Atomic Structure What is an atom Atom the












- Slides: 21

Atomic Structure

What is an atom? • Atom: the smallest unit of matter that retains the identity of the substance

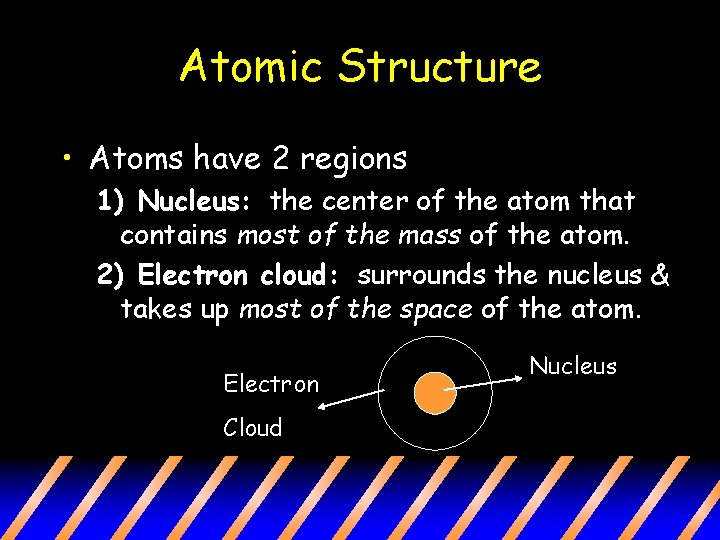
Atomic Structure • Atoms have 2 regions 1) Nucleus: the center of the atom that contains most of the mass of the atom. 2) Electron cloud: surrounds the nucleus & takes up most of the space of the atom. Electron Cloud Nucleus

What’s in the Nucleus? • In the nucleus we find: • Protons: positively charged subatomic particles • Mass of 1 amu • Neutrons: neutrally charged subatomic particles • Mass of 1 amu

What’s in the Electron Cloud? • In the electron cloud we find: • Electrons: the subatomic particle with a negative charge and relatively no mass • Mass of ~ 1/1836 amu

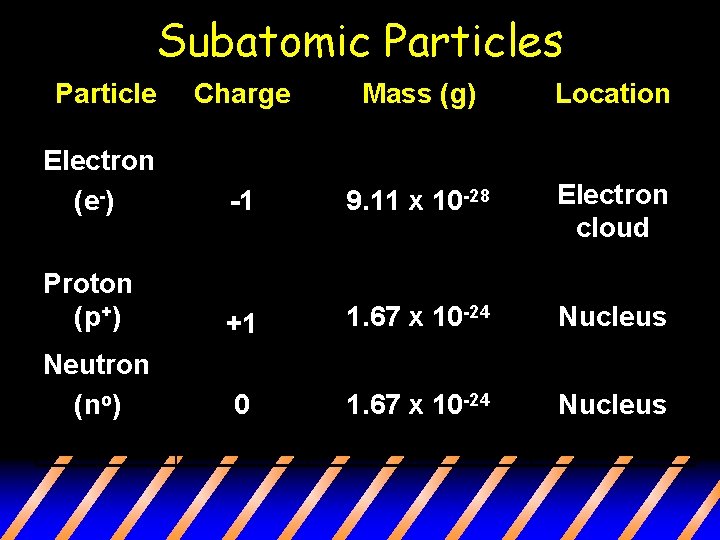
Subatomic Particles Particle Charge Mass (g) Location Electron (e-) -1 9. 11 x 10 -28 Electron cloud Proton (p+) +1 1. 67 x 10 -24 Nucleus Neutron (no) 0 1. 67 x 10 -24 Nucleus

How do we know the number of protons in an atom? • Atomic number (#)= # of protons in an atom • Ex: Hydrogen’s atomic # is 1 • hydrogen has 1 proton • Ex: Carbon’s atomic # is 6 • carbon has 6 protons **The number of protons identifies the atom-it’s an atom’s fingerprint.

How do we know the number of neutrons in an atom? • Mass #: the # of protons plus neutrons in the nucleus • # of neutrons = mass # - atomic # Example • Li has a mass # of 7 and an atomic # of 3 • Protons = 3 (same as atomic #) • Neutrons= 7 -3 = 4 (mass # - atomic #)

Mass # vs. Atomic Mass # ? = The Atomic mass on the periodic table rounded either up or down

How do we find the number of electrons in an atom? • Most atoms are neutral (have no overall charge) • Because the only charged subatomic particles are the protons and electrons… they must balance each other out in an electrically neutral atom. • Therefore. . • # Electrons = # Protons * (in a neutral atom. . ) *

Examples • He has a mass # of 4 and an atomic # of 2 • p+ =2 no = 2 e- = 2 • Cl has a mass # of 35 and an atomic # of 17 • p+ = 17, no = 18, e- = 17

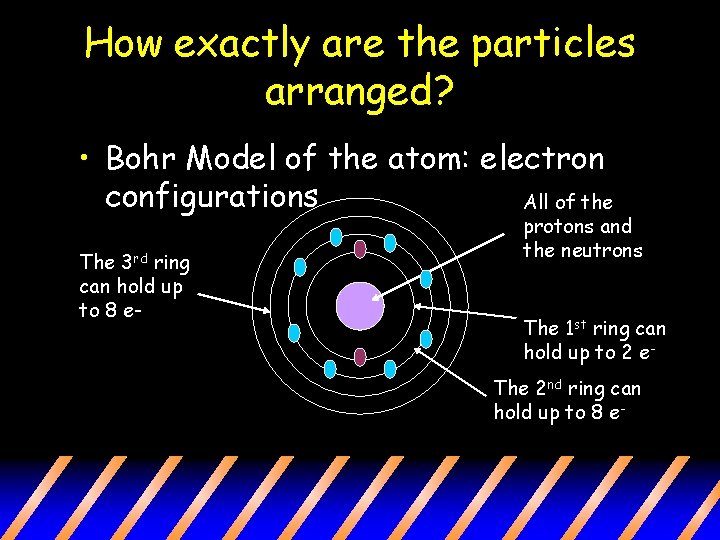
How exactly are the particles arranged? • Bohr Model of the atom: electron configurations All of the The 3 rd ring can hold up to 8 e- protons and the neutrons The 1 st ring can hold up to 2 e. The 2 nd ring can hold up to 8 e-

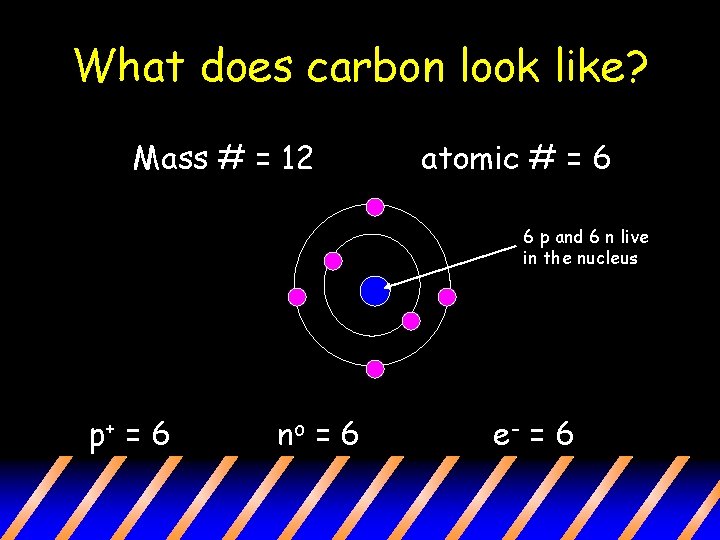
What does carbon look like? Mass # = 12 atomic # = 6 6 p and 6 n live in the nucleus p+ = 6 no = 6 e- = 6

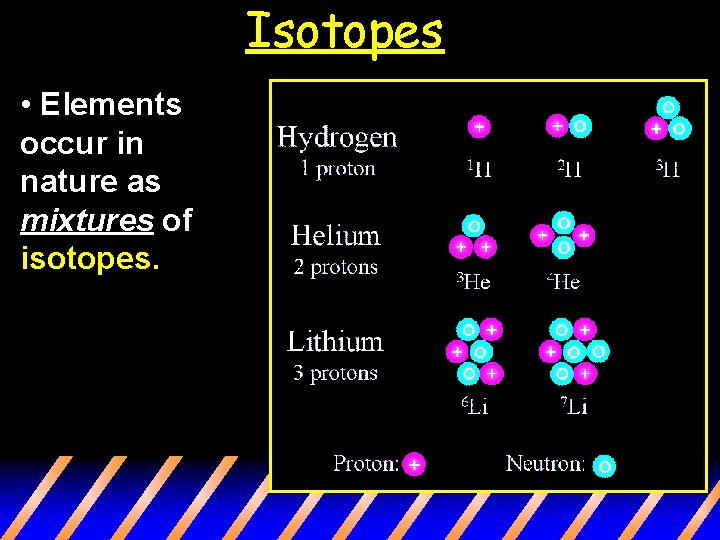
Isotopes • Dalton’s postulate was wrong. Atoms of the same element can be different (they can have different # of neutrons) st 1 • Thus, different mass numbers. • These are called isotopes.

Isotopes are atoms of the same element having different masses, due to varying numbers of neutrons.

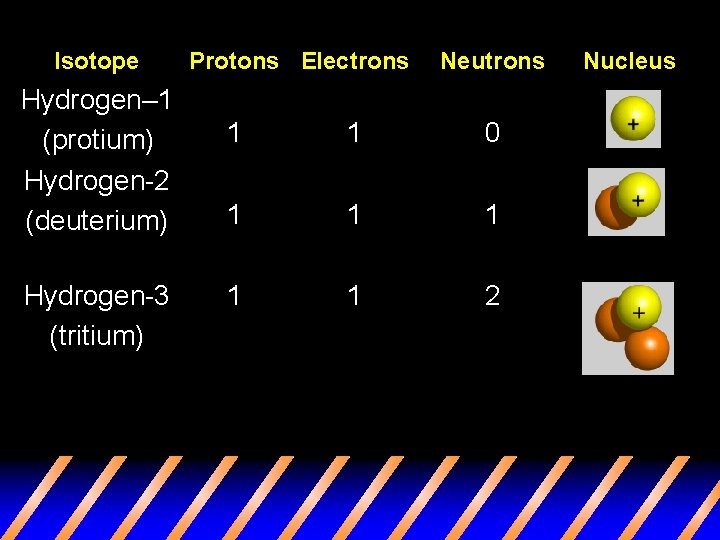
Isotope Hydrogen– 1 (protium) Hydrogen-2 (deuterium) Hydrogen-3 (tritium) Protons Electrons Neutrons 1 1 0 1 1 1 2 Nucleus

Naming Isotopes • We name the isotope based on its mass number • carbon-12 • carbon-14 • uranium-235

Isotopes • Elements occur in nature as mixtures of isotopes.

Atomic Mass § How heavy is an atom of oxygen? § It depends. . b/c there are different oxygen isotopes. § We are more concerned with the average atomic mass. § This is determined based on the abundance of each isotope § We don’t use grams for this mass because the numbers would be too small.

Measuring Atomic Mass • Instead we use the Atomic Mass Unit (amu) • defined as one-twelfth the mass of a carbon-12 atom. • Each isotope has its own atomic mass, thus we determine the average from percent abundance.

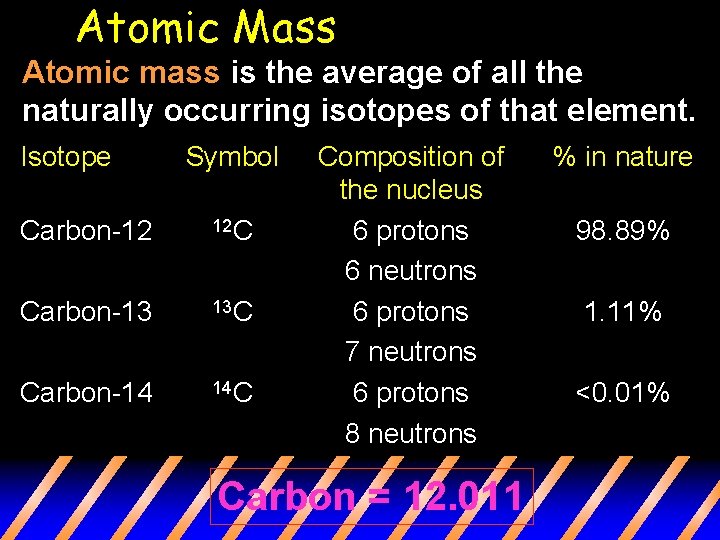
Atomic Mass Atomic mass is the average of all the naturally occurring isotopes of that element. Isotope Symbol Carbon-12 12 C Carbon-13 13 C Carbon-14 14 C Composition of the nucleus 6 protons 6 neutrons 6 protons 7 neutrons 6 protons 8 neutrons Carbon = 12. 011 % in nature 98. 89% 1. 11% <0. 01%