AP Physics Chapter 10 Temperature Chapter 10 Temperature























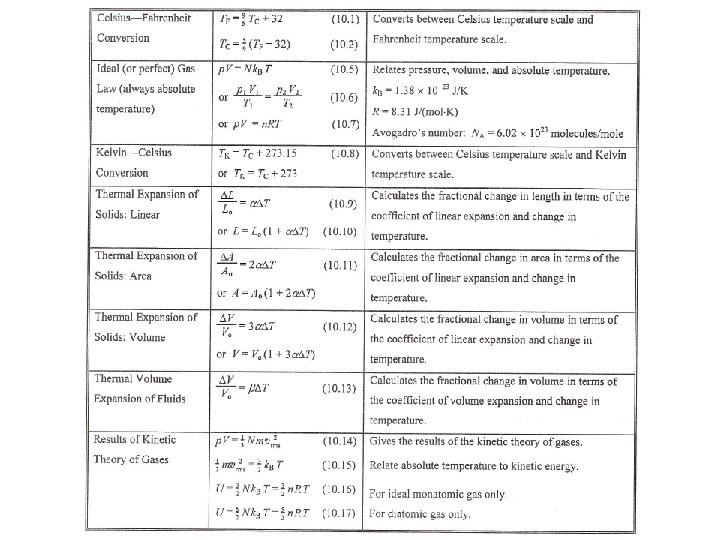
- Slides: 68

AP Physics Chapter 10 Temperature

Chapter 10: Temperature 10. 1 Temperature and Heat 10. 2 The Celsius and Fahrenheit Temperature Scales 10. 3 Gas Laws and Absolute Temperature 10. 4 Thermal Expansion 10. 5 The Kinetic Theory of Gases 10. 6 (omit)

Learning Objectives Kinetic Theory and Thermodynamics: Ideal Gases Students will understand the kinetic theory model of an ideal gas, so they can: a) State the assumptions of the model. b) State the connection between temperature and mean translational kinetic energy, and apply it to determine the mean speed of gas molecules as a function of their mass and the temperature of the gas.

Learning Objectives Kinetic Theory and Thermodynamics: Ideal Gases Students will understand the kinetic theory model of an ideal gas, so they can: c) State the relationship among Avogadri’s number, Boltzmann’s constant, and the gas constant R, and express the energy of a mole of a monatomic ideal gas as a function of its temperature. d) Explain qualitatively how the model explains the pressure of a gas in terms of collisions with the container walls, and explain how the model predicts that, for fixed volume, pressure must be proportional to temperature.

Learning Objectives Heat Transfer and Thermal Expansion Students will understand heat transfer and thermal expansion so they can: a) Calculate how the flow of heat through a slab of material is affected by changes in the thickness or area of the slab, or the temperature difference between the two faces of the slab. b) Analyze what happens to the size and shape of an object when it is heated.

Homework for Chapter 10 • Read Chapter 10 • HW 10. A: 7, 8, 17, 22, 24, 27 -32, 34, 36, 38. • HW 10. B: 45 -49, 65 -68, 71.

10. 1 Temperature and Heat

Warmup: Sky High Cooking Physics Warmup #89 The boiling temperature for water is dependent on the atmospheric pressure. At standard atmospheric pressure, the boiling temperature is 100°C. At altitudes below sea level, where the atmospheric pressure is greater, the boiling temperature is higher. Altitudes above sea level would result in water boiling below 100°C. *********************************************** Pressure cookers allow the cook to regulate the air pressure inside the cooker at levels greater than standard atmospheric pressure. When a recipe calls for cooking something in boiling water, it is assumed that the water is at a temperature of 100°C. Suppose you had a cooking thermometer and a pressure cooker available. Explain how you could stay true to the intent of a recipe, which called for cooking in boiling water if you were in Death Valley, California (significantly below sea level) and Denver, Colorado (significantly above sea level). Answer: Use thermometer to regulate temperature at 100°C, even though it is not boiling. Denver: Use the pressure cooker to raise the pressure to 1 atm.

10. 1 Temperature and Heat temperature – a relative measure, or indication, of hotness and coldness • Temperature is associated with molecular motion (translational, linear vibration, rotation). heat – the net energy transferred from one object to another because of a temperature difference internal energy – the total energy (kinetic plus potential) of all molecules of a body or a system • When heat is transferred out of or into a system while there is no other physical process present, the internal energy of the system will change. thermal contact – when heat is transferred between two objects, even if they are not physically touching thermal equilibrium – when there is no longer a net heat transfer between objects in thermal contact, and they are at the same temperature

10. 2 The Celcius and Fahrenheit Temperature Scales

Warmup: Up on the Roof Physics Warmup #90 The color of your roof can play a major role in how hot or cold your house becomes when the sun shines on it. Dark colors absorb much of the sun’s heat energy, while lighter colors will reflect a good portion of that energy. On hot summer days it would be better to have a light roof, while on cold winter days it would be better to have a darker roof. *********************************************** Obviously, you can only have one color of roof on your house. For your geographic location, explain which color roof would be better in terms of energy efficiency over the course of a year. Answer: In the Northern U. S. , a darker roof would be better due to longer winters, shorter summers, and less direct sunlight. In the South, a lighter roof would be better due to longer summers, shorter winters, and more direct sunlight. In the Central U. S. it would not make much of a difference either way.

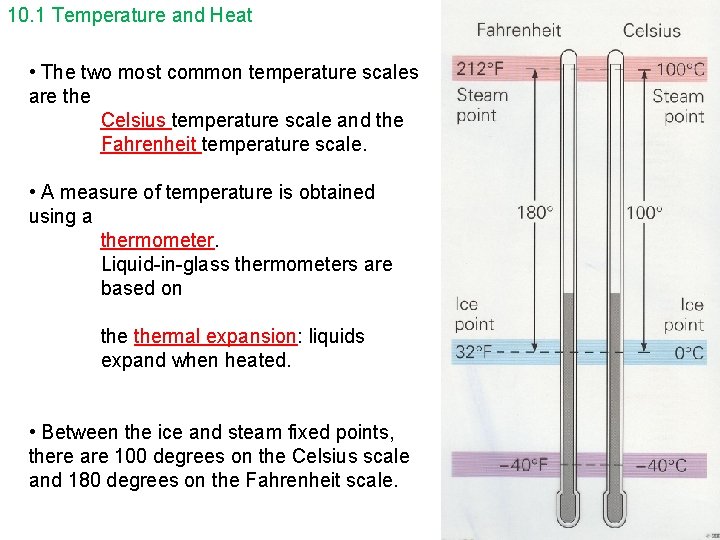
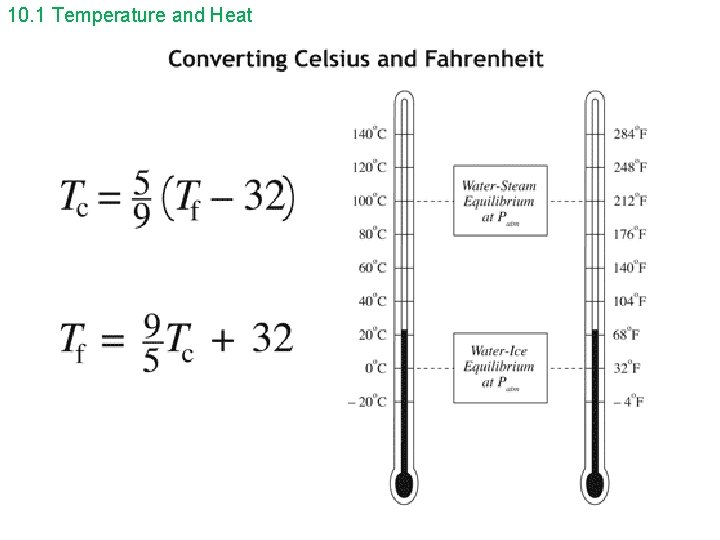
10. 1 Temperature and Heat • The two most common temperature scales are the Celsius temperature scale and the Fahrenheit temperature scale. • A measure of temperature is obtained using a thermometer. Liquid-in-glass thermometers are based on thermal expansion: liquids expand when heated. • Between the ice and steam fixed points, there are 100 degrees on the Celsius scale and 180 degrees on the Fahrenheit scale.

10. 1 Temperature and Heat

10. 1 Temperature and Heat

10. 1 Temperature and Heat Example 10. 1: What is the temperature 50. 0°F on the Celsius scale? Example 10. 2: The temperature changes from 35°F during the night to 75°F during the day. What is the temperature change on the Celsius scale?

10. 3 Gas Laws and Absolute Temperature

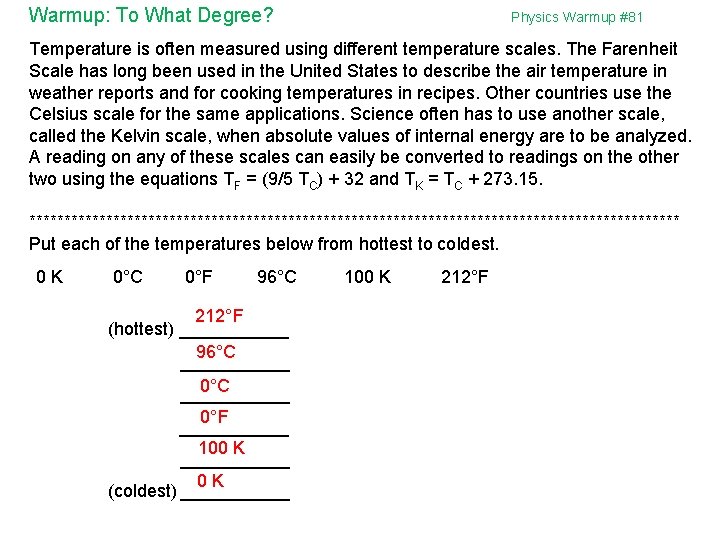
Warmup: To What Degree? Physics Warmup #81 Temperature is often measured using different temperature scales. The Farenheit Scale has long been used in the United States to describe the air temperature in weather reports and for cooking temperatures in recipes. Other countries use the Celsius scale for the same applications. Science often has to use another scale, called the Kelvin scale, when absolute values of internal energy are to be analyzed. A reading on any of these scales can easily be converted to readings on the other two using the equations TF = (9/5 TC) + 32 and TK = TC + 273. 15. *********************************************** Put each of the temperatures below from hottest to coldest. 0 K 0°C 0°F 96°C 212°F (hottest) ______ 96°C ___________ 0°F ______ 100 K ______ 0 K (coldest) ______ 100 K 212°F

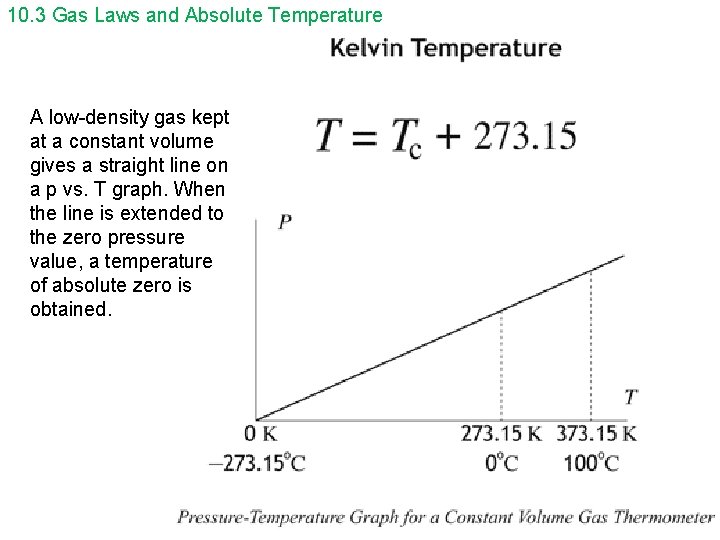
10. 3 Gas Laws and Absolute Temperature A low-density gas kept at a constant volume gives a straight line on a p vs. T graph. When the line is extended to the zero pressure value, a temperature of absolute zero is obtained.

10. 1 Temperature and Heat The Kelvin scale also uses the triple point of water as a fixed point of reference. The triple point of water is a unique set of conditions where water exists simultaneously in equilibrium as a solid, liquid, and gas. This point is 610 Pa, at temperature of 273. 16 K (or 0. 01 °C).

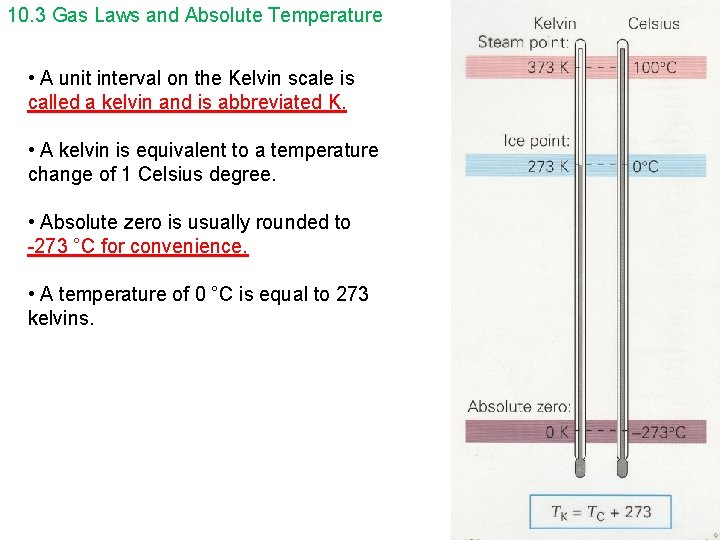
10. 3 Gas Laws and Absolute Temperature • A unit interval on the Kelvin scale is called a kelvin and is abbreviated K. • A kelvin is equivalent to a temperature change of 1 Celsius degree. • Absolute zero is usually rounded to -273 °C for convenience. • A temperature of 0 °C is equal to 273 kelvins.

10. 3 Gas Laws and Absolute Temperature Example 10. 3: What is -40°F on the Kelvin scale?

10. 3 Gas Laws and Absolute Temperature Activity: Go to Nova’s Website, “Absolute Zero”. http: //www. pbs. org/wgbh/nova/zero/ Click on “A Sense of Scale” interactive. Find in F, C, & K: a) the temperature of a lightning bolt b) the coldest surface temperature on earth ever recorded c) the hottest temperature achieved in a lab Click on “A Matter of Degrees”. Create and name your own temperature scale.

10. 3 Gas Laws and Absolute Temperature Assignment: Read the article, “Absolute Hot” and write a Ten Percent Summary. Writer’s Purpose: You will write the main ideas in your own words. Include the most important details if the word limit permits. You will want clear and accurate information with no opinion. Remember, this is a summary – not an evaluation. Writer’s Role: You will take the role of a researcher or analyst who has been hired by Main. Ideas. com to distill information for interested adults. Audience: Your audience will be busy, smart adults who want to get the main ideas of articles to see if they should read the article completely. Form: You will write a summary of your assigned article in approximately ten percent of the words – not exactly ten percent, but approximately ten percent. This article is approximately 1500 words, so you will write 130170 words.

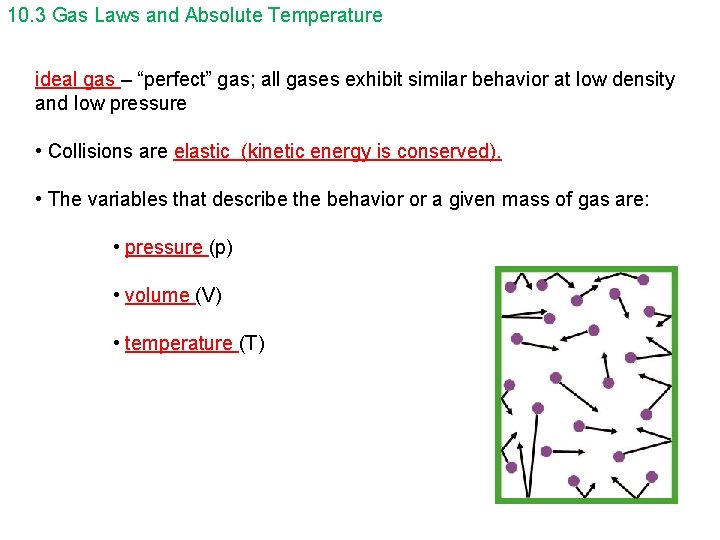
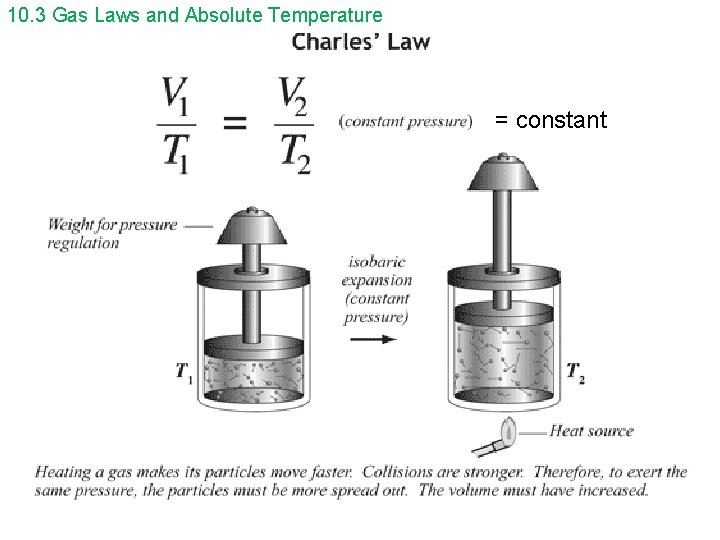
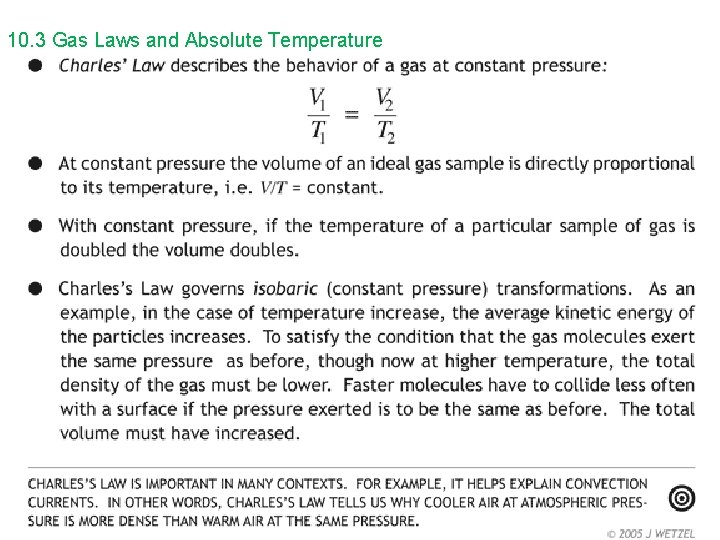
10. 3 Gas Laws and Absolute Temperature ideal gas – “perfect” gas; all gases exhibit similar behavior at low density and low pressure • Collisions are elastic (kinetic energy is conserved). • The variables that describe the behavior or a given mass of gas are: • pressure (p) • volume (V) • temperature (T)

10. 3 Gas Laws and Absolute Temperature

10. 3 Gas Laws and Absolute Temperature

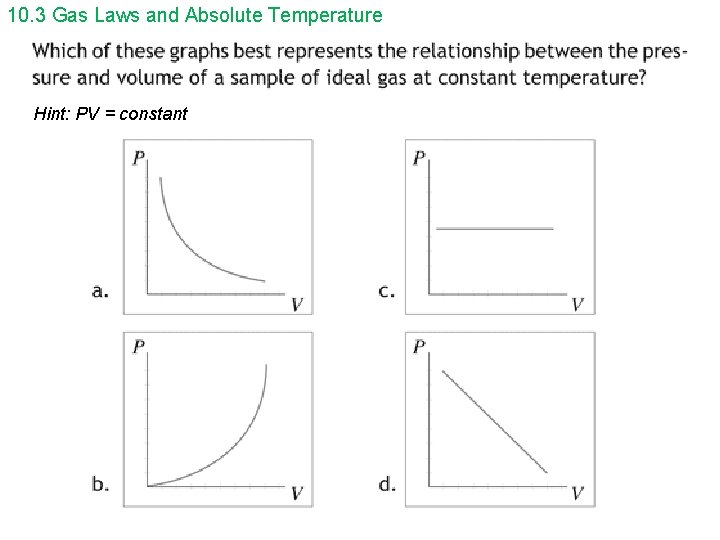
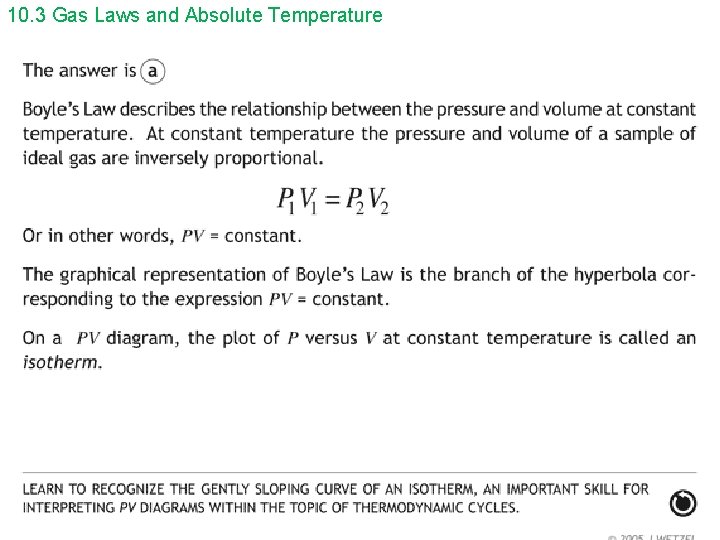
10. 3 Gas Laws and Absolute Temperature Hint: PV = constant

10. 3 Gas Laws and Absolute Temperature

10. 3 Gas Laws and Absolute Temperature = constant

10. 3 Gas Laws and Absolute Temperature

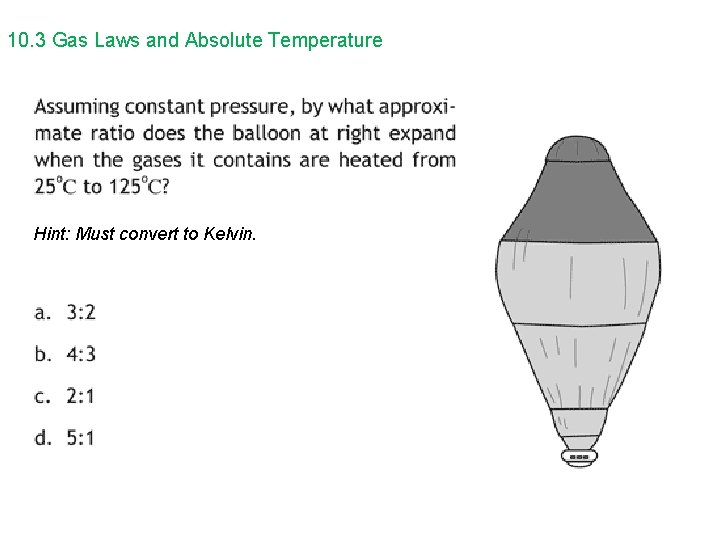
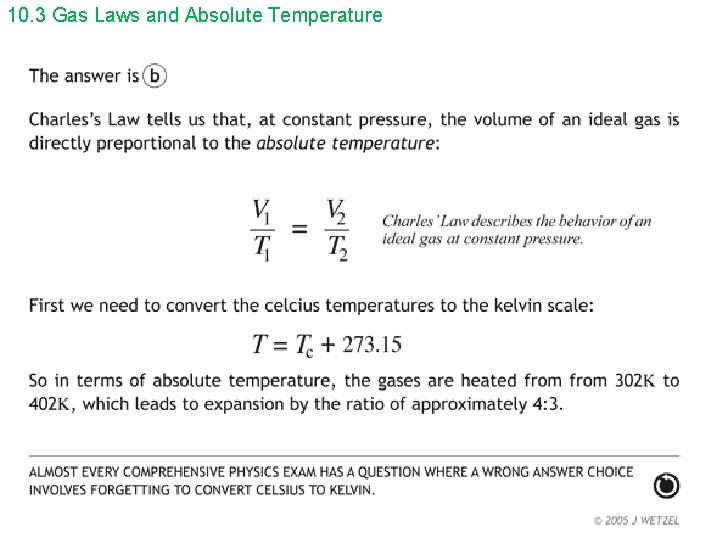
10. 3 Gas Laws and Absolute Temperature Hint: Must convert to Kelvin.

10. 3 Gas Laws and Absolute Temperature

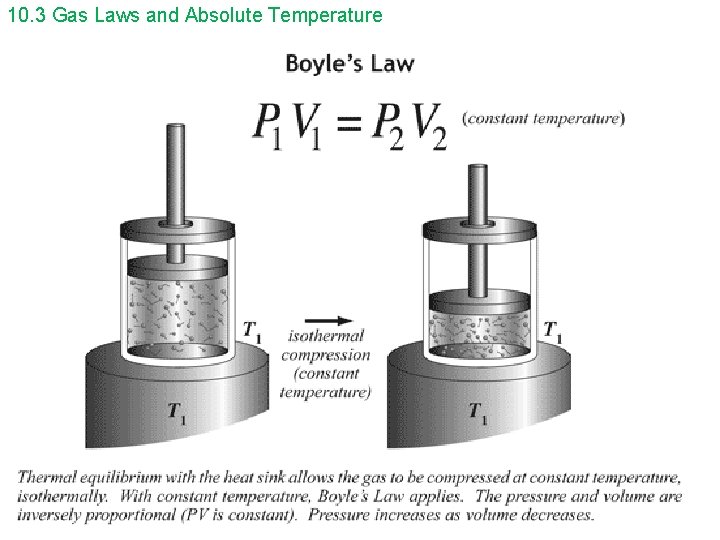
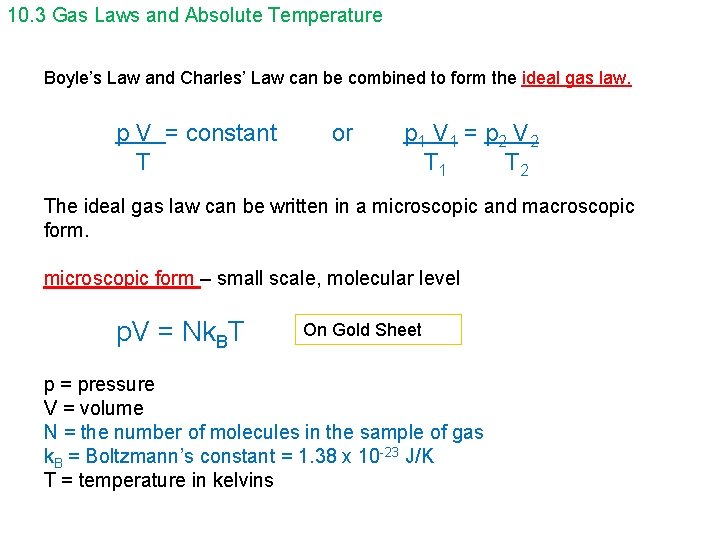
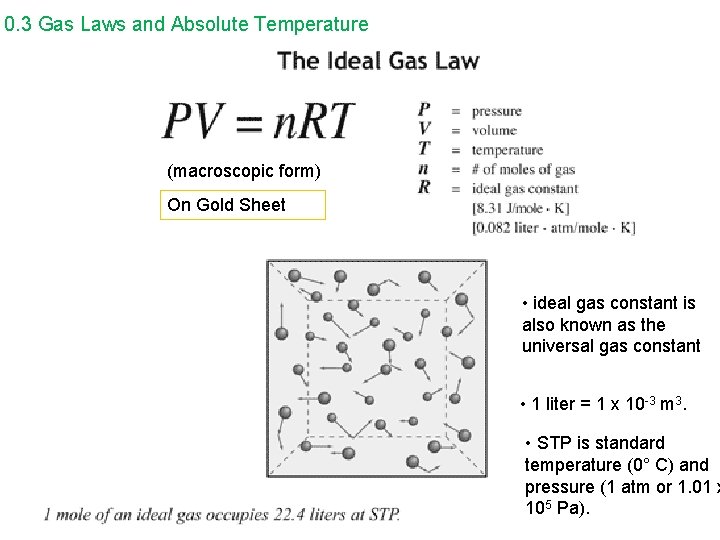
10. 3 Gas Laws and Absolute Temperature Boyle’s Law and Charles’ Law can be combined to form the ideal gas law. p V = constant T or p 1 V 1 = p 2 V 2 T 1 T 2 The ideal gas law can be written in a microscopic and macroscopic form. microscopic form – small scale, molecular level p. V = Nk. BT On Gold Sheet p = pressure V = volume N = the number of molecules in the sample of gas k. B = Boltzmann’s constant = 1. 38 x 10 -23 J/K T = temperature in kelvins

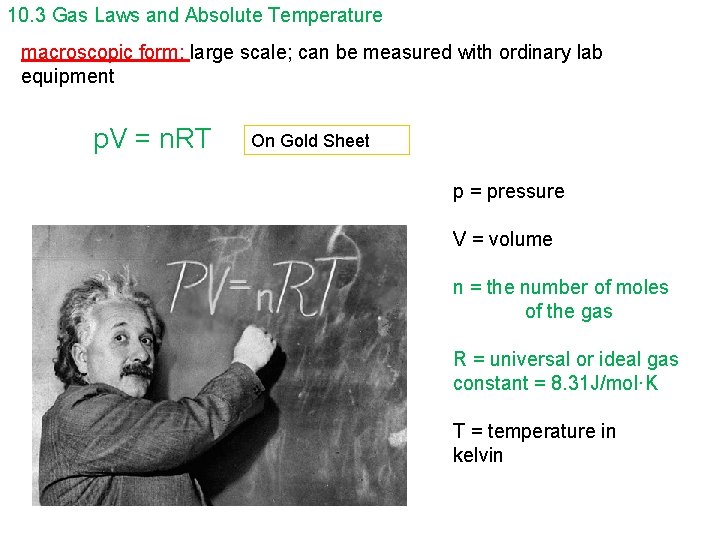
10. 3 Gas Laws and Absolute Temperature macroscopic form: large scale; can be measured with ordinary lab equipment p. V = n. RT On Gold Sheet p = pressure V = volume n = the number of moles of the gas R = universal or ideal gas constant = 8. 31 J/mol·K T = temperature in kelvin

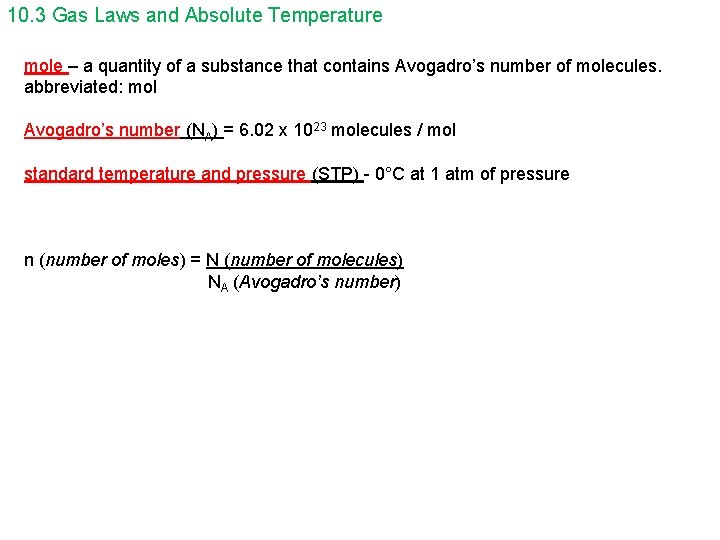
10. 3 Gas Laws and Absolute Temperature mole – a quantity of a substance that contains Avogadro’s number of molecules. abbreviated: mol Avogadro’s number (NA) = 6. 02 x 1023 molecules / mol standard temperature and pressure (STP) - 0°C at 1 atm of pressure n (number of moles) = N (number of molecules) NA (Avogadro’s number)

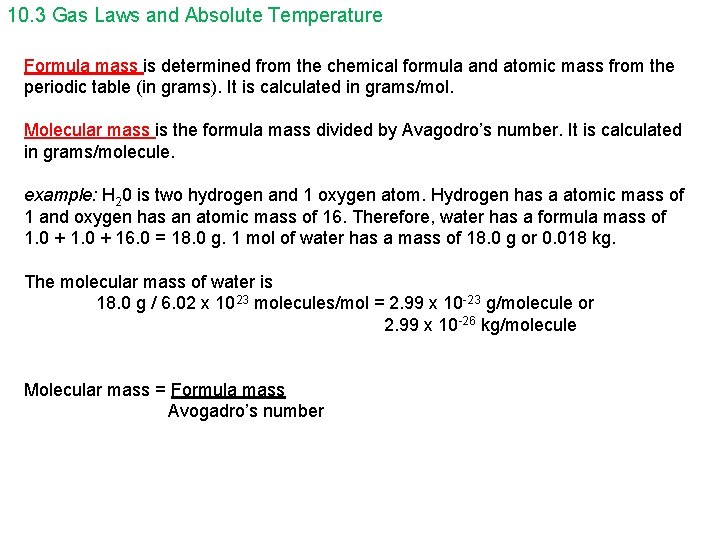
10. 3 Gas Laws and Absolute Temperature Formula mass is determined from the chemical formula and atomic mass from the periodic table (in grams). It is calculated in grams/mol. Molecular mass is the formula mass divided by Avagodro’s number. It is calculated in grams/molecule. example: H 20 is two hydrogen and 1 oxygen atom. Hydrogen has a atomic mass of 1 and oxygen has an atomic mass of 16. Therefore, water has a formula mass of 1. 0 + 16. 0 = 18. 0 g. 1 mol of water has a mass of 18. 0 g or 0. 018 kg. The molecular mass of water is 18. 0 g / 6. 02 x 1023 molecules/mol = 2. 99 x 10 -23 g/molecule or 2. 99 x 10 -26 kg/molecule Molecular mass = Formula mass Avogadro’s number

10. 3 Gas Laws and Absolute Temperature (macroscopic form) On Gold Sheet • ideal gas constant is also known as the universal gas constant • 1 liter = 1 x 10 -3 m 3. • STP is standard temperature (0° C) and pressure (1 atm or 1. 01 x 105 Pa).

10. 3 Gas Laws and Absolute Temperature

10. 3 Gas Laws and Absolute Temperature Example 10. 4: A gas has a volume of 0. 20 m 3, a temperature of 30°C, and a pressure of 1. 0 atm. It is heated to 60°C and is compressed to a volume of 0. 15 m 3. Find the new pressure in atmospheres.

10. 3 Gas Laws and Absolute Temperature Example 10. 5: An ideal gas in a container of volume 1000 cm 3 (one liter) at 20. 0°C has a pressure of 1. 00 x 104 N/m 2. Determine the number of gas molecules and the number of moles of gas in the container.

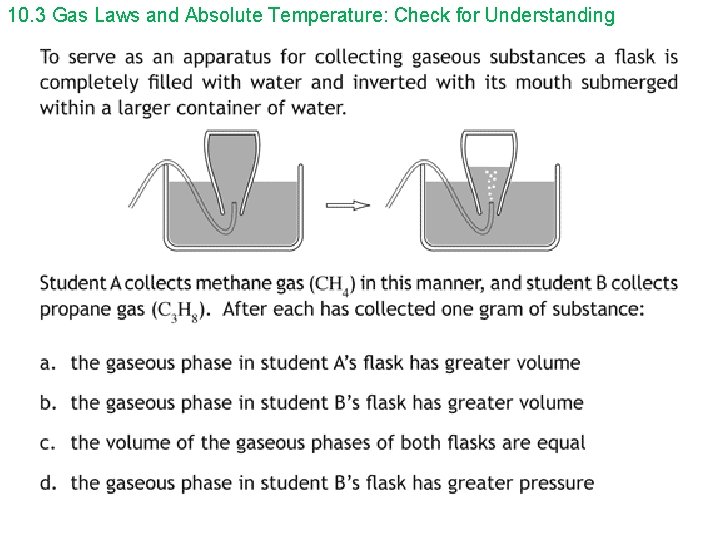
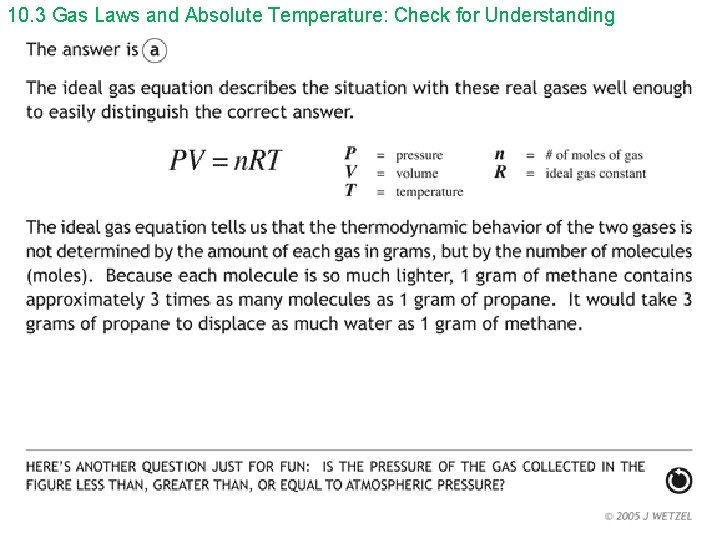
10. 3 Gas Laws and Absolute Temperature: Check for Understanding 1. The temperature used in the ideal gas law is: a) Celcius b) Fahrenheit c) Kelvin d) any of the preceding Answer: c

10. 3 Gas Laws and Absolute Temperature: Check for Understanding 2. When the temperature of a quantity of gas is increased: a) the pressure must increase b) the volume must increase c) both the pressure and volume must increase d) none of the preceding Answer: d ; p. V/T = constant

10. 3 Gas Laws and Absolute Temperature: Check for Understanding

10. 3 Gas Laws and Absolute Temperature: Check for Understanding

Homework 10. A • HW 10. A: 7, 8, 17, 22, 24, 27 -32, 34, 36, 38.

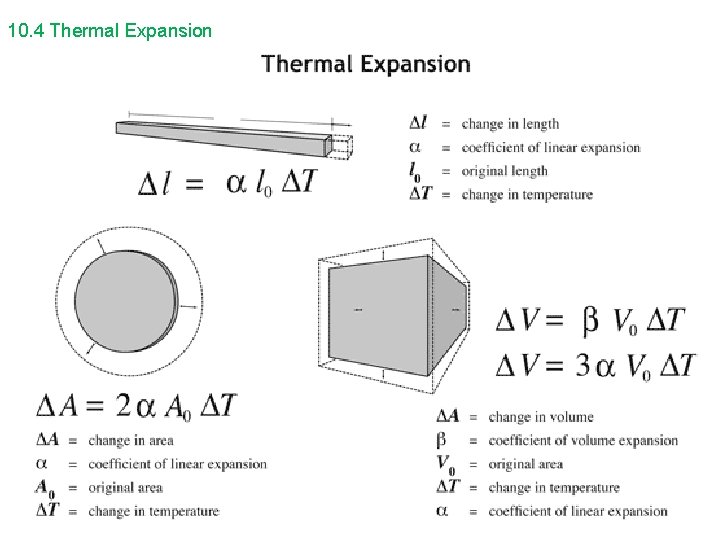
10. 4 Thermal Expansion

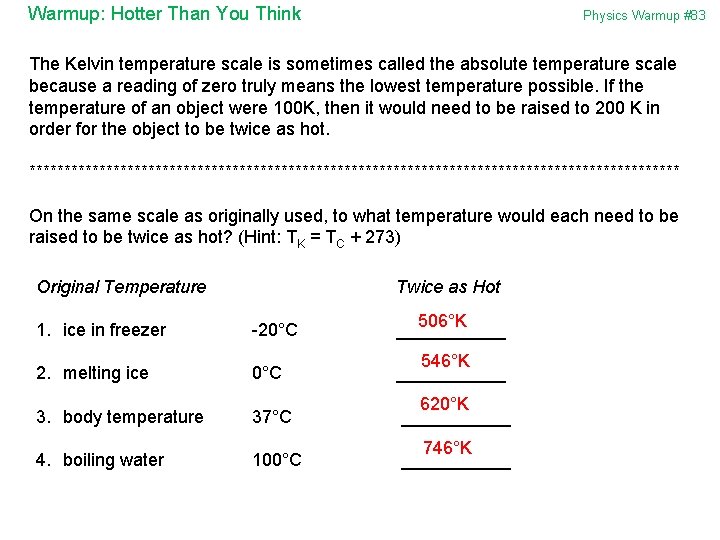
Warmup: Hotter Than You Think Physics Warmup #83 The Kelvin temperature scale is sometimes called the absolute temperature scale because a reading of zero truly means the lowest temperature possible. If the temperature of an object were 100 K, then it would need to be raised to 200 K in order for the object to be twice as hot. *********************************************** On the same scale as originally used, to what temperature would each need to be raised to be twice as hot? (Hint: TK = TC + 273) Original Temperature Twice as Hot 1. ice in freezer -20°C 506°K ______ 2. melting ice 0°C 546°K ______ 3. body temperature 37°C 620°K ______ 4. boiling water 100°C 746°K ______

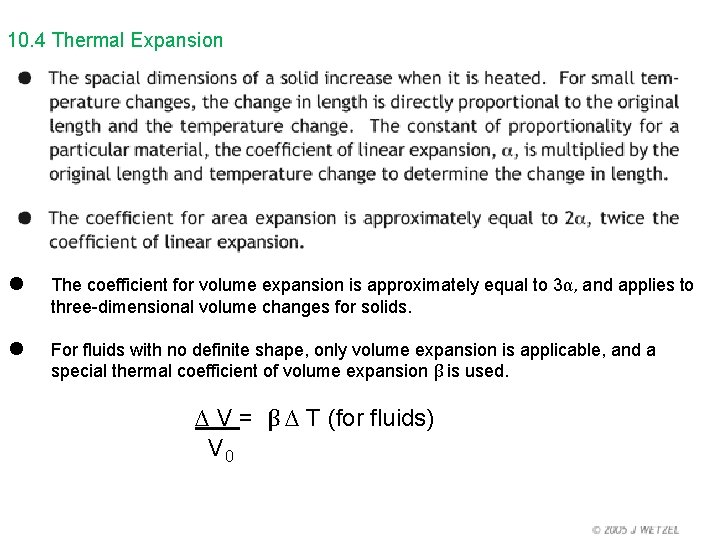
10. 4 Thermal Expansion

10. 4 Thermal Expansion • • The coefficient for volume expansion is approximately equal to 3α, and applies to three-dimensional volume changes for solids. For fluids with no definite shape, only volume expansion is applicable, and a special thermal coefficient of volume expansion β is used. ∆ V = β ∆ T (for fluids) V 0

10. 4 Thermal Expansion Example 10. 6: You are installing some outdoor copper electric wire to a backyard fish pond on a hot 40°C summer day. The temperature could be as low as -20°C in your area during a cold winter night. How much extra wire (minimum) do you have to include to allow for thermal expansion if the distance from the electric service to the pond is 100 m?

10. 4 Thermal Expansion Example 10. 7: A 500 -milliliter glass beaker of water is filled to the rim at a temperature of 0°C. How much water will overflow if the water is heated to a temperature of 95°C? (Ignore the expansion of the beaker, why? )

10. 5 The Kinetic Theory of Gases

Warmup: Loosen Up! I Physics Warmup #82 When energy is added to most objects, they expand. For equal changes in temperature, the amount of expansion depends in part on the dimensions of the object as well as the material it is made of. Two rods of different metals but of equal length would expand different amounts due to the difference in material. Two rods made of the same material but of different lengths would expand different amounts due to the difference in their lengths. *********************************************** A common way to loosen a metal lid that is screwed tightly to a glass jar is to run hot water over the lid and jar top. Explain why this loosens the lid. Answer: The rim of the lid is a circle. As the metal expands, the circumference expands. Metal expands more than glass.

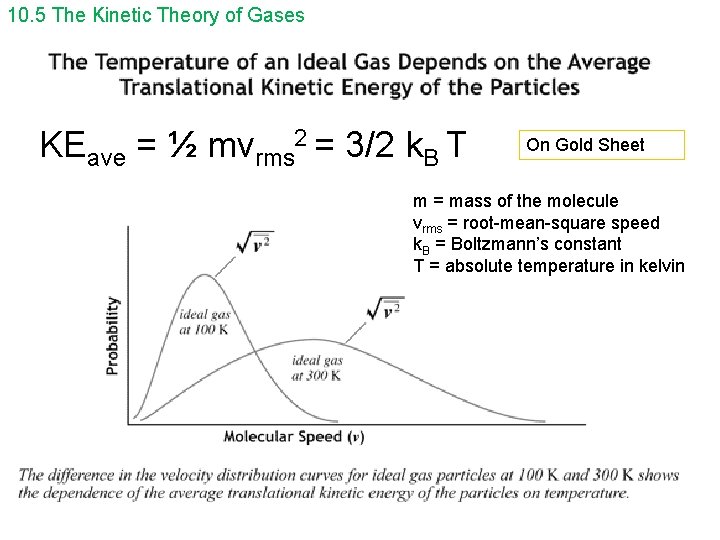
10. 5 The Kinetic Theory of Gases KEave = ½ mvrms 2 = 3/2 k. B T On Gold Sheet m = mass of the molecule vrms = root-mean-square speed k. B = Boltzmann’s constant T = absolute temperature in kelvin

10. 5 The Kinetic Theory of Gases

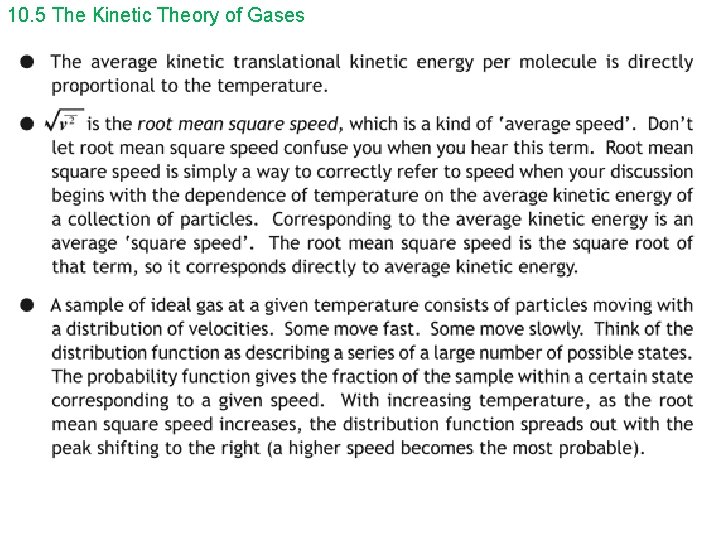
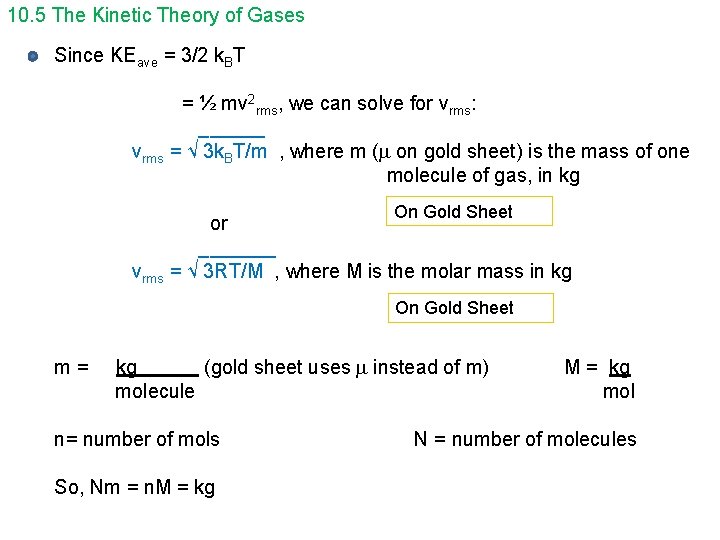
10. 5 The Kinetic Theory of Gases Since KEave = 3/2 k. BT = ½ mv 2 rms, we can solve for vrms: ______ vrms = √ 3 k. BT/m , where m ( on gold sheet) is the mass of one molecule of gas, in kg On Gold Sheet or _______ vrms = √ 3 RT/M , where M is the molar mass in kg On Gold Sheet m= kg (gold sheet uses instead of m) molecule n= number of mols So, Nm = n. M = kg mol N = number of molecules

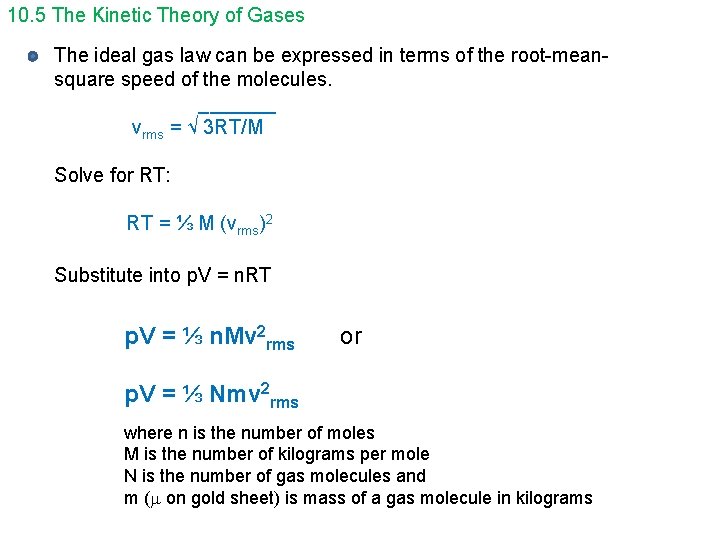
10. 5 The Kinetic Theory of Gases The ideal gas law can be expressed in terms of the root-meansquare speed of the molecules. _______ vrms = √ 3 RT/M Solve for RT: RT = ⅓ M (vrms)2 Substitute into p. V = n. RT p. V = ⅓ n. Mv 2 rms or p. V = ⅓ Nmv 2 rms where n is the number of moles M is the number of kilograms per mole N is the number of gas molecules and m ( on gold sheet) is mass of a gas molecule in kilograms

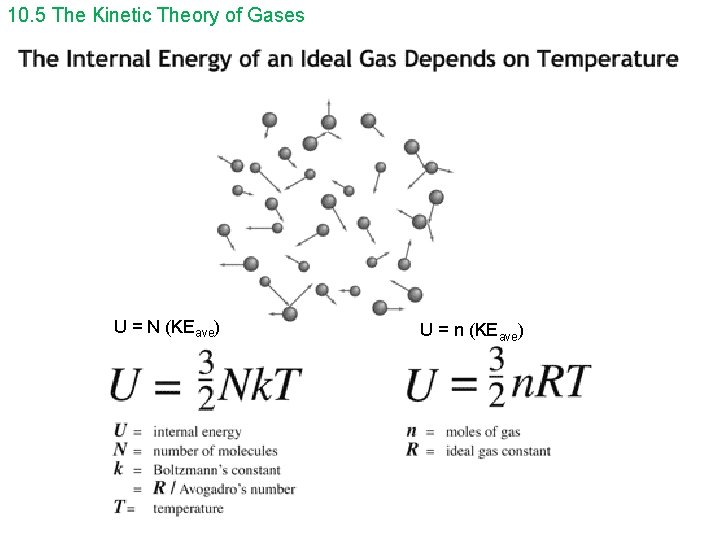
10. 5 The Kinetic Theory of Gases U = N (KEave) U = n (KEave)

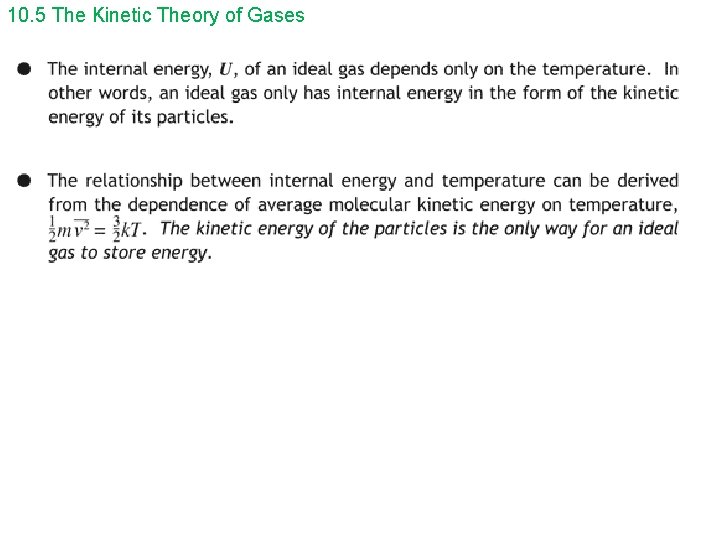
10. 5 The Kinetic Theory of Gases

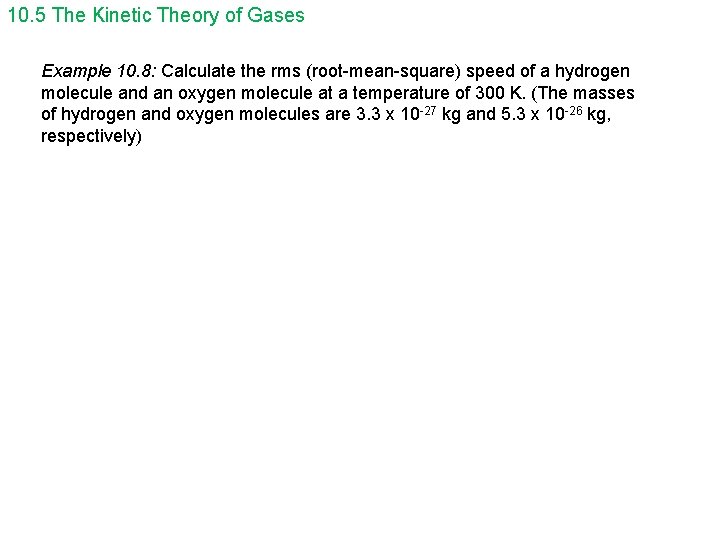
10. 5 The Kinetic Theory of Gases Example 10. 8: Calculate the rms (root-mean-square) speed of a hydrogen molecule and an oxygen molecule at a temperature of 300 K. (The masses of hydrogen and oxygen molecules are 3. 3 x 10 -27 kg and 5. 3 x 10 -26 kg, respectively)

10. 5 The Kinetic Theory of Gases Example 10. 9: If the temperature of a gas increases from 20°C to 40°C, by what factor does the rms speed increase?

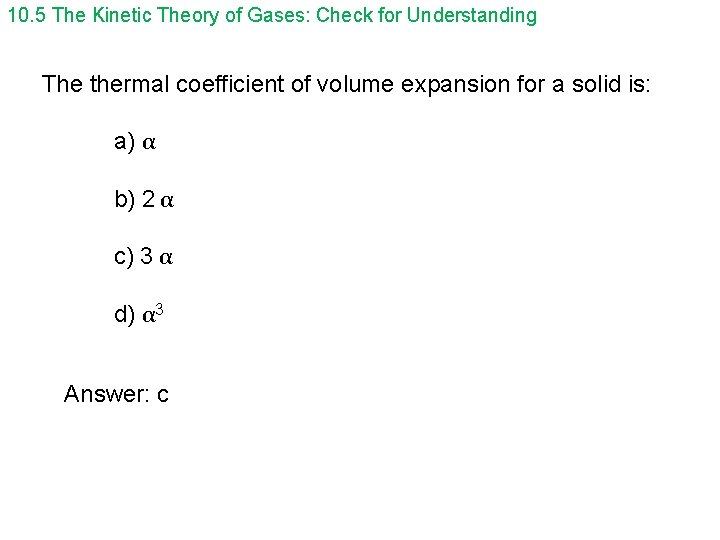
10. 5 The Kinetic Theory of Gases: Check for Understanding The thermal coefficient of volume expansion for a solid is: a) α b) 2 α c) 3 α d) α 3 Answer: c

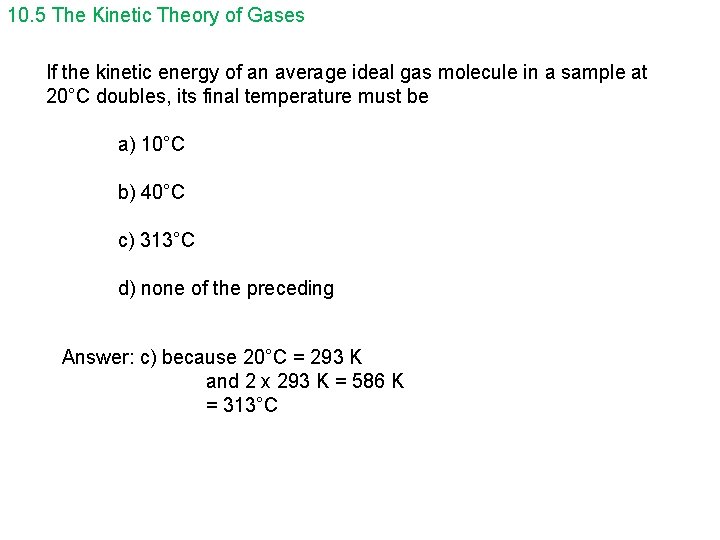
10. 5 The Kinetic Theory of Gases If the kinetic energy of an average ideal gas molecule in a sample at 20°C doubles, its final temperature must be a) 10°C b) 40°C c) 313°C d) none of the preceding Answer: c) because 20°C = 293 K and 2 x 293 K = 586 K = 313°C

10. 5 The Kinetic Theory of Gases If the temperature of a quantity of ideal gas is raised from 20°C to 40°C, its internal energy is a) doubled b) tripled c) unchanged d) none of the preceding Answer: d) Internal energy is proportional to the Kelvin temperature.

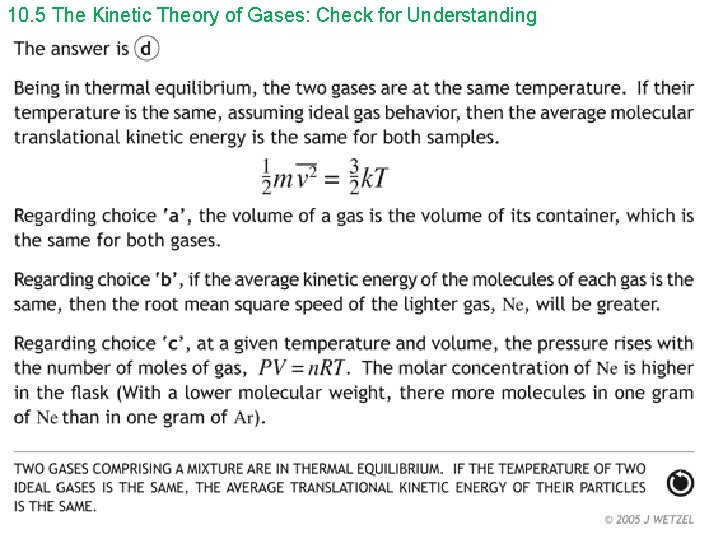
10. 5 The Kinetic Theory of Gases: Check for Understanding

10. 5 The Kinetic Theory of Gases: Check for Understanding

Homework 10. B • HW 10. B: 45 -49, 65 -68, 71.
