ANTIPSYCHOTIC DRUG CLASSES 4 th Year Pharmacy 2015




![Phenothiazines Propyl dialkylamino side chain • Promazine Hydrochloride 10 -[3 -(dimethylamino)propyl]phenothiazine monohydrochloride Phenothiazines Propyl dialkylamino side chain • Promazine Hydrochloride 10 -[3 -(dimethylamino)propyl]phenothiazine monohydrochloride](https://slidetodoc.com/presentation_image_h/24be7ce4931bb7dfa64c05611adcc186/image-11.jpg)
![Phenothiazines Propyl piperazine side chain Prochlorperazine maleate 2 -chloro-10 -[3 -(4 -methyl-1 -piperazinyl)propyl]phenothiazine Phenothiazines Propyl piperazine side chain Prochlorperazine maleate 2 -chloro-10 -[3 -(4 -methyl-1 -piperazinyl)propyl]phenothiazine](https://slidetodoc.com/presentation_image_h/24be7ce4931bb7dfa64c05611adcc186/image-12.jpg)

![Thioxanthene Derivatives Thiothixene (Navane) 2 -N, N-dimethyl-9 -[3 -(4 -methyl-1 -piprazinyl)propylene]thioxanthene- 2 sulfonamide Thioxanthene Derivatives Thiothixene (Navane) 2 -N, N-dimethyl-9 -[3 -(4 -methyl-1 -piprazinyl)propylene]thioxanthene- 2 sulfonamide](https://slidetodoc.com/presentation_image_h/24be7ce4931bb7dfa64c05611adcc186/image-21.jpg)
- Slides: 25

ANTIPSYCHOTIC DRUG CLASSES 4 th Year Pharmacy 2015 -2016

PHENOTHIAZINE DERIVATIVES The first-used class of efficacious antipsychotic • agents – Chlorpromazine, the prototypical member of this class, was first used to treat • mental disease in 1951. • Nonselective DA-receptor antagonists; also • act at other neurotransmitter receptors giving rise to significant AR profiles •

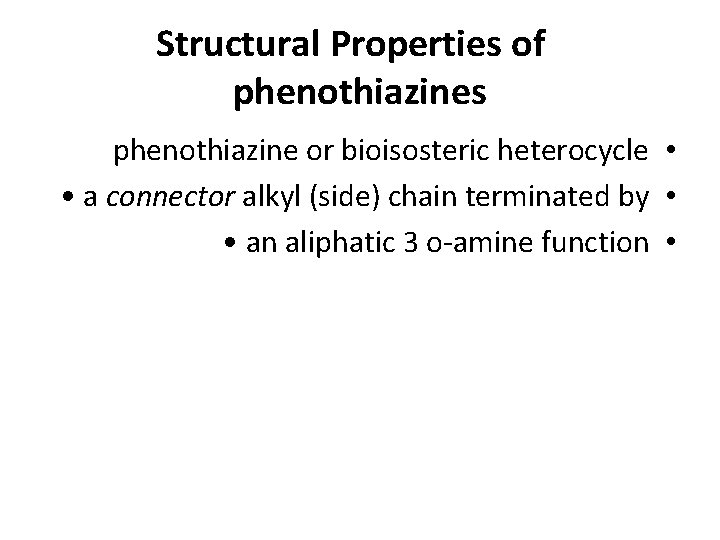
Structural Properties of phenothiazines phenothiazine or bioisosteric heterocycle • • a connector alkyl (side) chain terminated by • • an aliphatic 3 o-amine function •

The most important types of psychosis are: Schizophrenia. i Affective disorders (e. g. depression mania). ii Organic psychoses (mental disturbances caused . iii by head injury or alcoholism ……. .

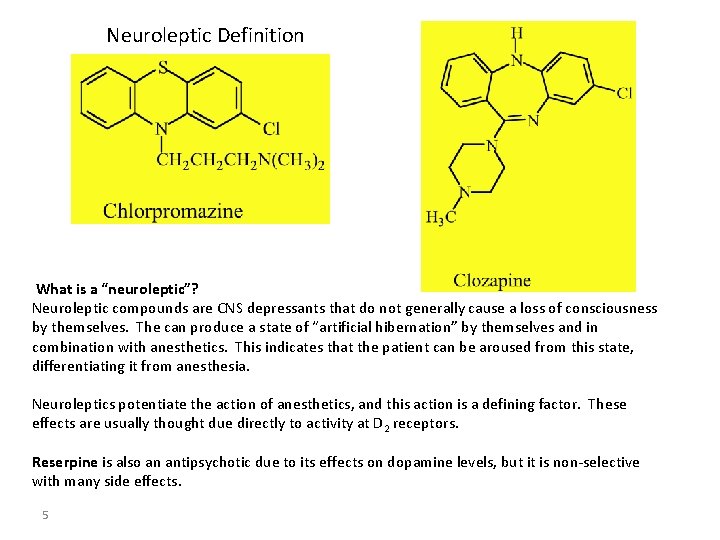
Neuroleptic Definition What is a “neuroleptic”? Neuroleptic compounds are CNS depressants that do not generally cause a loss of consciousness by themselves. The can produce a state of “artificial hibernation” by themselves and in combination with anesthetics. This indicates that the patient can be aroused from this state, differentiating it from anesthesia. Neuroleptics potentiate the action of anesthetics, and this action is a defining factor. These effects are usually thought due directly to activity at D 2 receptors. Reserpine is also an antipsychotic due to its effects on dopamine levels, but it is non-selective with many side effects. 5

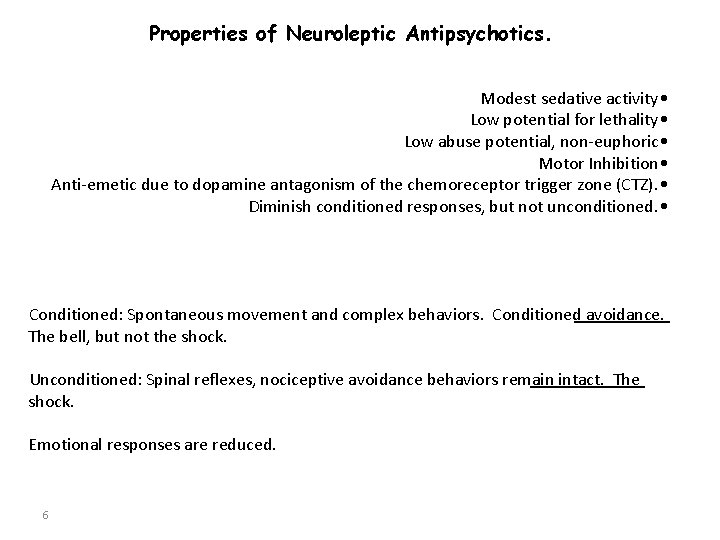
Properties of Neuroleptic Antipsychotics. Modest sedative activity • Low potential for lethality • Low abuse potential, non-euphoric • Motor Inhibition • Anti-emetic due to dopamine antagonism of the chemoreceptor trigger zone (CTZ). • Diminish conditioned responses, but not unconditioned. • Conditioned: Spontaneous movement and complex behaviors. Conditioned avoidance. The bell, but not the shock. Unconditioned: Spinal reflexes, nociceptive avoidance behaviors remain intact. The shock. Emotional responses are reduced. 6

Classification Rauwolfia alkaloids and synthetic analogues. 1 Phenothiazine and thioxanthene derivatives. 2 Butyrophenones. 3 Diphenylmethane derivatives. 4 Diphenylbutylpiperidines. 5 Miscellaneous (benzamide derivatives and . 6 tricyclic heterocyclic analogues)

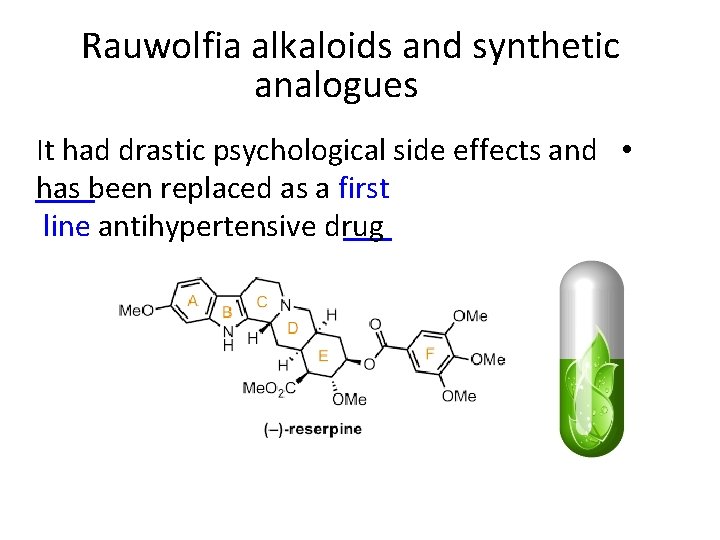
Rauwolfia alkaloids and synthetic analogues It had drastic psychological side effects and • has been replaced as a first line antihypertensive drug

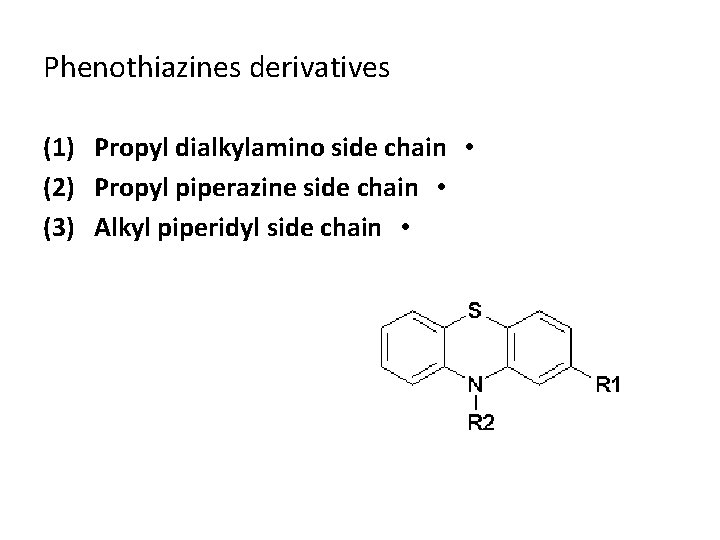
Phenothiazines derivatives (1) (2) (3) Propyl dialkylamino side chain • Propyl piperazine side chain • Alkyl piperidyl side chain •

Phenothiazines Propyl dialkylamino side chain • Chlorpromazine Hydrochloride ( Thorazine ) 2 -chloro-10 -[3 -(dimethylamino)propyl] phenothiazine
![Phenothiazines Propyl dialkylamino side chain Promazine Hydrochloride 10 3 dimethylaminopropylphenothiazine monohydrochloride Phenothiazines Propyl dialkylamino side chain • Promazine Hydrochloride 10 -[3 -(dimethylamino)propyl]phenothiazine monohydrochloride](https://slidetodoc.com/presentation_image_h/24be7ce4931bb7dfa64c05611adcc186/image-11.jpg)
Phenothiazines Propyl dialkylamino side chain • Promazine Hydrochloride 10 -[3 -(dimethylamino)propyl]phenothiazine monohydrochloride
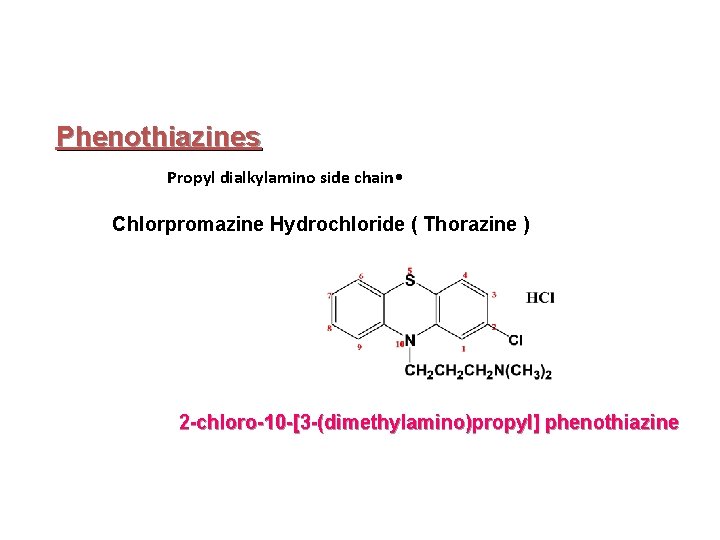
![Phenothiazines Propyl piperazine side chain Prochlorperazine maleate 2 chloro10 3 4 methyl1 piperazinylpropylphenothiazine Phenothiazines Propyl piperazine side chain Prochlorperazine maleate 2 -chloro-10 -[3 -(4 -methyl-1 -piperazinyl)propyl]phenothiazine](https://slidetodoc.com/presentation_image_h/24be7ce4931bb7dfa64c05611adcc186/image-12.jpg)
Phenothiazines Propyl piperazine side chain Prochlorperazine maleate 2 -chloro-10 -[3 -(4 -methyl-1 -piperazinyl)propyl]phenothiazine

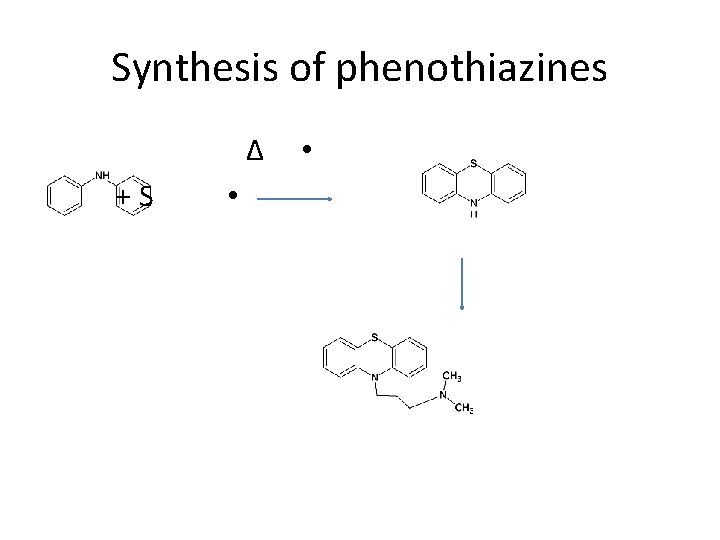
Synthesis of phenothiazines Δ • + S •

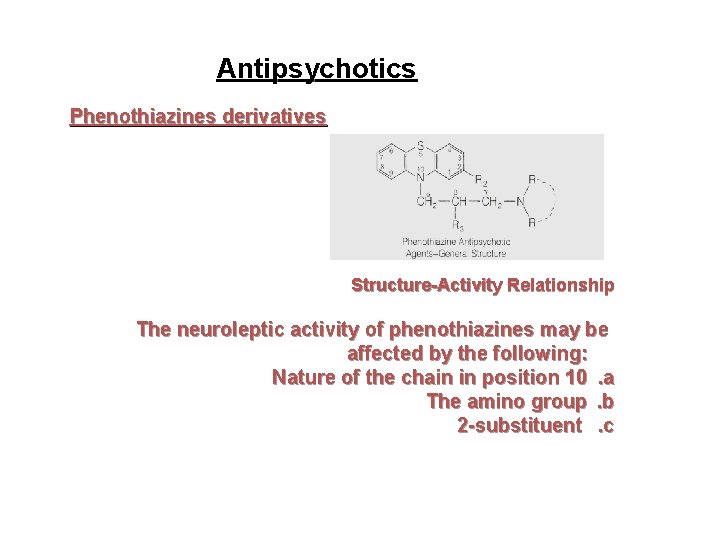
Antipsychotics Phenothiazines derivatives Structure-Activity Relationship The neuroleptic activity of phenothiazines may be affected by the following: Nature of the chain in position 10. a The amino group. b 2 -substituent. c

Replacement of H in position 2 Substitution at position 3 Substitution at position 1 Three carbon chain… Branching with larger groups. . Replacement of terminal alkyl amino group • • •

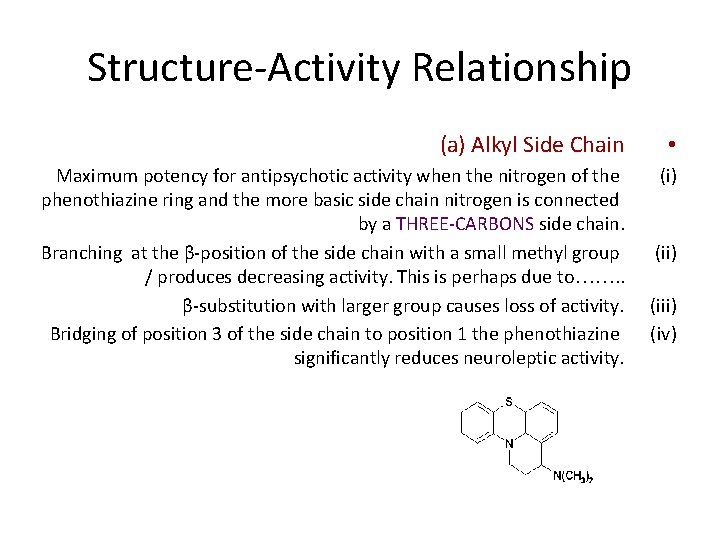
Structure-Activity Relationship (a) Alkyl Side Chain • Maximum potency for antipsychotic activity when the nitrogen of the phenothiazine ring and the more basic side chain nitrogen is connected by a THREE-CARBONS side chain. Branching at the β-position of the side chain with a small methyl group / produces decreasing activity. This is perhaps due to……. . β-substitution with larger group causes loss of activity. Bridging of position 3 of the side chain to position 1 the phenothiazine significantly reduces neuroleptic activity. (i) (iii) (iv)

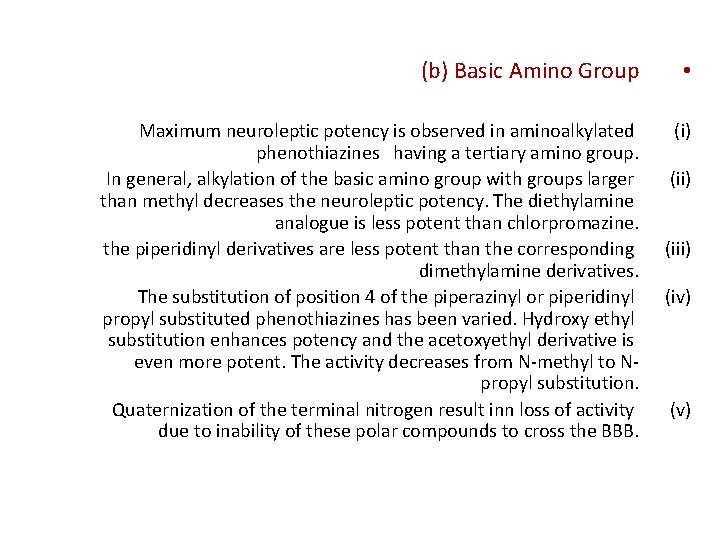
(b) Basic Amino Group • Maximum neuroleptic potency is observed in aminoalkylated phenothiazines having a tertiary amino group. In general, alkylation of the basic amino group with groups larger than methyl decreases the neuroleptic potency. The diethylamine analogue is less potent than chlorpromazine. the piperidinyl derivatives are less potent than the corresponding dimethylamine derivatives. The substitution of position 4 of the piperazinyl or piperidinyl propyl substituted phenothiazines has been varied. Hydroxy ethyl substitution enhances potency and the acetoxyethyl derivative is even more potent. The activity decreases from N-methyl to Npropyl substitution. Quaternization of the terminal nitrogen result inn loss of activity due to inability of these polar compounds to cross the BBB. (i) (iii) (iv) (v)

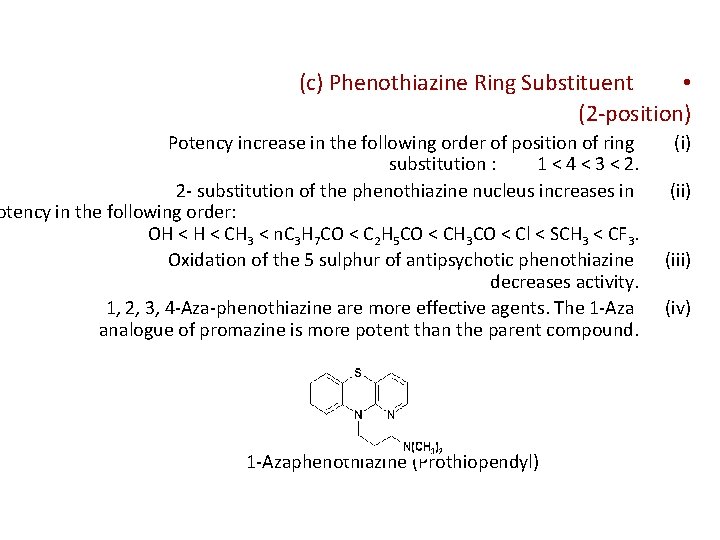
(c) Phenothiazine Ring Substituent • (2 -position) Potency increase in the following order of position of ring substitution : 1 < 4 < 3 < 2. 2 - substitution of the phenothiazine nucleus increases in otency in the following order: OH < CH 3 < n. C 3 H 7 CO < C 2 H 5 CO < CH 3 CO < Cl < SCH 3 < CF 3. Oxidation of the 5 sulphur of antipsychotic phenothiazine decreases activity. 1, 2, 3, 4 -Aza-phenothiazine are more effective agents. The 1 -Aza analogue of promazine is more potent than the parent compound. 1 -Azaphenothiazine (Prothiopendyl) (ii) (iii) (iv)

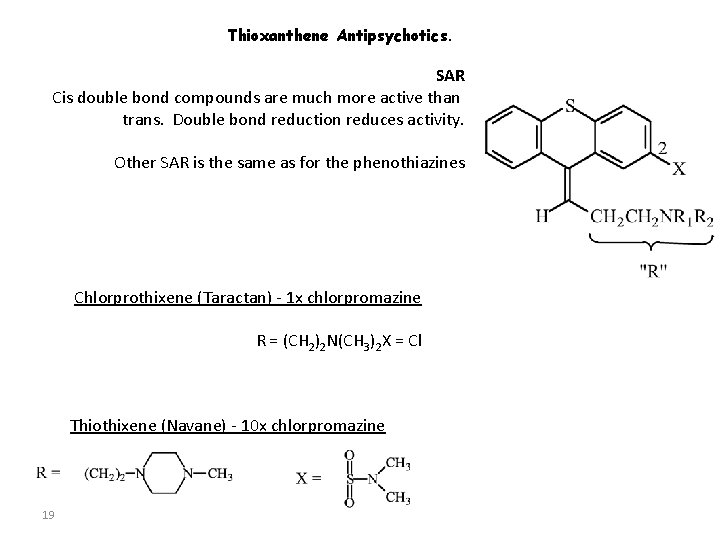
Thioxanthene Antipsychotics. SAR Cis double bond compounds are much more active than trans. Double bond reduction reduces activity. Other SAR is the same as for the phenothiazines Chlorprothixene (Taractan) - 1 x chlorpromazine R = (CH 2)2 N(CH 3)2 X = Cl Thiothixene (Navane) - 10 x chlorpromazine 19

Thioxanthenes derived by isosteric replacement of • one atom in the structure of phenothiazine. The compounds possess may clinical useful pharmacologic properties in common with phenothiazines. They are indicated for the management of the • manifestation of acute and chronic schizophrenia.
![Thioxanthene Derivatives Thiothixene Navane 2 N Ndimethyl9 3 4 methyl1 piprazinylpropylenethioxanthene 2 sulfonamide Thioxanthene Derivatives Thiothixene (Navane) 2 -N, N-dimethyl-9 -[3 -(4 -methyl-1 -piprazinyl)propylene]thioxanthene- 2 sulfonamide](https://slidetodoc.com/presentation_image_h/24be7ce4931bb7dfa64c05611adcc186/image-21.jpg)
Thioxanthene Derivatives Thiothixene (Navane) 2 -N, N-dimethyl-9 -[3 -(4 -methyl-1 -piprazinyl)propylene]thioxanthene- 2 sulfonamide

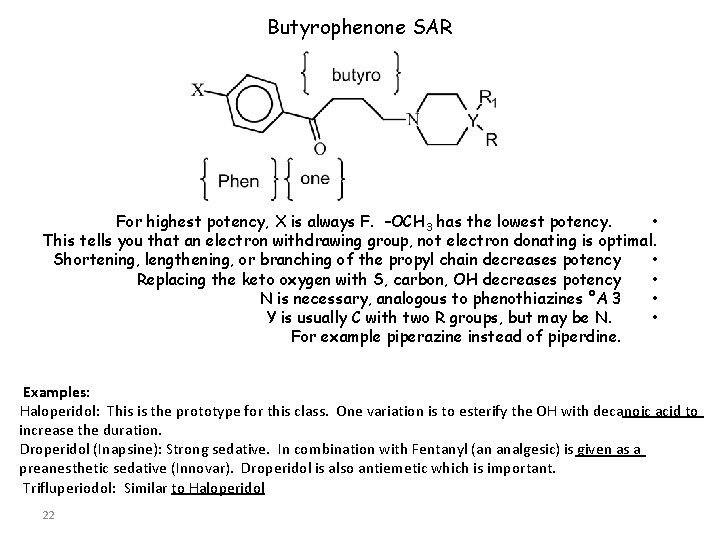
Butyrophenone SAR For highest potency, X is always F. –OCH 3 has the lowest potency. • This tells you that an electron withdrawing group, not electron donating is optimal. Shortening, lengthening, or branching of the propyl chain decreases potency • Replacing the keto oxygen with S, carbon, OH decreases potency • N is necessary, analogous to phenothiazines °A 3 • Y is usually C with two R groups, but may be N. • For example piperazine instead of piperdine. Examples: Haloperidol: This is the prototype for this class. One variation is to esterify the OH with decanoic acid to increase the duration. Droperidol (Inapsine): Strong sedative. In combination with Fentanyl (an analgesic) is given as a preanesthetic sedative (Innovar). Droperidol is also antiemetic which is important. Trifluperiodol: Similar to Haloperidol 22

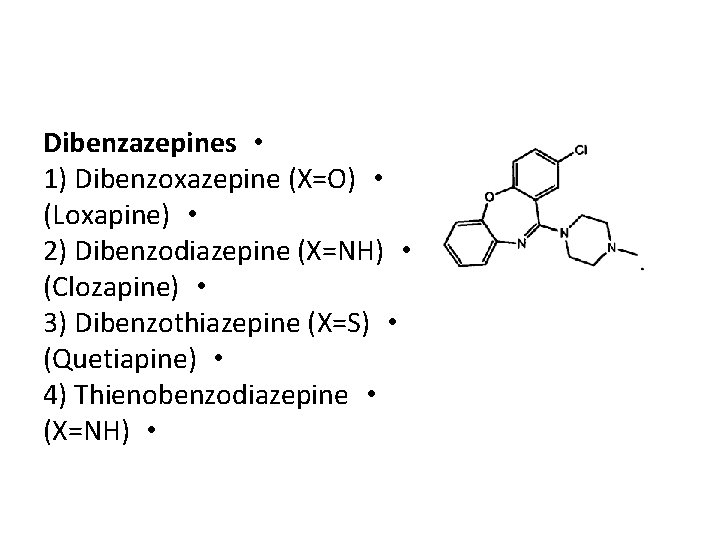
Dibenzazepines • 1) Dibenzoxazepine (X=O) • (Loxapine) • 2) Dibenzodiazepine (X=NH) • (Clozapine) • 3) Dibenzothiazepine (X=S) • (Quetiapine) • 4) Thienobenzodiazepine • (X=NH) •

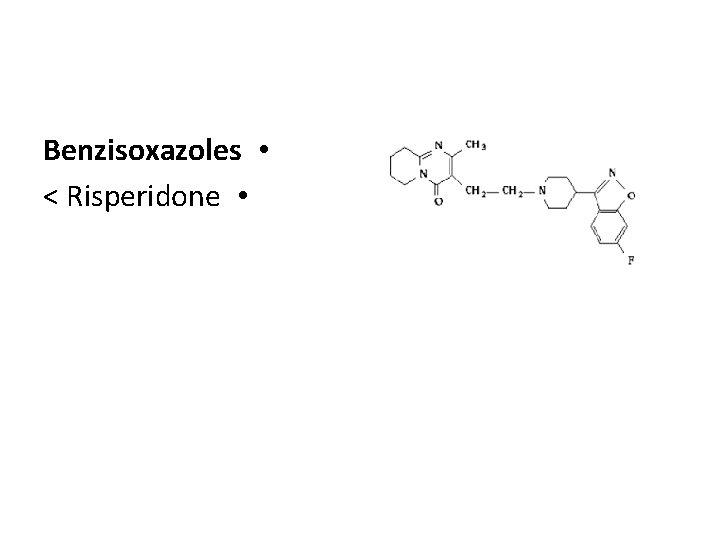
Benzisoxazoles • < Risperidone •

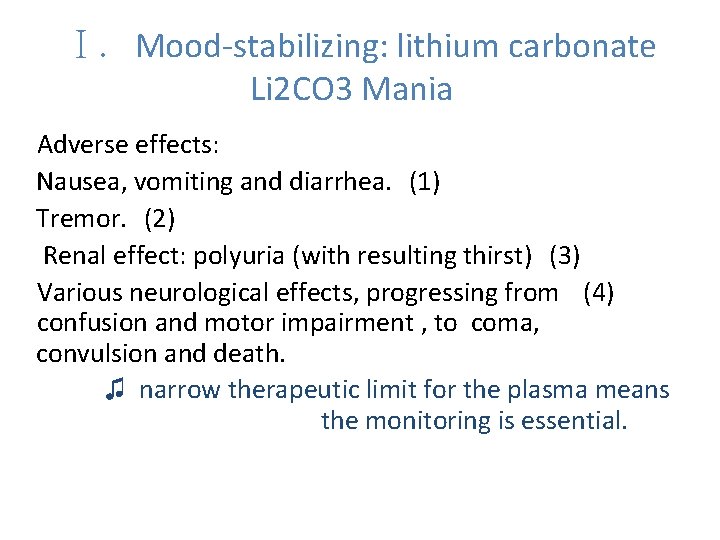
Ⅰ. Mood-stabilizing: lithium carbonate Li 2 CO 3 Mania Adverse effects: Nausea, vomiting and diarrhea. (1) Tremor. (2) Renal effect: polyuria (with resulting thirst) (3) Various neurological effects, progressing from (4) confusion and motor impairment , to coma, convulsion and death. ♫ narrow therapeutic limit for the plasma means the monitoring is essential.
 Antipsychotic drugs classification
Antipsychotic drugs classification Types of adulteration of crude drugs
Types of adulteration of crude drugs M
M Classe e subclasse de palavras 3.o ano
Classe e subclasse de palavras 3.o ano Pre ap classes vs regular classes
Pre ap classes vs regular classes How to transfer prescription to costco
How to transfer prescription to costco Complete floor stock system
Complete floor stock system Drug basket method
Drug basket method 2015 calendar year
2015 calendar year Tiny feet poem
Tiny feet poem Iso 37001 audit checklist
Iso 37001 audit checklist Saasta astro quiz 2015 answers
Saasta astro quiz 2015 answers Human development report 2015
Human development report 2015 Perkap no 18 tahun 2015
Perkap no 18 tahun 2015 Dovitinib
Dovitinib Forrester identity governance
Forrester identity governance Spring break 2015
Spring break 2015 Ncae test results
Ncae test results 2 novembre 2015
2 novembre 2015 Afs 2015 4
Afs 2015 4 Paebes 2015
Paebes 2015 Imbens rubin 2015
Imbens rubin 2015 Child care and protection act 3 of 2015
Child care and protection act 3 of 2015 2014 geografijos egzamino atsakymai
2014 geografijos egzamino atsakymai Ccslc results
Ccslc results Gallup employee engagement index 2012
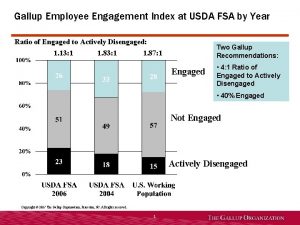
Gallup employee engagement index 2012