AP Chemistry th Zumdahl Notes 9 ed A









- Slides: 17

AP Chemistry th Zumdahl Notes, 9 ed. A Brief Collection of notes, Chapter 8 Feel free to open these files and annotate as you feel the need…this is for your success.

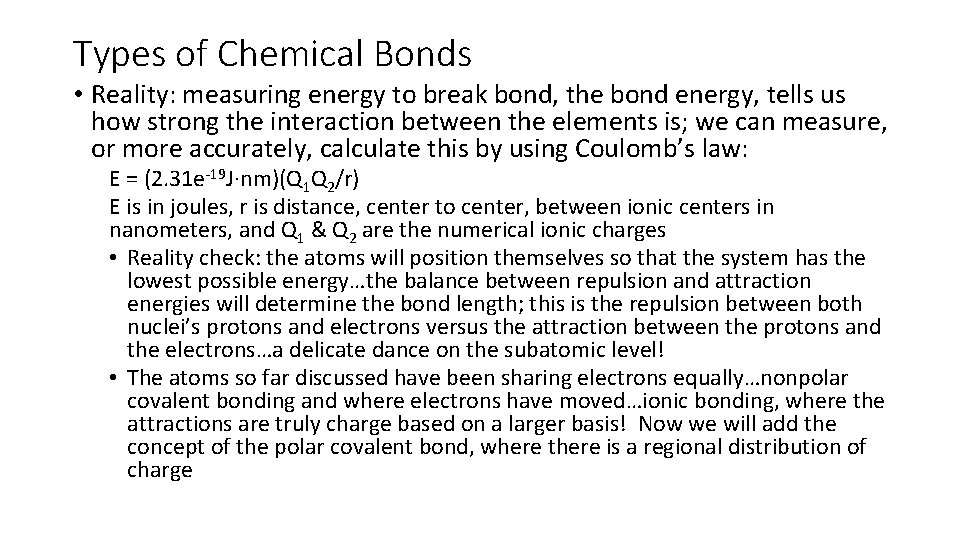
Types of Chemical Bonds • Reality: measuring energy to break bond, the bond energy, tells us how strong the interaction between the elements is; we can measure, or more accurately, calculate this by using Coulomb’s law: E = (2. 31 e-19 J·nm)(Q 1 Q 2/r) E is in joules, r is distance, center to center, between ionic centers in nanometers, and Q 1 & Q 2 are the numerical ionic charges • Reality check: the atoms will position themselves so that the system has the lowest possible energy…the balance between repulsion and attraction energies will determine the bond length; this is the repulsion between both nuclei’s protons and electrons versus the attraction between the protons and the electrons…a delicate dance on the subatomic level! • The atoms so far discussed have been sharing electrons equally…nonpolar covalent bonding and where electrons have moved…ionic bonding, where the attractions are truly charge based on a larger basis! Now we will add the concept of the polar covalent bond, where there is a regional distribution of charge

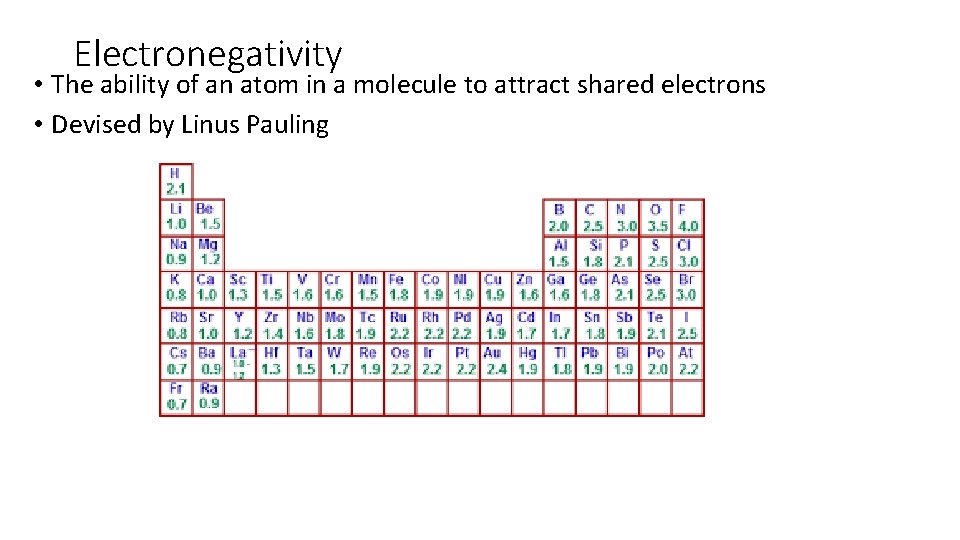
Electronegativity • The ability of an atom in a molecule to attract shared electrons • Devised by Linus Pauling

Bond Polarity and Dipole Moments • The differential between two atoms in a bond, or the overall differential across a molecule…if the differential ≠ zero, there is some polarity and the existence of a dipole moment • Reality check: there can be polar bonds and no net polarity on the molecule…they cancel each other out! • Don’t forget resonance structures…may end out similarly

Ions: electron configurations • Realization: when most atoms form a stable compound, they achieve a noble gas configuration of their valence electrons, either through gain/loss or sharing • Realization: some transition metals will vary from these expectations…it is part of their “deal” with the d-orbital electrons, that they can have options, which means we have to remember to consider these situations…memorizing them, or at least being cognizant of their existence

Ions: sizes • Considerations • Original size of atom • Did it gain or lose electrons? • Isoelectronic ions: may have the same number of electrons, but size will vary due to effective nuclear charge…more protons typically means smaller size

Energy Effects in Binary ionic compounds • Lattice energy: energy to form ionic solid from separated gaseous ions • Realize steps involved: solid metal must sublimate…this takes energy! • Ionization of metal in gas phase • For formation of molecule, may involve dissociation of covalent nonmetal molecule into separate atoms • Sublimation or vaporization if not already in gas phase • Ionization of nonmetal in gas phase • Formation of solid compound from gaseous ions • (see table/explanation, page 366) • Read explanation on 366 -367 for lattice energy calculations…lattice energy greatly exceeds energy of ionization, whether first or second, for an element with more than one valence electron…need to realize that if the compound is not favored, it won’t form

Partial Ionic Character of Covalent Bonds • Can be calculated: see 369 for equation • Reality check: there are likely no totally ionic nor covalent bonds…between intramolecular attractions, etc, we must all recognize that electrons are not stationary • Be thoughtful…if not the same element, any bond has some degree of ionic character aka polarity…right?

Covalent chemical bond: a model • First question: why do bonds occur? Bonds are the result of a tendency to seek a lower energy place…this should be intuitive at this point. • Why use a model? • We are attempting to show something we have difficulty understanding otherwise • To give us a framework for better understanding…allowing us to categorize, predict, etc, so that we know where we are going

Covalent chemical bond: a model: realities! • Models are invented…they are not reality, usually • Models are frequently incorrect in their entirety…oversimplified, at the least! • As they age, we tend to patch and add more detail, as this “works!” • Prior to using any model, we need to consider its assumptions…the simpler the model, the more likely there to be larger assumptions, which therefore leads to the opportunity for greater distortion from what “really” is happening; we also need to realize that even with rules, we can have exceptions • Sometimes our greatest learning comes from when something disobeys what we think is a rule!

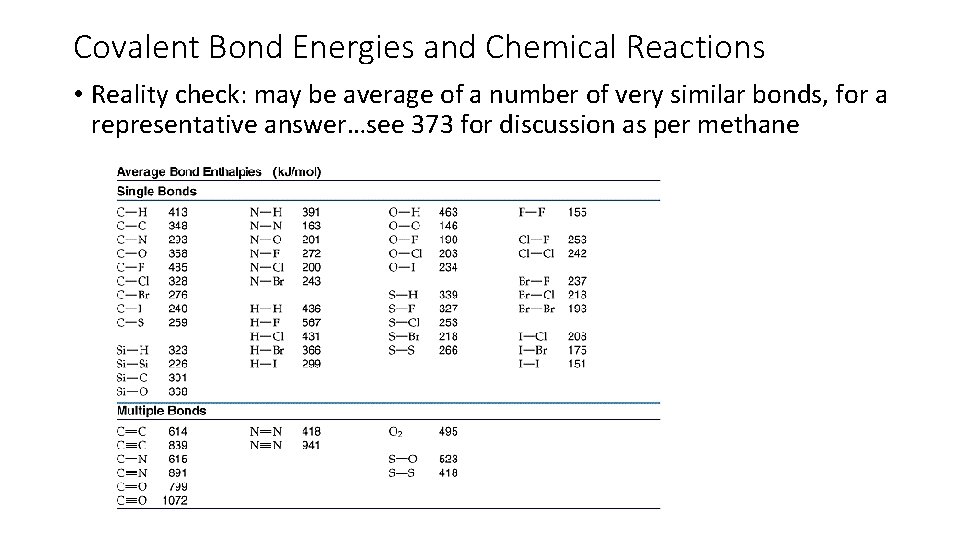
Covalent Bond Energies and Chemical Reactions • Reality check: may be average of a number of very similar bonds, for a representative answer…see 373 for discussion as per methane

Bond energies and enthalpy • Review: ΔH is the sum of the energies to break old bonds (+) plus the sum of the energies released in the formation of new bonds (-) • Examples: see 375 -376

Localized electron bonding model & Lewis Structures • General idea: atoms comprising a molecule are bound together by the shared pairs of electrons using atomic orbitals of these bonded atoms • 3 parts • Lewis structures can describe the structure of the molecule • VSEPR can redict the molecule’s shape • We will be able to describe the types of atomic orbitals used to either share pairs of electrons or hold lone/un-bonded pairs of electrons • Lewis structures • Symbol represents nucleus, inner filled levels of electrons; dots represent valence electrons only • Duet, octet rules; electrons may or may not originate from a given atom to make the correct structure; some molecules/elements may deviate from following the octet rule: P, S, Cl, Br, I, Kr, Xe, Se; violators of duet rule: H, He, Li, Be, B; important caveat: these atoms must be the central atom!

Exceptions to the Octet Rule • Some molecules/elements deviate from duet rule: H, He, Li, Be, B; • Others may deviate from following the octet rule: P, S, Cl, Br, I, Kr, Xe, Se; these occur as they have d orbitals available to allow foe expansion; note rules, page 382 • Important caveat: these atoms must be the central atom!

Resonance • Resonance is considered to occur when there is more than one valid Lewis structure which can be written for a given molecule…the resulting structure is considered an average of the structures. This is borne out by experimental results measuring the bonds of the actual molecules…see drawing, page 385 • There a few molecules where we do not have enough electrons…nitric oxide comes to mind…realize that this is an extremely reactive molecule and it has a short half-life before it reacts with more oxygen

Formal charge • Defined as the difference between the number of valence electrons on the free atom and the number of valence electrons assigned to the atom in the given molecule • Lone pairs belong entirely to the given atom • Shared electrons are dived equally between the two atoms sharing them • Valence electronsassigned = (# lone pair electrons) + ½(# shared electrons) • Resources • http: //www. chem. sc. edu/faculty/shimizu/333/Chem_333/1 a. iii. html • http: //www. masterorganicchemistry. com/2010/09/24/how-to-calculateformal-charge/ • http: //www. mhhe. com/physsci/chemistry/carey/student/olc/ch 01 lewis. html

VSEPR • Review: determining the structure based on the repulsion of the electron domains around the central atom • Resources • http: //chemed. chem. purdue. edu/genchem/topicreview/bp/ch 8/vsepr. html • http: //intro. chem. okstate. edu/1314 f 97/chapter 9/vsepr. html • Practice: https: //phet. colorado. edu/en/simulation/molecule-shapes