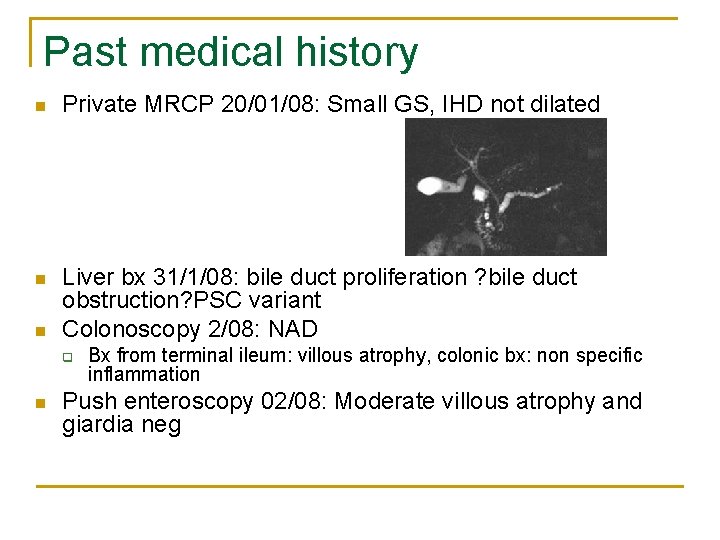
Past medical history n Private MRCP 20/01/08: Small GS, IHD not dilated n Liver bx 31/1/08: bile duct proliferation ? bile duct obstruction? PSC variant Colonoscopy 2/08: NAD n q n Bx from terminal ileum: villous atrophy, colonic bx: non specific inflammation Push enteroscopy 02/08: Moderate villous atrophy and giardia neg

Past medical history n n Xylose absorption test borderline normal 5 days stool x Alpha antitrypsin clearance study in QMH 18 (NR <13) q n Suggestive of Protein losing enteropathy Albumin scan and small bowel enema scheduled 04/08

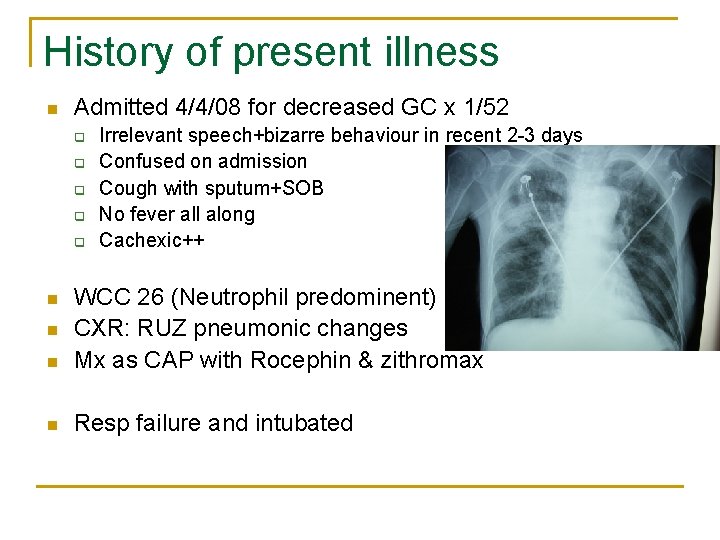
History of present illness n Admitted 4/4/08 for decreased GC x 1/52 q q q Irrelevant speech+bizarre behaviour in recent 2 -3 days Confused on admission Cough with sputum+SOB No fever all along Cachexic++ n WCC 26 (Neutrophil predominent) CXR: RUZ pneumonic changes Mx as CAP with Rocephin & zithromax n Resp failure and intubated n n

History of present illness n Tracheal aspirate q C/ST, TB PCR, AFB smear, Influenza/parainfluenza: neg n Urine x Legionella Ag neg Mycoplasma <10 Blood x C/ST: neg n TPN n n q n n n 5/4/08 Clinomel NT 1000 Clinically improved with good ventilation & oxygenation Sedation off 07/4/08 Remained comatose>48 hrs ? Reason

? Reasons of coma n n C-CO 2 narcosis O-Overdose of medications/Sedations M-Metabolic: Hypoglycaemia, DKA, hypothyroidism, hypercalcaemia, adrenal failure, uraemia, hepatic coma A-Apoplexy: HI, CVA, ICH, CNS infection, epilepsy

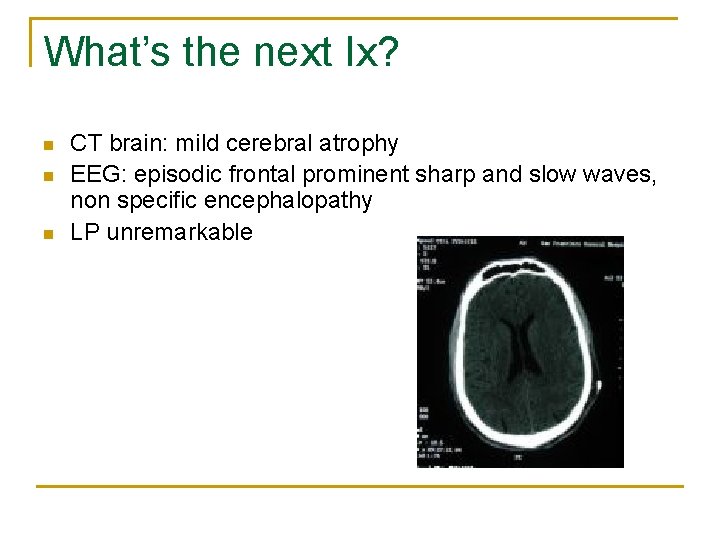
What’s the next Ix? n n n CT brain: mild cerebral atrophy EEG: episodic frontal prominent sharp and slow waves, non specific encephalopathy LP unremarkable

Reasons of coma n A blood test was performed

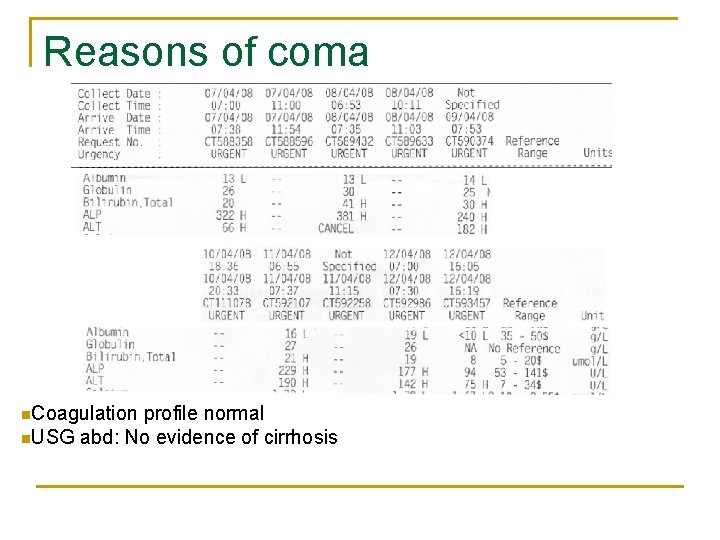
Reasons of coma

Reasons of coma n. Coagulation profile normal n. USG abd: No evidence of cirrhosis

History n Stopped and given patient some Px n Extubated 11/04/08 Sitting out, watching TV ? Happy ending n n

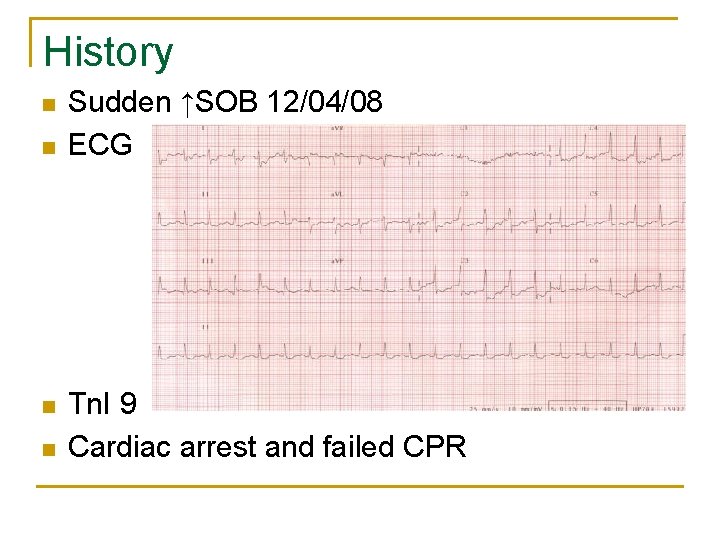
History n n Sudden ↑SOB 12/04/08 ECG Tn. I 9 Cardiac arrest and failed CPR

The forgotten area in ICU……Fertility? Hyperammonemia in the ICU

Ammonia and fertility

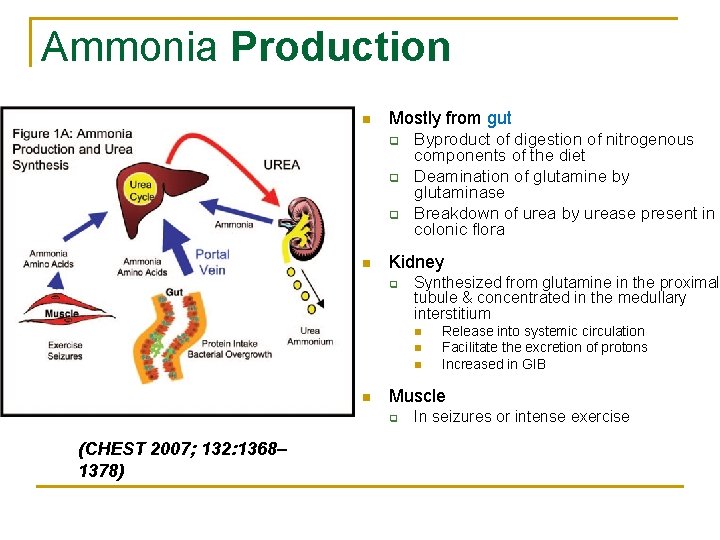
Ammonia Production n Mostly from gut q q q n Byproduct of digestion of nitrogenous components of the diet Deamination of glutamine by glutaminase Breakdown of urea by urease present in colonic flora Kidney q Synthesized from glutamine in the proximal tubule & concentrated in the medullary interstitium n n Muscle q (CHEST 2007; 132: 1368– 1378) Release into systemic circulation Facilitate the excretion of protons Increased in GIB In seizures or intense exercise

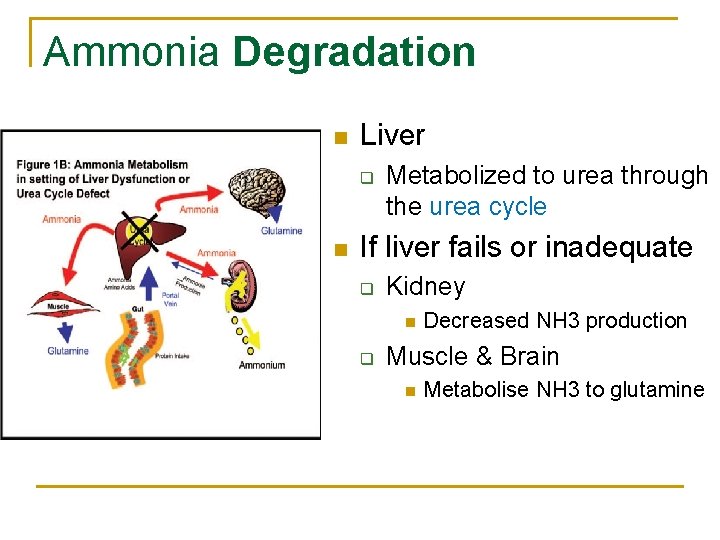
Ammonia Degradation n Liver q n Metabolized to urea through the urea cycle If liver fails or inadequate q Kidney n q Decreased NH 3 production Muscle & Brain n Metabolise NH 3 to glutamine

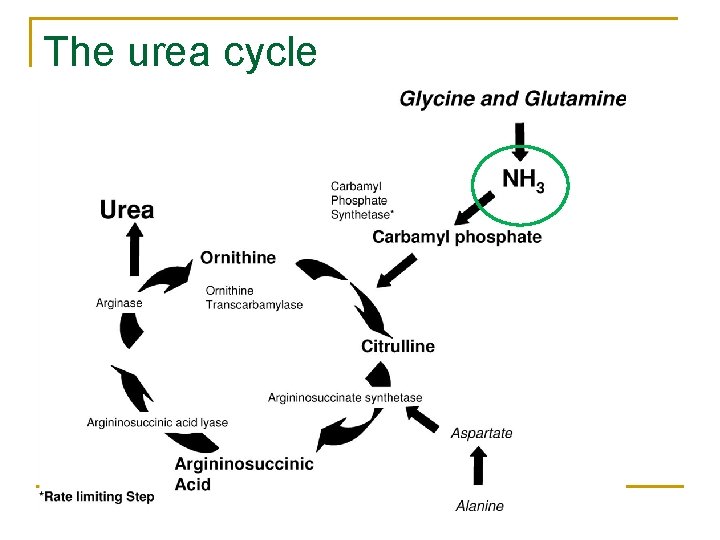
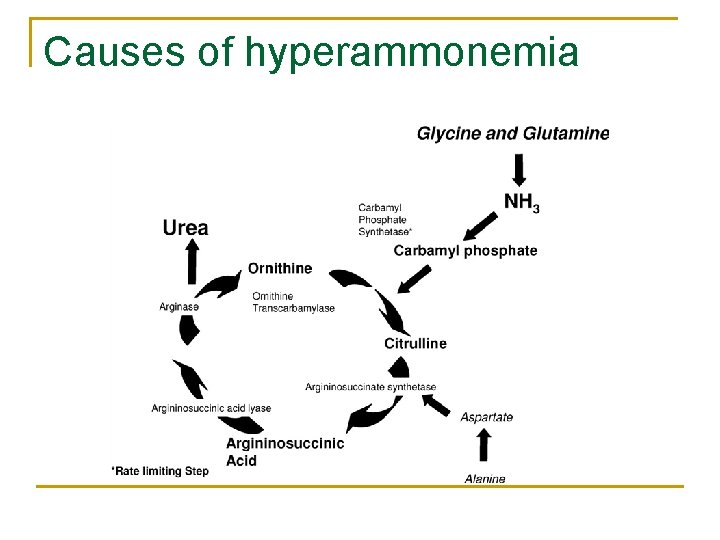
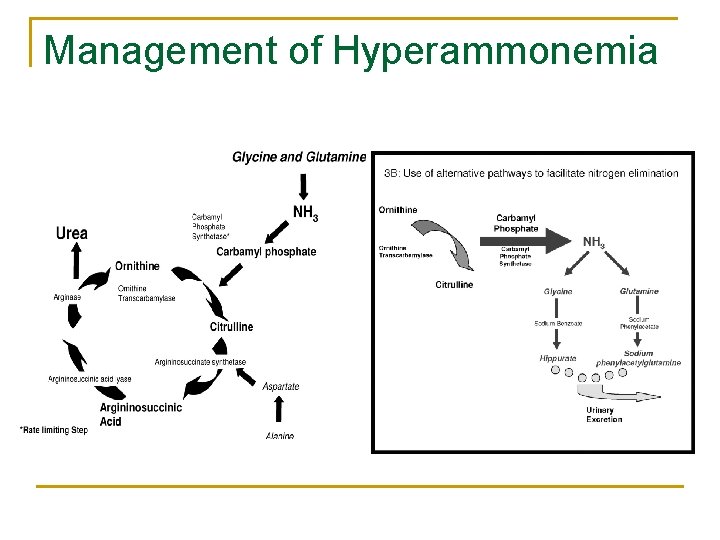
The urea cycle

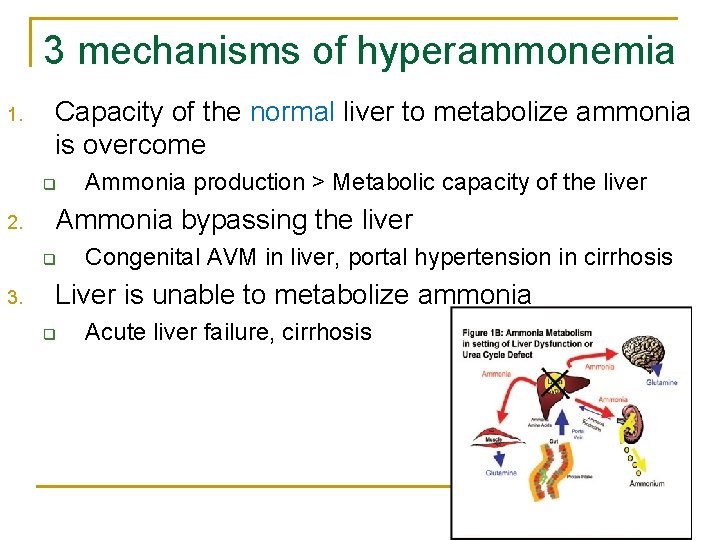
3 mechanisms of hyperammonemia 1. Capacity of the normal liver to metabolize ammonia is overcome q 2. Ammonia bypassing the liver q 3. Ammonia production > Metabolic capacity of the liver Congenital AVM in liver, portal hypertension in cirrhosis Liver is unable to metabolize ammonia q Acute liver failure, cirrhosis

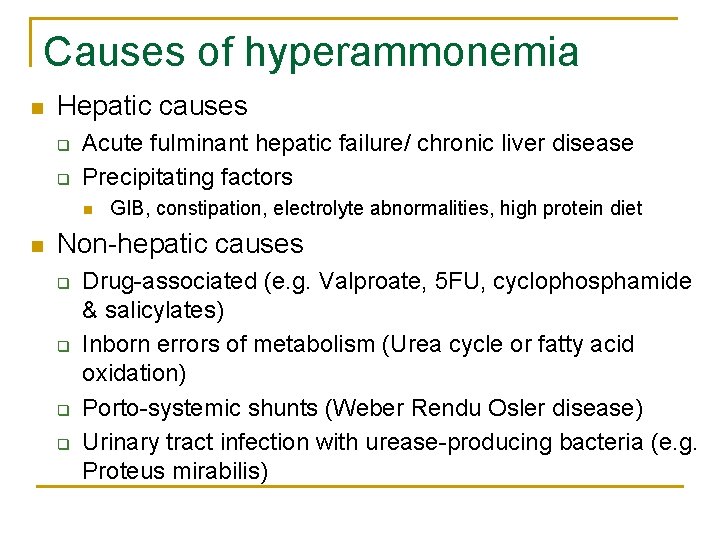
Causes of hyperammonemia n Hepatic causes q q Acute fulminant hepatic failure/ chronic liver disease Precipitating factors n n GIB, constipation, electrolyte abnormalities, high protein diet Non-hepatic causes q q Drug-associated (e. g. Valproate, 5 FU, cyclophosphamide & salicylates) Inborn errors of metabolism (Urea cycle or fatty acid oxidation) Porto-systemic shunts (Weber Rendu Osler disease) Urinary tract infection with urease-producing bacteria (e. g. Proteus mirabilis)

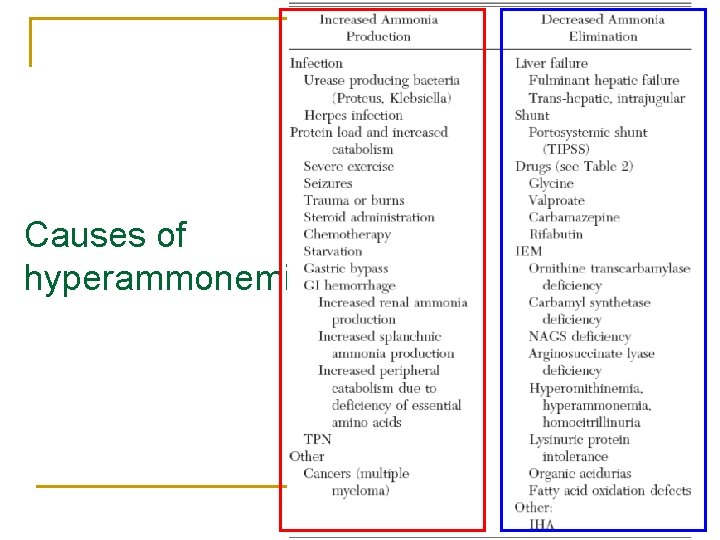
Causes of hyperammonemia

Causes of hyperammonemia n n n Fulminant liver failure Drugs IEM Infection Idiopathic

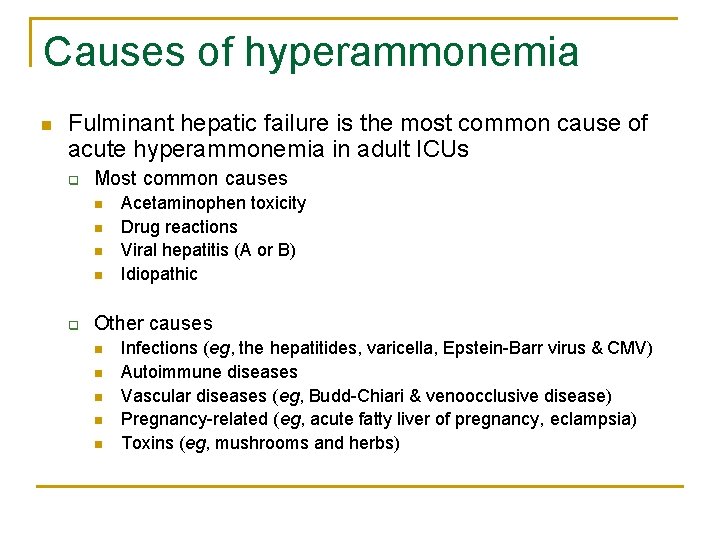
Causes of hyperammonemia n Fulminant hepatic failure is the most common cause of acute hyperammonemia in adult ICUs q Most common causes n n q Acetaminophen toxicity Drug reactions Viral hepatitis (A or B) Idiopathic Other causes n n n Infections (eg, the hepatitides, varicella, Epstein-Barr virus & CMV) Autoimmune diseases Vascular diseases (eg, Budd-Chiari & venoocclusive disease) Pregnancy-related (eg, acute fatty liver of pregnancy, eclampsia) Toxins (eg, mushrooms and herbs)

Causes of hyperammonemia n n n Fulminant liver failure Drugs IEM Infection Idiopathic

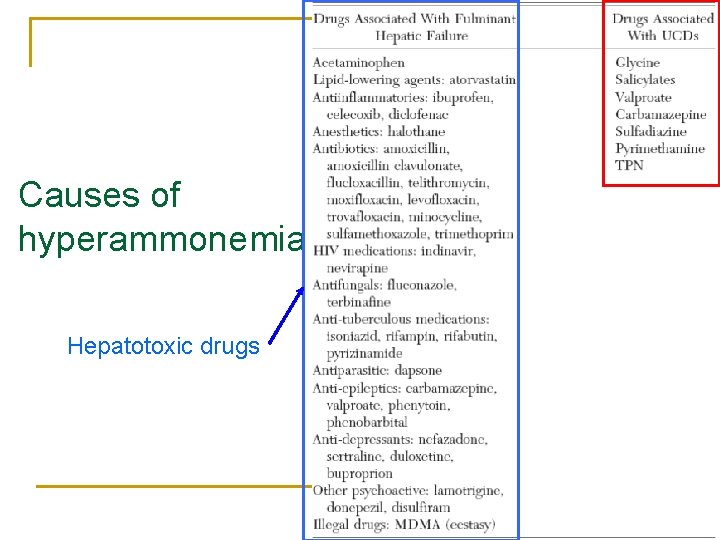
Causes of hyperammonemia Hepatotoxic drugs

Causes of hyperammonemia n Several drugs cause hyperammonemia by disrupting the urea cycle q q q Glycine stimulates ammonia production Salicylates can reduce mitochondrial function in the liver e. g. Reye syndrome Carbamazepine, ribavirin, sulfadiazine with pyrimethamine: Mechanisms not known

Causes of hyperammonemia n Valproate may rarely cause hyperammonemic coma q In chronic dosing n n q In healthy patients n q Asymptomatic hyperammonemia occurs in 50% of patients Chronic use leads to carnitine deficiency, impairs urea cycle Overdose increases propionic acid levels, which inhibit mitochrondrial CPS In heterozygote females with asymptomatic OTC deficiency, therapeutic doses of valproate may also cause acute hyperammonemia

Causes of hyperammonemia n n n Fulminant liver failure Drugs IEM Infection Idiopathic

Causes of hyperammonemia

Causes of hyperammonemia n Inborn errors of metabolism (IEM) q Most common UCDs in adults n q q Hyperammonemia is most severe when the enzyme defect occurs in the early steps of the urea cycle (eg OTC deficiency) Clinical presentations of different IEM are quite similar n n q OTC deficiency (X-linked), ASS deficiency(AR), and carbamyl phosphate deficiency(AR) In the fulminant form, coma and encephalopathy In the milder forms, intermittent periods of confusion or bizarre behavior, presumably from hyperammonemia May present in adulthood when unmasked by precipitants

Causes of hyperammonemia n Inborn errors of metabolism (IEM) q Physiologic stressors that provoke hyperammonemia n Infection: urease-splitting organisms, URI or pneumonia Dietary changes Fever Pregnancy GI bleeding Insults to the liver, eg alcohol or acetaminophen n TPN n n n q q q Provides more protein than consumes enterally Provoked hyperammonemia in many patients with UCDs, most often OTC The presence of hyperammonemia following TPN should prompt an investigation of a UCD

Causes of hyperammonemia n Inborn errors of metabolism q Other presentations n Seizure disorders, including complex partial seizures n A history of repetitive or cyclical vomiting n Intellectual limitations n Prolonged clinical course with a seemingly routine illness n Family history of early infant mortality n Voluntarily limit their protein intake (called autovegetarianism) to avoid postprandial headaches or somnolence q Patients with citrullinemia (ie, ASS deficiency) often have a history of preferring beans, provide arginine which is an essential amino acid in these patients

Causes of hyperammonemia n n n Fulminant liver failure Drugs IEM Infection Idiopathic

Causes of hyperammonemia n Urea splitting urinary tract infection q q q Urea splitting organism e. g. Proteus mirabilis, Pseudomonas aeruginosa, Klebsiella Cause rise in urine ammonia conc Prerequisite of hyperammonaemia n n Distended bladder with large surface area for NH 3 diffusion e. g. bladder or pouch retention Diffusion facilitated by alkaline urine

Causes of hyperammonemia n n n Fulminant liver failure Drugs IEM Infection Idiopathic

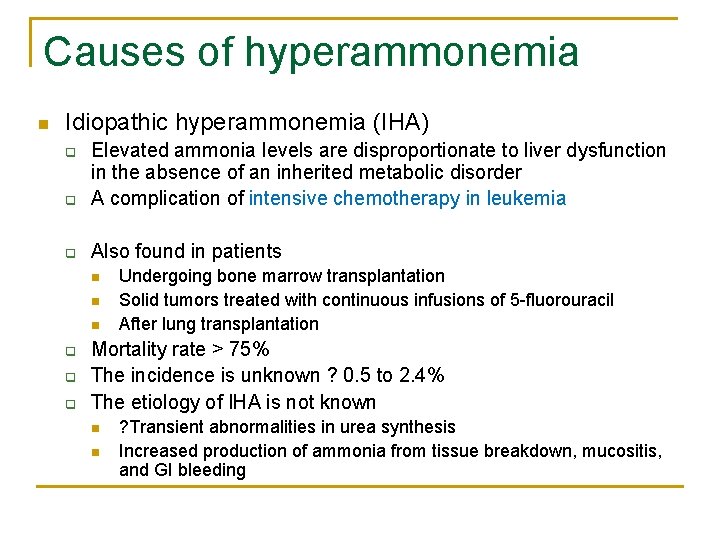
Causes of hyperammonemia n Idiopathic hyperammonemia (IHA) q Elevated ammonia levels are disproportionate to liver dysfunction in the absence of an inherited metabolic disorder A complication of intensive chemotherapy in leukemia q Also found in patients q n n n q q q Undergoing bone marrow transplantation Solid tumors treated with continuous infusions of 5 -fluorouracil After lung transplantation Mortality rate > 75% The incidence is unknown ? 0. 5 to 2. 4% The etiology of IHA is not known n n ? Transient abnormalities in urea synthesis Increased production of ammonia from tissue breakdown, mucositis, and GI bleeding

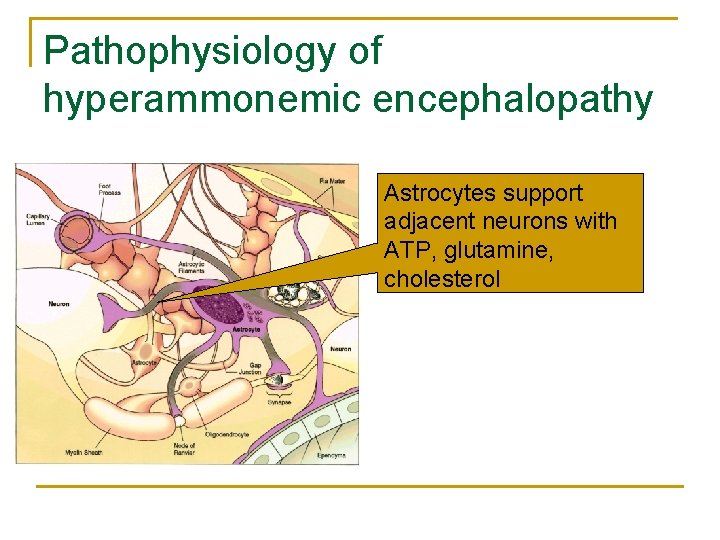
Pathophysiology of hyperammonemic encephalopathy Astrocytes support adjacent neurons with ATP, glutamine, cholesterol

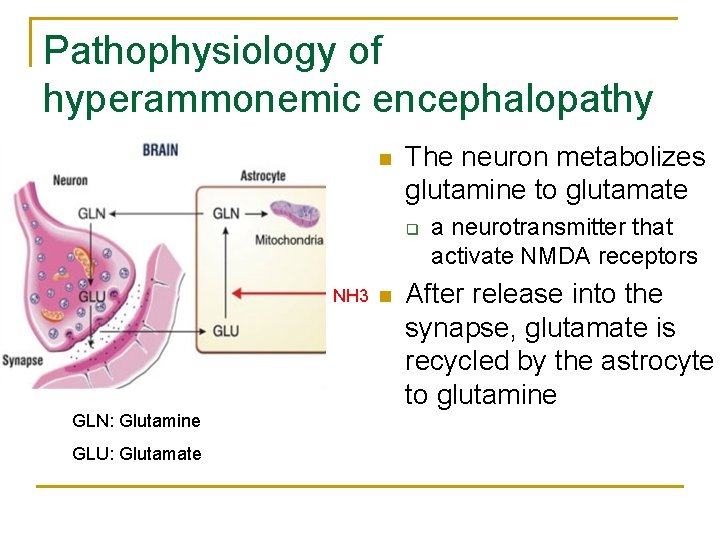
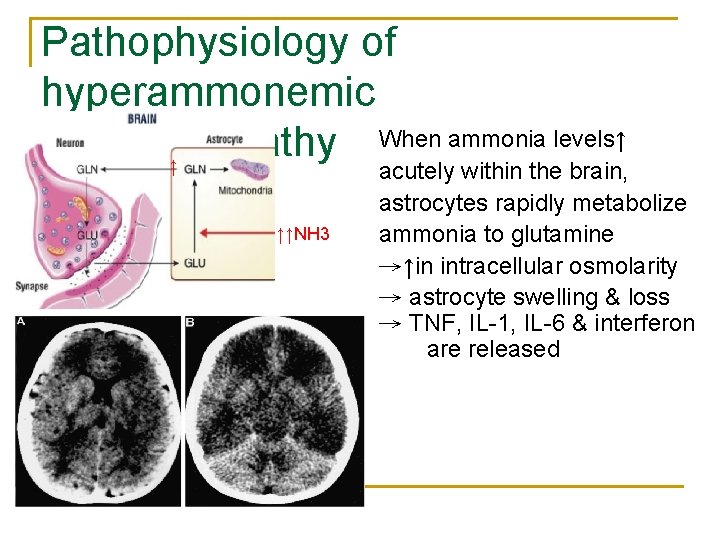
Pathophysiology of hyperammonemic encephalopathy n The neuron metabolizes glutamine to glutamate q NH 3 n GLN: Glutamine GLU: Glutamate a neurotransmitter that activate NMDA receptors After release into the synapse, glutamate is recycled by the astrocyte to glutamine

Pathophysiology of hyperammonemic When ammonia levels↑ encephalopathy ↑ ↑↑NH 3 acutely within the brain, astrocytes rapidly metabolize ammonia to glutamine →↑in intracellular osmolarity → astrocyte swelling & loss → TNF, IL-1, IL-6 & interferon are released

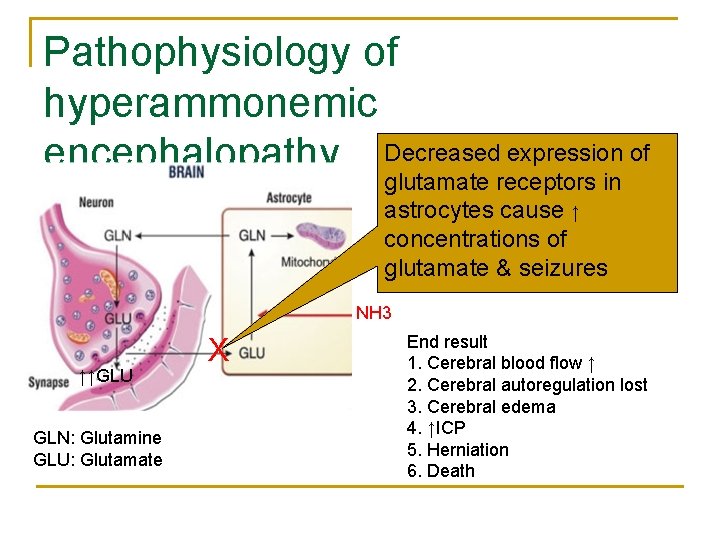
Pathophysiology of hyperammonemic encephalopathy Decreased expression of glutamate receptors in astrocytes cause ↑ concentrations of glutamate & seizures NH 3 ↑↑GLU GLN: Glutamine GLU: Glutamate X End result 1. Cerebral blood flow ↑ 2. Cerebral autoregulation lost 3. Cerebral edema 4. ↑ICP 5. Herniation 6. Death

Clinical feature n Acute hyperammonemia q q Cerebral edema, herniation & seizures Usually occur only when arterial NH 3 are > 200 umol/L Elevations of glutamine & osmolarity Excitatory effect of glutamine n Chronic effect of hyperammonemia on the brain q q q Osmolarity does not rise acutely Down-regulation of NMDA receptors results in less neuroexcitation from glutamate NH 3 has more of an effect on neuroinhibitory GABA receptors

Dx: Ammonia level in blood n Experimentally, at least 85% of liver function must be impaired before ammonia starts to accumulate n Specimen q q n Arterial NH 3 do not correlate with venous NH 3 levels q q n Venous ammonia levels vary locally, e. g. muscle contraction Liver is adept at the metabolism of ammonia Acute hyperammonemia may be an exception q n Heparin (Reduce RBC ammonia production)/EDTA Placed on ice (stable <1 hr in 4°C) and plasma separated within 15 mins (NH 3 concentrations increases spontaneously in standing blood and plasma) In fulminant hepatic failure, venous ammonia levels correlate with arterial ammonia levels Arterial ammonia levels q q q More accurate assessment of the amount of ammonia at the blood brain barrier Correlate with glutamine levels Correlate with the development of Intracranial hypertension Hepatology 1999; 29: 648– 653 Am J Med 2003; 114: 188– 193 Gastroenterology 2001; 121: 1109– 1119 J Cereb Blood Flow Metab 2006; 26: 21– 27

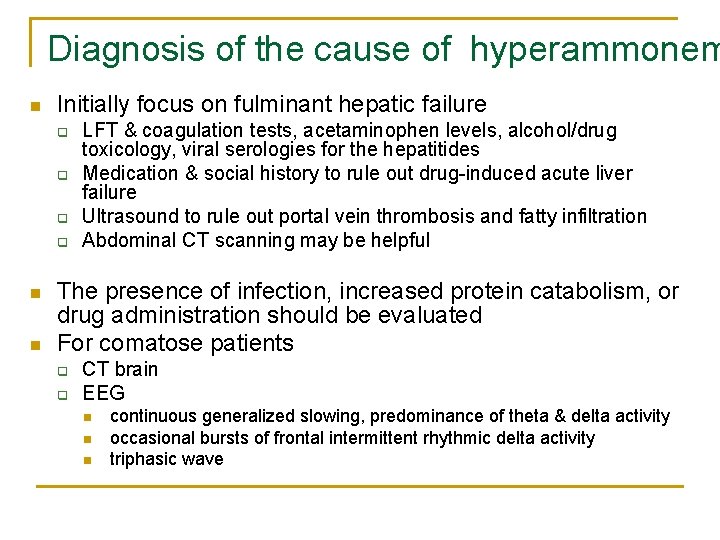
Diagnosis of the cause of hyperammonem n Initially focus on fulminant hepatic failure q q n n LFT & coagulation tests, acetaminophen levels, alcohol/drug toxicology, viral serologies for the hepatitides Medication & social history to rule out drug-induced acute liver failure Ultrasound to rule out portal vein thrombosis and fatty infiltration Abdominal CT scanning may be helpful The presence of infection, increased protein catabolism, or drug administration should be evaluated For comatose patients q q CT brain EEG n n n continuous generalized slowing, predominance of theta & delta activity occasional bursts of frontal intermittent rhythmic delta activity triphasic wave

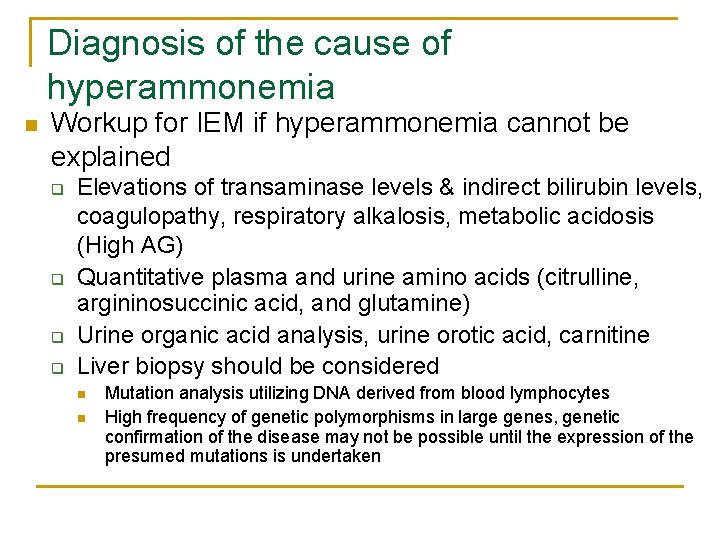
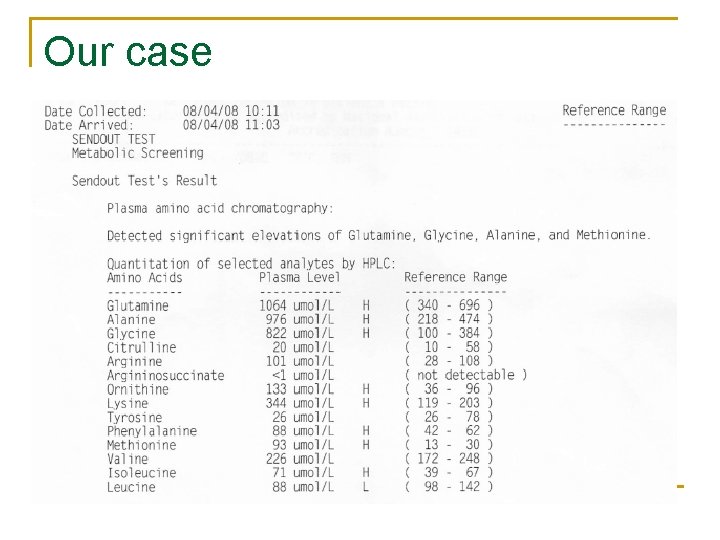
Diagnosis of the cause of hyperammonemia n Workup for IEM if hyperammonemia cannot be explained q q Elevations of transaminase levels & indirect bilirubin levels, coagulopathy, respiratory alkalosis, metabolic acidosis (High AG) Quantitative plasma and urine amino acids (citrulline, argininosuccinic acid, and glutamine) Urine organic acid analysis, urine orotic acid, carnitine Liver biopsy should be considered n n Mutation analysis utilizing DNA derived from blood lymphocytes High frequency of genetic polymorphisms in large genes, genetic confirmation of the disease may not be possible until the expression of the presumed mutations is undertaken

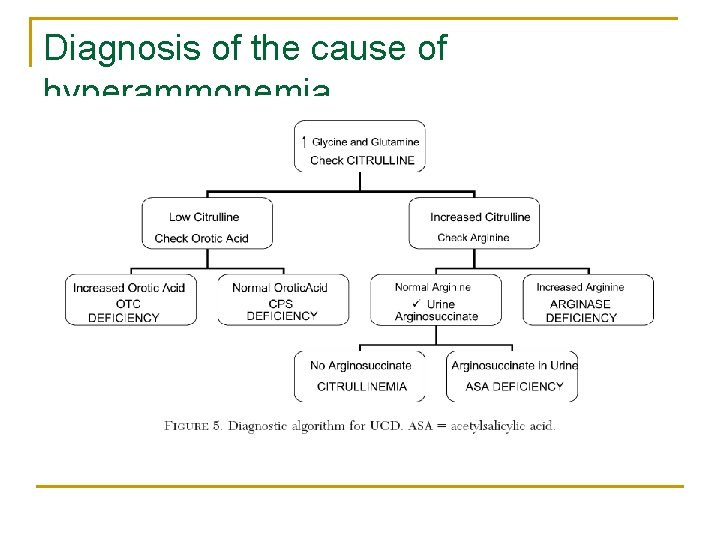
Diagnosis of the cause of hyperammonemia

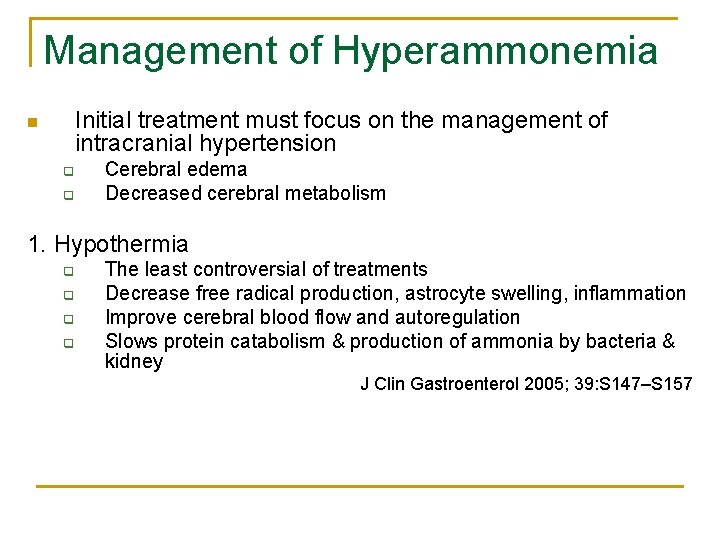
Management of Hyperammonemia Initial treatment must focus on the management of intracranial hypertension n q q Cerebral edema Decreased cerebral metabolism 1. Hypothermia q q The least controversial of treatments Decrease free radical production, astrocyte swelling, inflammation Improve cerebral blood flow and autoregulation Slows protein catabolism & production of ammonia by bacteria & kidney J Clin Gastroenterol 2005; 39: S 147–S 157

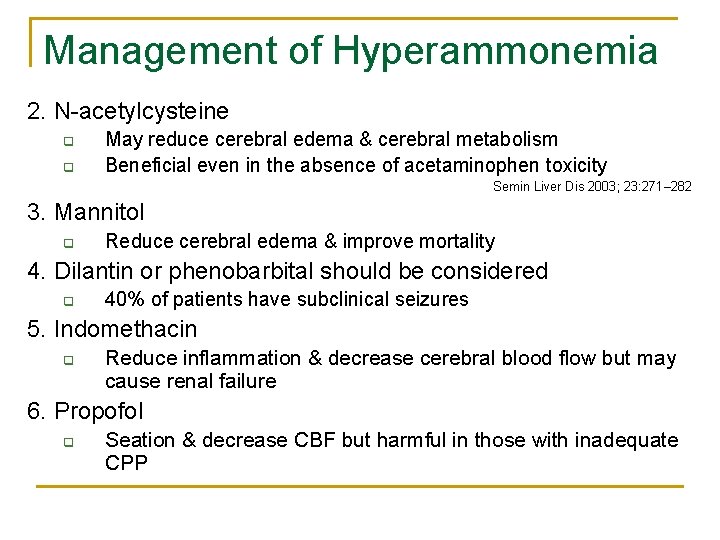
Management of Hyperammonemia 2. N-acetylcysteine q q May reduce cerebral edema & cerebral metabolism Beneficial even in the absence of acetaminophen toxicity Semin Liver Dis 2003; 23: 271– 282 3. Mannitol q Reduce cerebral edema & improve mortality 4. Dilantin or phenobarbital should be considered q 40% of patients have subclinical seizures 5. Indomethacin q Reduce inflammation & decrease cerebral blood flow but may cause renal failure 6. Propofol q Seation & decrease CBF but harmful in those with inadequate CPP


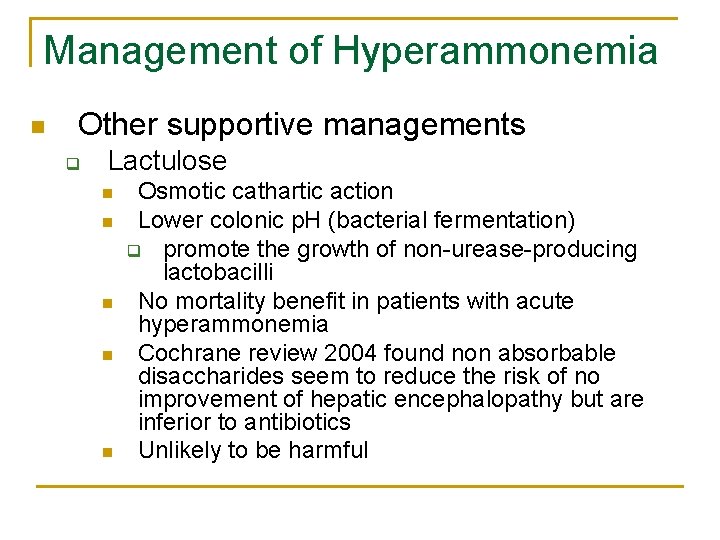
Management of Hyperammonemia n Other supportive managements q Lactulose n n n Osmotic cathartic action Lower colonic p. H (bacterial fermentation) q promote the growth of non-urease-producing lactobacilli No mortality benefit in patients with acute hyperammonemia Cochrane review 2004 found non absorbable disaccharides seem to reduce the risk of no improvement of hepatic encephalopathy but are inferior to antibiotics Unlikely to be harmful

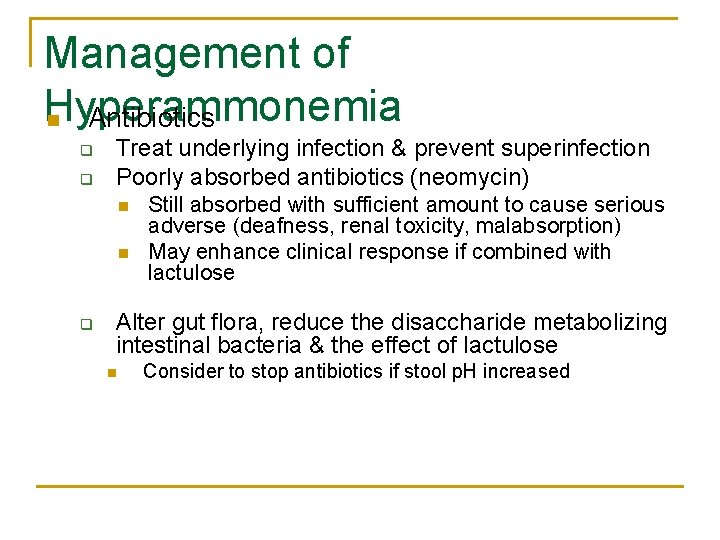
Management of Hyperammonemia n Antibiotics q q Treat underlying infection & prevent superinfection Poorly absorbed antibiotics (neomycin) n n q Still absorbed with sufficient amount to cause serious adverse (deafness, renal toxicity, malabsorption) May enhance clinical response if combined with lactulose Alter gut flora, reduce the disaccharide metabolizing intestinal bacteria & the effect of lactulose n Consider to stop antibiotics if stool p. H increased

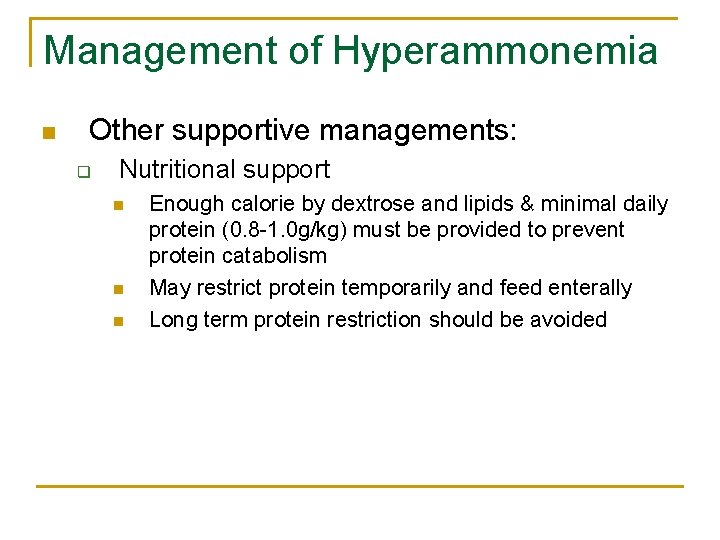
Management of Hyperammonemia n Other supportive managements: q Nutritional support n n n Enough calorie by dextrose and lipids & minimal daily protein (0. 8 -1. 0 g/kg) must be provided to prevent protein catabolism May restrict protein temporarily and feed enterally Long term protein restriction should be avoided

Management of Hyperammonemia n Ammonia reducing therapy q Renal replacement therapy n Peritoneal dialysis, hemodialysis, CVVHDF & CAVHDF q q effective to remove ammonia helpful in treating hyperammonemia associated with urea cycle disorders in children and adults serve as a potential bridge for adults with fulminant hepatic failure who are awaiting transplantation Sodium phenylacetate and sodium benzoate n n Promote the degradation of ammonia through “alternate” metabolic pathways Side effects: nausea, vomiting, and hypokalemia FDA: for hyperammonemic crisis in patients with IEMs May prevent the need for dialysis

Management of Hyperammonemia

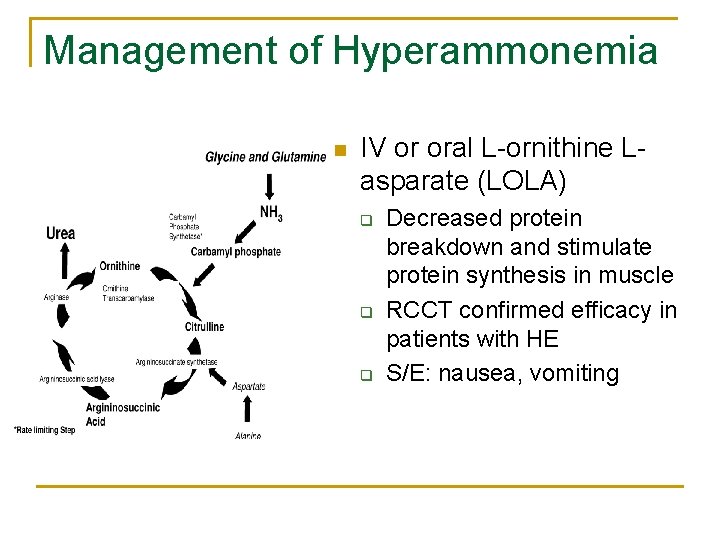
Management of Hyperammonemia n IV or oral L-ornithine Lasparate (LOLA) q q q Decreased protein breakdown and stimulate protein synthesis in muscle RCCT confirmed efficacy in patients with HE S/E: nausea, vomiting

Management of Hyperammonemia n Ammonia reducing therapy q L-carnitine n n q Facilitates lipid metabolism Reduce cerebral lactate levels by indirectly stimulating pyruvate dehydrogenase Facilitate transport of valporate into mitochondria & maintaining the ratio of acyl-Co. A to free Co. A in the mitochondria Use in Valproic acid induced hyperammonemic encephalopathy Zinc n n Cofactor for enzymes of urea cycle Deficiency common esp in alcoholic cirrhosis due to poor dietary intake, impaired absorption, excessive urinary loss Zinc supplement 600 mg daily speeds up the kinetics of urea formation from amino acids & ammonia No study performed in ICU setting

Management of Hyperammonemia n Ammonia reducing therapy q Artificial liver support n n n q Use extracorporeal blood purification to dialyse albumin bound hydrophobic substances Clinical benefit unclear Improve encephalopathy & as bridge to transplant Liver transplantation n Successful for cirrhosis & fulminant hepatic failure


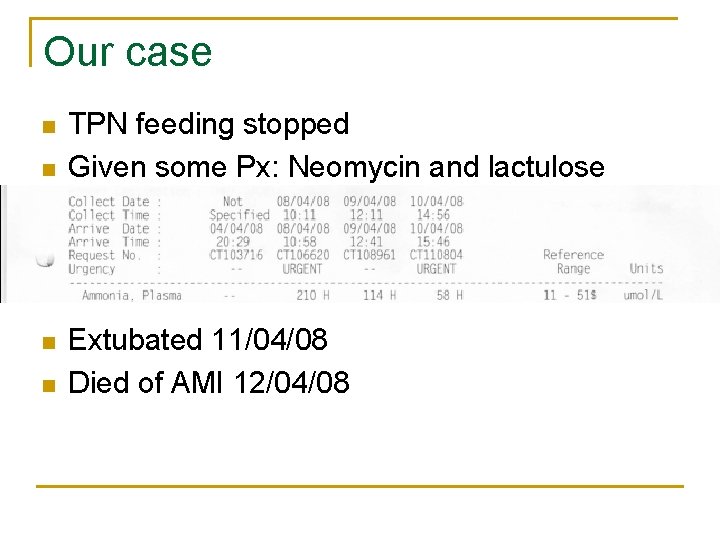
Our case n n TPN feeding stopped Given some Px: Neomycin and lactulose Extubated 11/04/08 Died of AMI 12/04/08

Our case

Our case

Our case

Our case: Cause of coma n Hyperammonaemic encephalopathy secondary to q q TPN (High protein content) Bacteria overgrowth n q q Hx of BII gastrectomy+ stagnant bowel loop syndrome ? Aspirin intake for migraine (Drugs) ? IEM/UCD (Underlying liver disease)

Our case: Cause of coma n Zn deficiency

Take home message n n C-CO 2 narcosis O-Overdose of medications/Sedations M-Metabolic: Hypoglycaemia, DKA, hypothyroidism, hypercalcaemia, adrenal failure, uraemia, hepatic coma A-Apoplexy: HI, CVA, ICH, CNS infection, epilepsy

Take home message n Comatose patient with normal LFT q q q Consider hyperammonaemic encephalopathy Common causes in ICU: TPN, drugs Ix and Novel management

The END
 Icu case presentation
Icu case presentation Icu case presentation
Icu case presentation Chatecholamine
Chatecholamine Enterprise ccm
Enterprise ccm Ccm certification construction
Ccm certification construction Ccm exemple
Ccm exemple Ccm evolution
Ccm evolution Ccm tutorials
Ccm tutorials Ccm crm
Ccm crm Model ccm
Model ccm Continuous control monitoring definition
Continuous control monitoring definition Bhutan ccm
Bhutan ccm Ccm eligibility requirements
Ccm eligibility requirements Kebutuhan tenaga perawat menurut gillies
Kebutuhan tenaga perawat menurut gillies Escala sas de riker
Escala sas de riker Icu localization
Icu localization Icu tersier
Icu tersier Information and communication university zambia location
Information and communication university zambia location 5hs and 5ts
5hs and 5ts Pressors icu
Pressors icu Escala de morse y braden
Escala de morse y braden Cam icu
Cam icu Cam icu escala
Cam icu escala Cheetah nicom monitor
Cheetah nicom monitor Cam icu
Cam icu Intensive care unit definition
Intensive care unit definition Icu orientation
Icu orientation Critical care for dummies
Critical care for dummies List of lecturers at icu zambia
List of lecturers at icu zambia Icu acuity tool
Icu acuity tool Icu library
Icu library Icu layout and design
Icu layout and design Icu transliterator
Icu transliterator Icu medical b3108
Icu medical b3108 Kp icu jpm
Kp icu jpm Ideal icu setup
Ideal icu setup Shanks algorithm example
Shanks algorithm example Escala de cam icu
Escala de cam icu Dobutamine dose
Dobutamine dose Hypertonic solution iv fluid
Hypertonic solution iv fluid Diet chart for icu patients
Diet chart for icu patients End of life care in icu
End of life care in icu Definition of physiology
Definition of physiology Icu unicode
Icu unicode Icu security group
Icu security group Alfred icu
Alfred icu The forgotten footnote plagiarism
The forgotten footnote plagiarism What is plagiarism in media and information literacy
What is plagiarism in media and information literacy Listening the forgotten skill
Listening the forgotten skill Once done never forgotten
Once done never forgotten Siachen the forgotten war
Siachen the forgotten war The forgotten princesses
The forgotten princesses Education is what remains after one has forgotten meaning
Education is what remains after one has forgotten meaning Darryl worley have you forgotten
Darryl worley have you forgotten Not forgotten carry
Not forgotten carry Unborn but not forgotten
Unborn but not forgotten Expenseondemand
Expenseondemand Forgotten altar
Forgotten altar Forgotten core circuitry disassembler
Forgotten core circuitry disassembler Nnn hypno
Nnn hypno Total fertility rate formula
Total fertility rate formula Imr formula
Imr formula Obando fertility rate
Obando fertility rate Birth rate problem
Birth rate problem Contoh cara menghitung tfr
Contoh cara menghitung tfr Fertility blend gnc
Fertility blend gnc Net reproductive rate
Net reproductive rate Cupping therapy and infertility
Cupping therapy and infertility Fertility rate calculation
Fertility rate calculation Christian views on fertility treatment
Christian views on fertility treatment Klasifikasi kapabilitas kesuburan tanah
Klasifikasi kapabilitas kesuburan tanah Types of speciation
Types of speciation