AP Chemistry th Zumdahl Notes 9 ed A





- Slides: 8

AP Chemistry th Zumdahl Notes, 9 ed. A Brief Collection of notes, Chapter 11 Feel free to open these files and annotate as you feel the need…this is for your success.

Solution Composition • Molarity: good with this? Moles solute/liters solution • Mass percent: (mass solute)/(mass solution)x 100=mass percent • Mole fraction: mole A/moles (A + B) • Molality: moles solute/kg solvent • Usually used for colligative property calculations (boiling pt elevation, freezing pt depression)

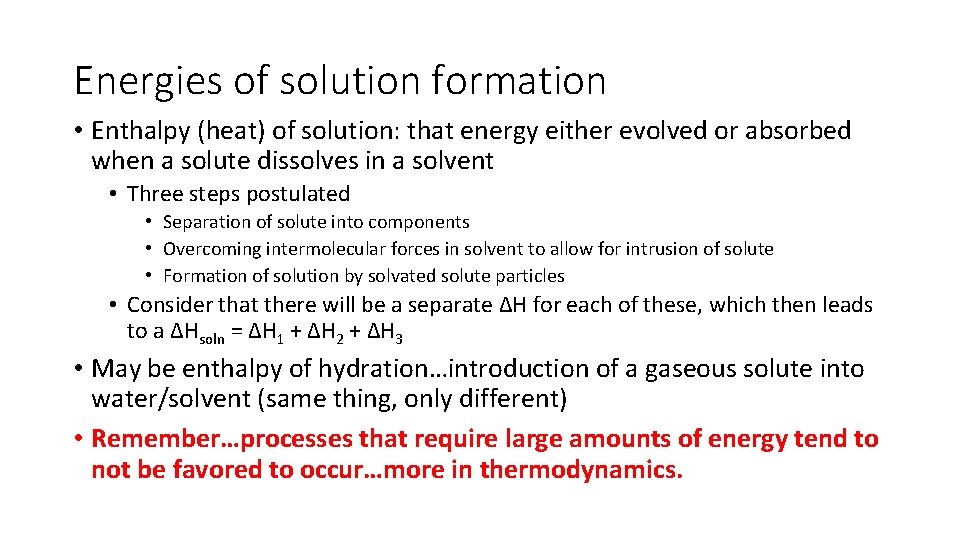
Energies of solution formation • Enthalpy (heat) of solution: that energy either evolved or absorbed when a solute dissolves in a solvent • Three steps postulated • Separation of solute into components • Overcoming intermolecular forces in solvent to allow for intrusion of solute • Formation of solution by solvated solute particles • Consider that there will be a separate ∆H for each of these, which then leads to a ∆Hsoln = ∆H 1 + ∆H 2 + ∆H 3 • May be enthalpy of hydration…introduction of a gaseous solute into water/solvent (same thing, only different) • Remember…processes that require large amounts of energy tend to not be favored to occur…more in thermodynamics.

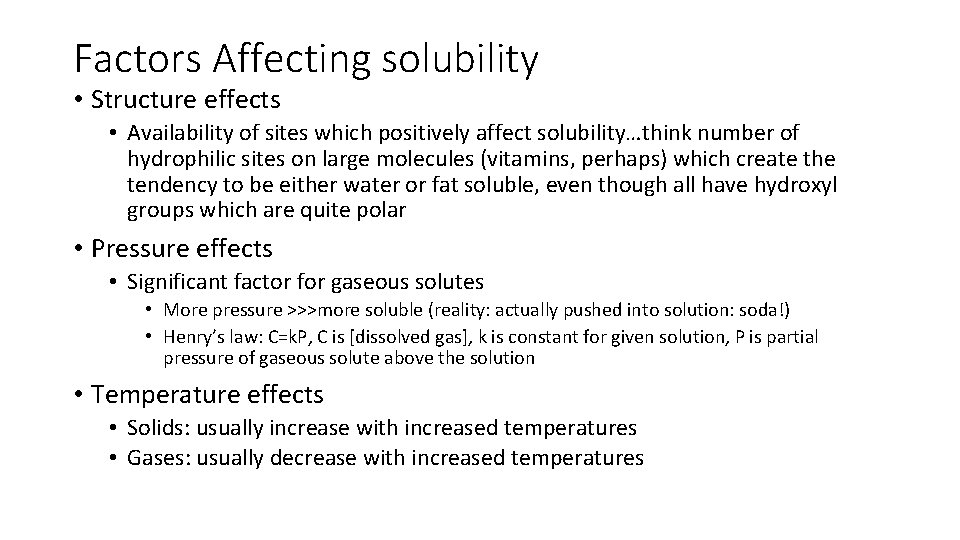
Factors Affecting solubility • Structure effects • Availability of sites which positively affect solubility…think number of hydrophilic sites on large molecules (vitamins, perhaps) which create the tendency to be either water or fat soluble, even though all have hydroxyl groups which are quite polar • Pressure effects • Significant factor for gaseous solutes • More pressure >>>more soluble (reality: actually pushed into solution: soda!) • Henry’s law: C=k. P, C is [dissolved gas], k is constant for given solution, P is partial pressure of gaseous solute above the solution • Temperature effects • Solids: usually increase with increased temperatures • Gases: usually decrease with increased temperatures

Vapor pressures of solutions • Raoult’s law: Psoln = Xsolvent. P⁰solvent where psoln is observed vapor pressure of solution, Xsolvent is mole fraction of solvent, P⁰solvent is vapor pressure of pure solvent (page 521) • Reality check…be thoughtful about number of particles evolved when a given solute dissolves…might be 2, 3 or… • Nonideal solutions…uh oh…suppose both components are volatile…now our equation gets tricky (page 534)

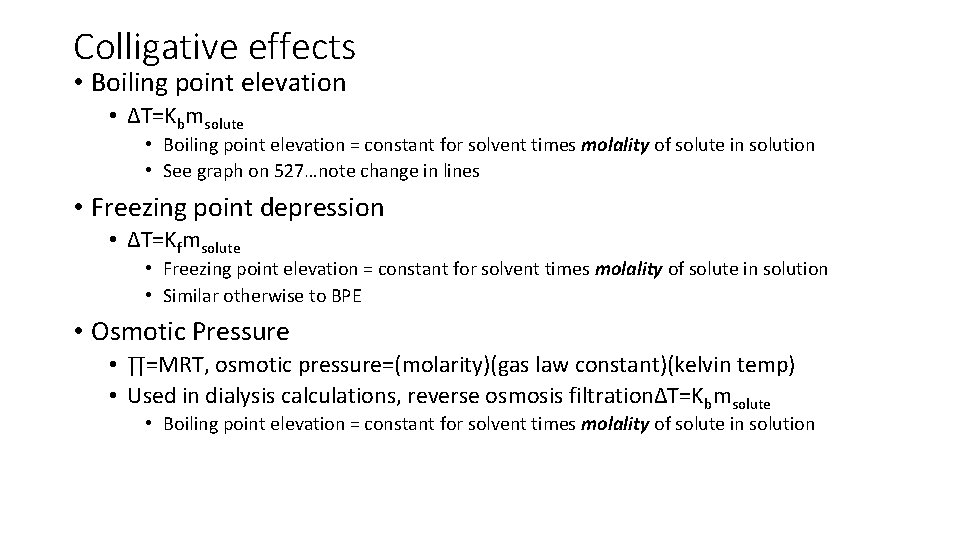
Colligative effects • Boiling point elevation • ∆T=Kbmsolute • Boiling point elevation = constant for solvent times molality of solute in solution • See graph on 527…note change in lines • Freezing point depression • ∆T=Kfmsolute • Freezing point elevation = constant for solvent times molality of solute in solution • Similar otherwise to BPE • Osmotic Pressure • ∏=MRT, osmotic pressure=(molarity)(gas law constant)(kelvin temp) • Used in dialysis calculations, reverse osmosis filtration∆T=Kbmsolute • Boiling point elevation = constant for solvent times molality of solute in solution

Colligative properties of electrolyte solutions • Van’t Hoff factor: i=(moles of particles in solution)/(moles solute dissolved) • What is this saying? If your solute dissolves into 2 ions, ideally 2 x ions. If it dissolves into 3 ions, 3 x effect (this is why we usually use Ca. Cl 2 instead of Na. Cl for road salting… 1. 5 x more effect)

Colloids • May also be called a colloidal suspension • Small particles which stay suspended in the solvent • Why? Usually seems to be electrostatic repulsion of the particles • Tyndall effect: scatters light • Milk, soot, fog, …see table, page 538 • Coagulation…when the colloid collapses, and the susupended material aggregates into large particles, usually settling out • Problems: (starting on page 543): 22, 32, 43, 57, 69, 91, 96, 108 a, as a group: 132