ANGIOLITE TRIAL Noninferiority Randomized Clinical Trial comparing the








- Slides: 28

ANGIOLITE TRIAL Non-inferiority Randomized Clinical Trial comparing the efficacy and safety of Angiolite vs Xience in patients with PCI indication (NCT 03049657)

Potential conflicts of interest Speaker's name : Jose, Moreu Burgos, Toledo ☑ I have the following potential conflicts of interest to report: Receipt of grants / research supports: Abbott, Boston Scientific, Cardiva, Edwards Lifesciences, Medtronic

ANGIOLITE Trial Leadership & Organization • Steering Committee – J. Moreu MD. (PI), A. Perez de Prado MD, R. Moreno MD, B. Garcia MD, E. Pinar MD, R. Trillo MD, J. Zueco MD, A. Merchan MD, JF Diaz- Fernandez MD, E. Molina MD, B. Ramos MD • Core Laboratory – ICICORELAB. Instituto Ciencias del Corazón. Escuela de Medicina de Valladolid • Study Management, Data Monitoring and Analysis – Effice Servicios Para la Investigación S. L • Clinical Events Committee – L. Rodriguez-Padial MD, F. Fernandez-Vazquez MD • Sponsor – CARDIVA 2

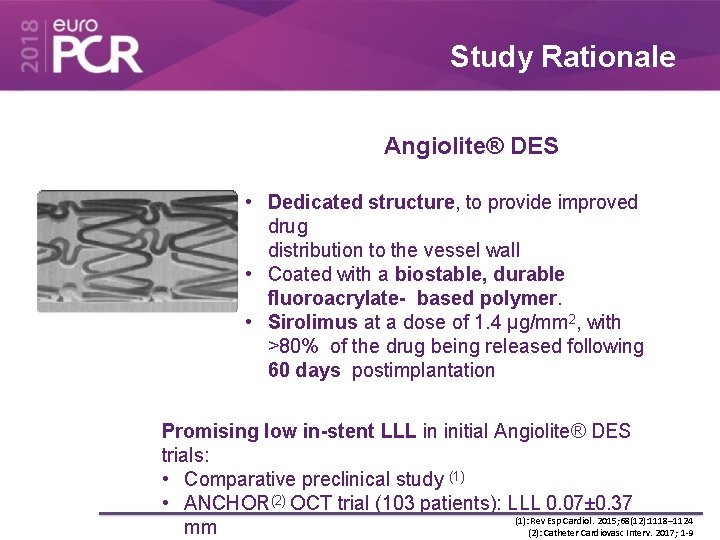
Study Rationale Angiolite® DES • Dedicated structure, to provide improved drug distribution to the vessel wall • Coated with a biostable, durable fluoroacrylate- based polymer. • Sirolimus at a dose of 1. 4 μg/mm 2, with >80% of the drug being released following 60 days postimplantation Promising low in-stent LLL in initial Angiolite® DES trials: • Comparative preclinical study (1) • ANCHOR(2) OCT trial (103 patients): LLL 0. 07± 0. 37 (1): Rev Esp Cardiol. 2015; 68(12): 1118– 1124 mm (2): Catheter Cardiovasc Interv. 2017; 1 -9

Study Rationale (con’t) • Need for a Randomized Clinical Trial to compare the efficacy of Angiolite stent versus a reference DES. • XIENCE is considered as world's leading DES – unparalleled safety record – twice as many implants as any other DES. We selected Xience DES as the gold standard and most challenging benchmark

Study Objective To demonstrate that Angiolite® drug eluting stent is “non inferior” to Xience Alpine® from safety and efficacy standpoints.

Study Design • ANGIOLITE Trial is a randomized, prospective and multicentric phase IV trial with CE-marked medical device, to be used according to the instructions for use and in the approved indications. • Angiolite® vs Xience Alpine® (1: 1) • Sample size: 176 patients Primary Endpoints • • Efficacy: 6 -month intra-stent late lumen loss (QCA analysis, LLL) Safety: Target Lesion Failure (TLF) at 12 months: cardiovascular death, target vessel-related myocardial infraction (MI) with definite thrombosis or ischemia- driven TLR Secondary Endpoints • • 6 -month BAR (Binary artery restenosis) mean neointimal thickness and % of covered struts in prespecified OCT substudy at 6 months 12 -month definite and probable thrombosis 12 -month MACE

Inclusion and exclusion criteria • • Inclusion criteria: Patients with clinical or subclinical ischemic heart disease with indication of percutaneous revascularization. De novo lesions ≥ 70% Reference diameters ≥ 2 mm and ≤ 4 mm. Signature of informed consent • • Exclusion criteria: Totally occluded artery Left main disease Cardiogenic shock Life expectancy less than 1 year DAPT intolerance Patient with previous restenosis. Patients in whom all lesions won’t be treated with Angiolite or Xience DES.

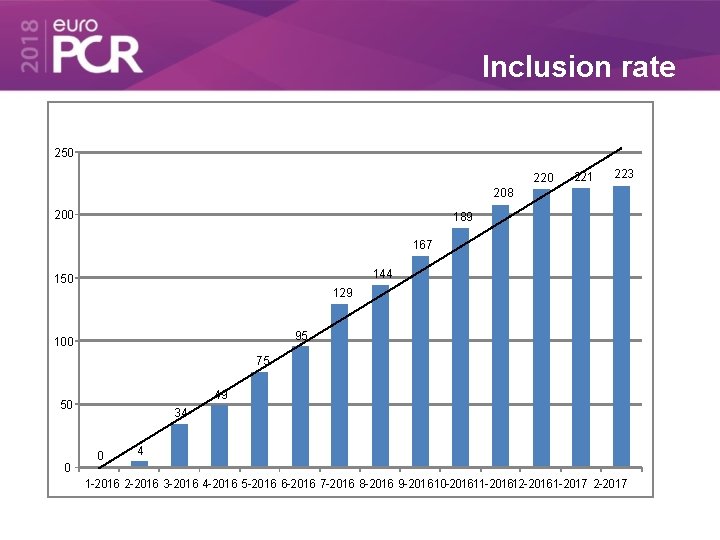
Inclusion rate 250 221 223 208 200 189 167 144 150 129 95 100 75 49 50 0 34 0 4 1 -2016 2 -2016 3 -2016 4 -2016 5 -2016 6 -2016 7 -2016 8 -2016 9 -2016 10 -201611 -201612 -2016 1 -2017 2 -2017

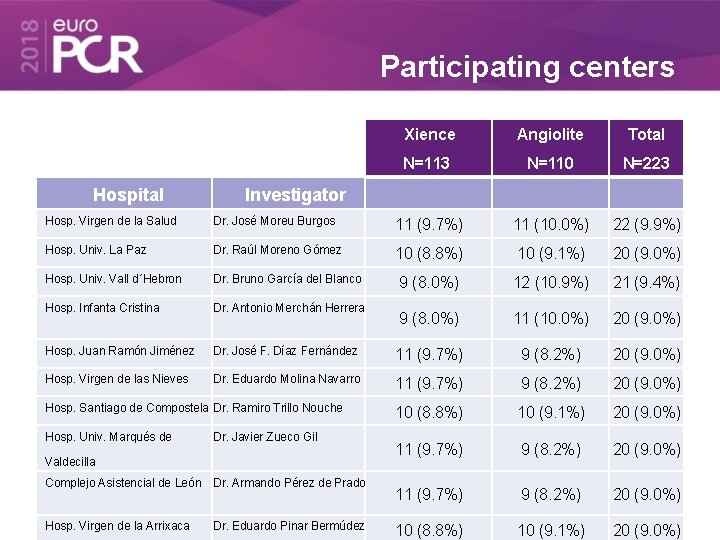
Participating centers Hospital Xience Angiolite Total N=113 N=110 N=223 Investigator Hosp. Virgen de la Salud Dr. José Moreu Burgos 11 (9. 7%) 11 (10. 0%) 22 (9. 9%) Hosp. Univ. La Paz Dr. Raúl Moreno Gómez 10 (8. 8%) 10 (9. 1%) 20 (9. 0%) Hosp. Univ. Vall d´Hebron Dr. Bruno García del Blanco 9 (8. 0%) 12 (10. 9%) 21 (9. 4%) Hosp. Infanta Cristina Dr. Antonio Merchán Herrera 9 (8. 0%) 11 (10. 0%) 20 (9. 0%) Hosp. Juan Ramón Jiménez Dr. José F. Díaz Fernández 11 (9. 7%) 9 (8. 2%) 20 (9. 0%) Hosp. Virgen de las Nieves Dr. Eduardo Molina Navarro 11 (9. 7%) 9 (8. 2%) 20 (9. 0%) 10 (8. 8%) 10 (9. 1%) 20 (9. 0%) Hosp. Santiago de Compostela Dr. Ramiro Trillo Nouche Hosp. Univ. Marqués de Dr. Javier Zueco Gil Valdecilla Complejo Asistencial de León Dr. Armando Pérez de Prado Hosp. Virgen de la Arrixaca Dr. Eduardo Pinar Bermúdez

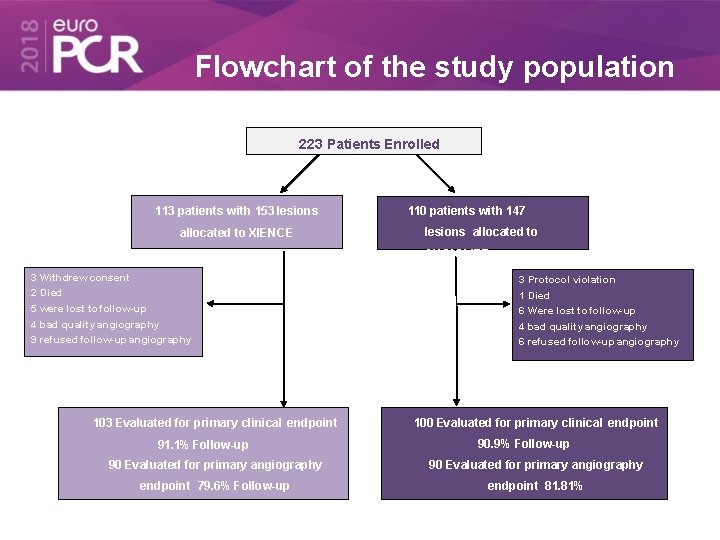
Flowchart of the study population 223 Patients Enrolled 113 patients with 153 lesions allocated to XIENCE 110 patients with 147 lesions allocated to ANGIOLITE 3 Withdrew consent 2 Died 5 were lost to follow-up 4 bad quality angiography 9 refused follow-up angiography 103 Evaluated for primary clinical endpoint 91. 1% Follow-up 3 Protocol violation 1 Died 6 Were lost to follow-up 4 bad quality angiography 6 refused follow-up angiography 100 Evaluated for primary clinical endpoint 90. 9% Follow-up 90 Evaluated for primary angiography endpoint 79. 6% Follow-up endpoint 81. 81%

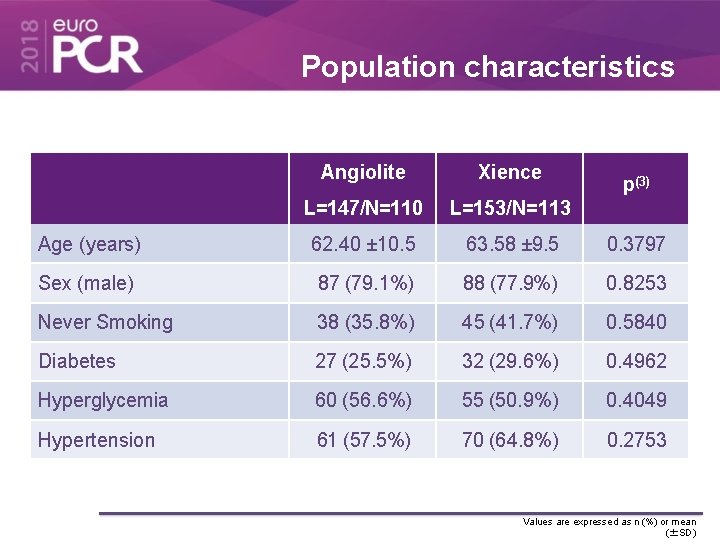
Population characteristics Angiolite Xience L=147/N=110 L=153/N=113 Age (years) 62. 40 ± 10. 5 63. 58 ± 9. 5 0. 3797 Sex (male) 87 (79. 1%) 88 (77. 9%) 0. 8253 Never Smoking 38 (35. 8%) 45 (41. 7%) 0. 5840 Diabetes 27 (25. 5%) 32 (29. 6%) 0. 4962 Hyperglycemia 60 (56. 6%) 55 (50. 9%) 0. 4049 Hypertension 61 (57. 5%) 70 (64. 8%) 0. 2753 p(3) Values are expressed as n (%) or mean (±SD)

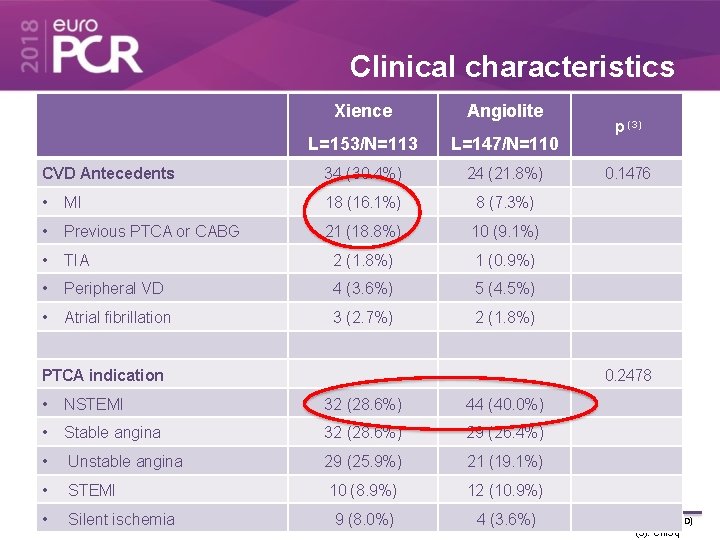
Clinical characteristics Xience Angiolite L=153/N=113 L=147/N=110 CVD Antecedents 34 (30. 4%) 24 (21. 8%) • MI 18 (16. 1%) 8 (7. 3%) • Previous PTCA or CABG 21 (18. 8%) 10 (9. 1%) • TIA 2 (1. 8%) 1 (0. 9%) • Peripheral VD 4 (3. 6%) 5 (4. 5%) • Atrial fibrillation 3 (2. 7%) 2 (1. 8%) PTCA indication p (3) 0. 1476 0. 2478 • NSTEMI 32 (28. 6%) 44 (40. 0%) • Stable angina 32 (28. 6%) 29 (26. 4%) • Unstable angina 29 (25. 9%) 21 (19. 1%) • STEMI 10 (8. 9%) 12 (10. 9%) • Silent ischemia 9 (8. 0%) Values are expre ssed as n (%) or mean (±SD) 4 (3. 6%) (3): Chi. Sq

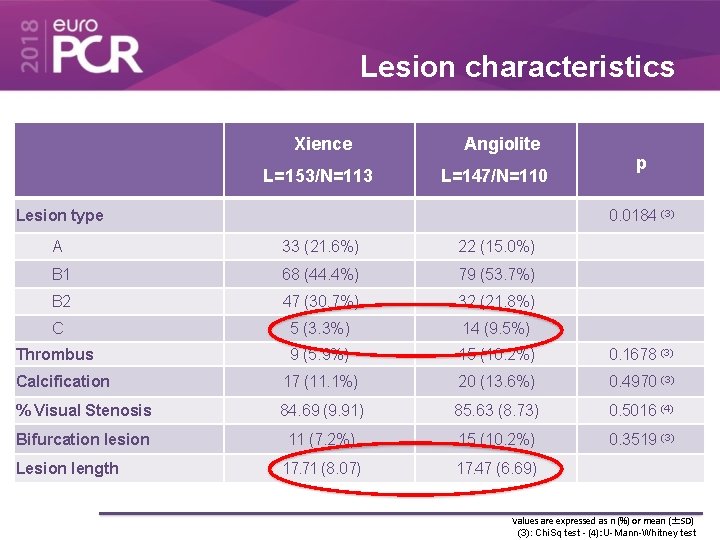
Lesion characteristics Xience L=153/N=113 Angiolite L=147/N=110 Lesion type p 0. 0184 (3) A 33 (21. 6%) 22 (15. 0%) B 1 68 (44. 4%) 79 (53. 7%) B 2 47 (30. 7%) 32 (21. 8%) C 5 (3. 3%) 14 (9. 5%) Thrombus 9 (5. 9%) 15 (10. 2%) 0. 1678 (3) Calcification 17 (11. 1%) 20 (13. 6%) 0. 4970 (3) % Visual Stenosis 84. 69 (9. 91) 85. 63 (8. 73) 0. 5016 (4) Bifurcation lesion 11 (7. 2%) 15 (10. 2%) 0. 3519 (3) 17. 71 (8. 07) 17. 47 (6. 69) Lesion length Values are expressed as n (%) or mean (±SD) (3): Chi. Sq test - (4): U-Mann-Whitney test

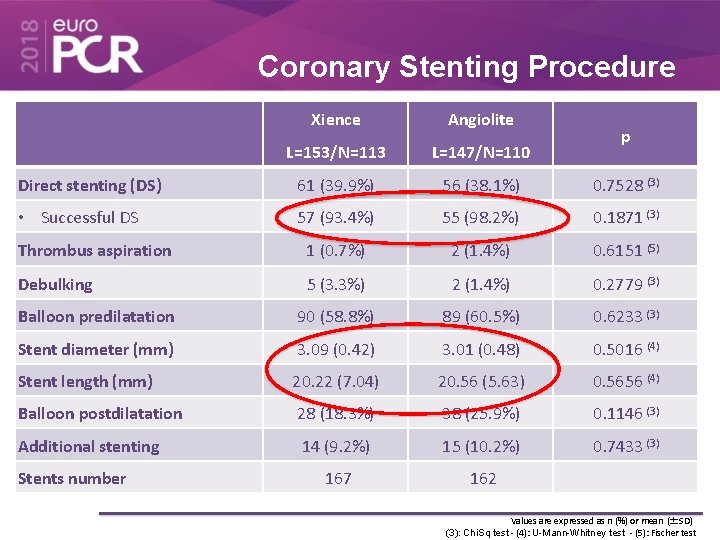
Coronary Stenting Procedure Xience Angiolite L=153/N=113 L=147/N=110 Direct stenting (DS) 61 (39. 9%) 56 (38. 1%) 0. 7528 (3) • Successful DS 57 (93. 4%) 55 (98. 2%) 0. 1871 (3) Thrombus aspiration 1 (0. 7%) 2 (1. 4%) 0. 6151 (5) Debulking 5 (3. 3%) 2 (1. 4%) 0. 2779 (3) Balloon predilatation 90 (58. 8%) 89 (60. 5%) 0. 6233 (3) Stent diameter (mm) 3. 09 (0. 42) 3. 01 (0. 48) 0. 5016 (4) Stent length (mm) 20. 22 (7. 04) 20. 56 (5. 63) 0. 5656 (4) Balloon postdilatation 28 (18. 3%) 38 (25. 9%) 0. 1146 (3) Additional stenting 14 (9. 2%) 15 (10. 2%) 0. 7433 (3) 167 162 Stents number p Values are expressed as n (%) or mean (±SD) (3): Chi. Sq test - (4): U-Mann-Whitney test - (5): Fischer test

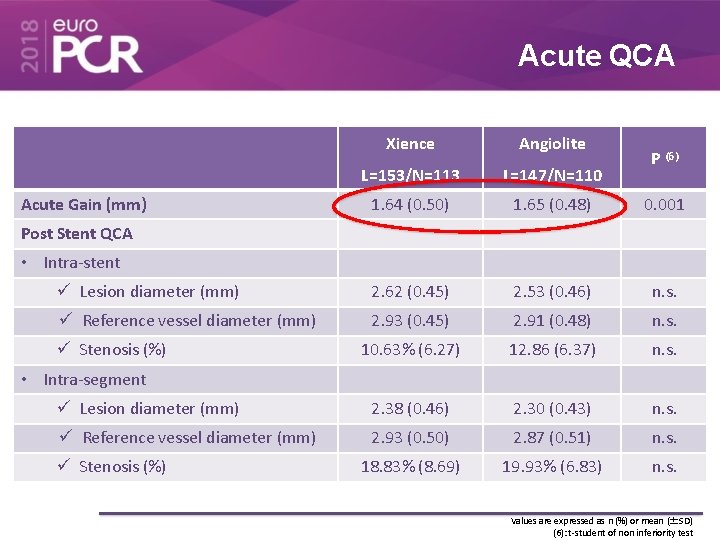
Acute QCA Xience Angiolite L=153/N=113 L=147/N=110 1. 64 (0. 50) 1. 65 (0. 48) 0. 001 Lesion diameter (mm) 2. 62 (0. 45) 2. 53 (0. 46) n. s. Reference vessel diameter (mm) 2. 93 (0. 45) 2. 91 (0. 48) n. s. 10. 63% (6. 27) 12. 86 (6. 37) n. s. Lesion diameter (mm) 2. 38 (0. 46) 2. 30 (0. 43) n. s. Reference vessel diameter (mm) 2. 93 (0. 50) 2. 87 (0. 51) n. s. 18. 83% (8. 69) 19. 93% (6. 83) n. s. Acute Gain (mm) P (6) Post Stent QCA • Intra-stent Stenosis (%) • Intra-segment Stenosis (%) Values are expressed as n (%) or mean (±SD) (6): t-student of non inferiority test

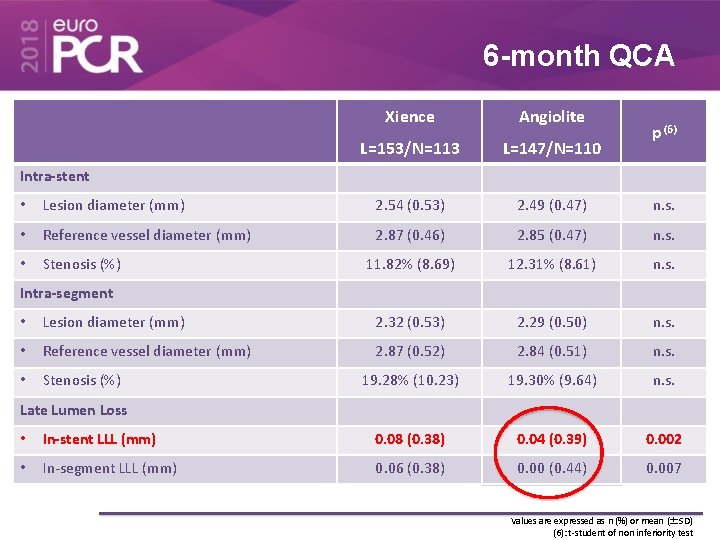
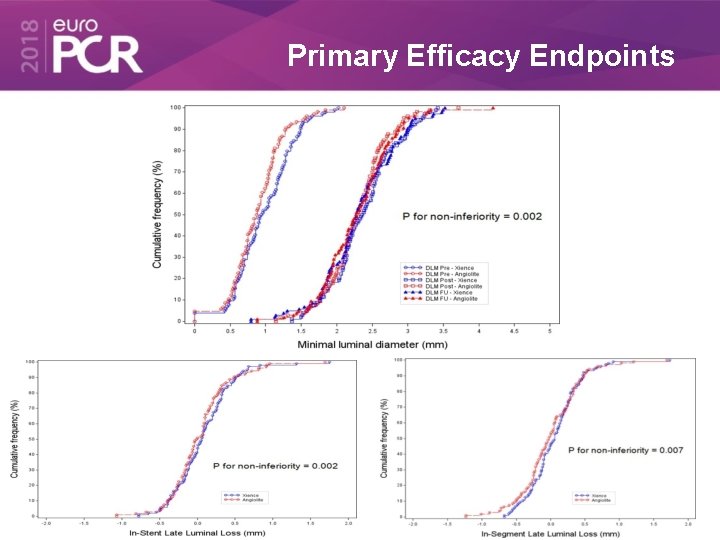
6 -month QCA Xience Angiolite L=153/N=113 L=147/N=110 p (6) Intra-stent • Lesion diameter (mm) 2. 54 (0. 53) 2. 49 (0. 47) n. s. • Reference vessel diameter (mm) 2. 87 (0. 46) 2. 85 (0. 47) n. s. • Stenosis (%) 11. 82% (8. 69) 12. 31% (8. 61) n. s. Intra-segment • Lesion diameter (mm) 2. 32 (0. 53) 2. 29 (0. 50) n. s. • Reference vessel diameter (mm) 2. 87 (0. 52) 2. 84 (0. 51) n. s. • Stenosis (%) 19. 28% (10. 23) 19. 30% (9. 64) n. s. Late Lumen Loss • In-stent LLL (mm) 0. 08 (0. 38) 0. 04 (0. 39) 0. 002 • In-segment LLL (mm) 0. 06 (0. 38) 0. 00 (0. 44) 0. 007 Values are expressed as n (%) or mean (±SD) (6): t-student of non inferiority test

Primary Efficacy Endpoints

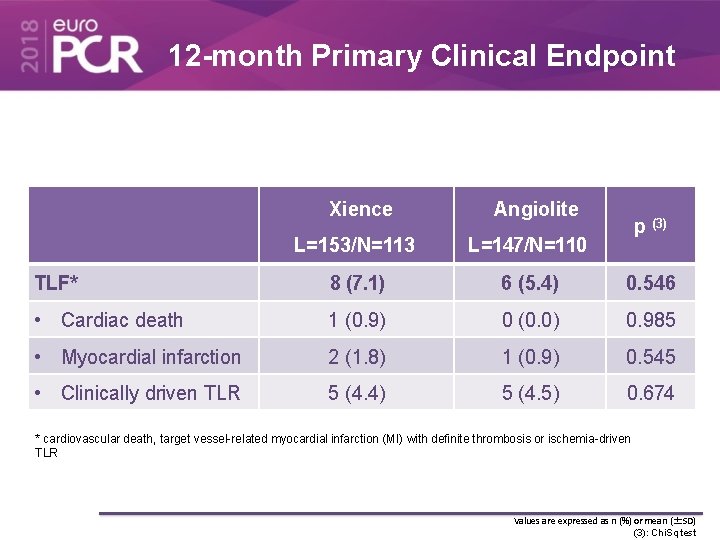
12 -month Primary Clinical Endpoint Xience Angiolite p (3) L=153/N=113 L=147/N=110 TLF* 8 (7. 1) 6 (5. 4) 0. 546 • Cardiac death 1 (0. 9) 0 (0. 0) 0. 985 • Myocardial infarction 2 (1. 8) 1 (0. 9) 0. 545 • Clinically driven TLR 5 (4. 4) 5 (4. 5) 0. 674 * cardiovascular death, target vessel-related myocardial infarction (MI) with definite thrombosis or ischemia-driven TLR Values are expressed as n (%) or mean (±SD) (3): Chi. Sq test

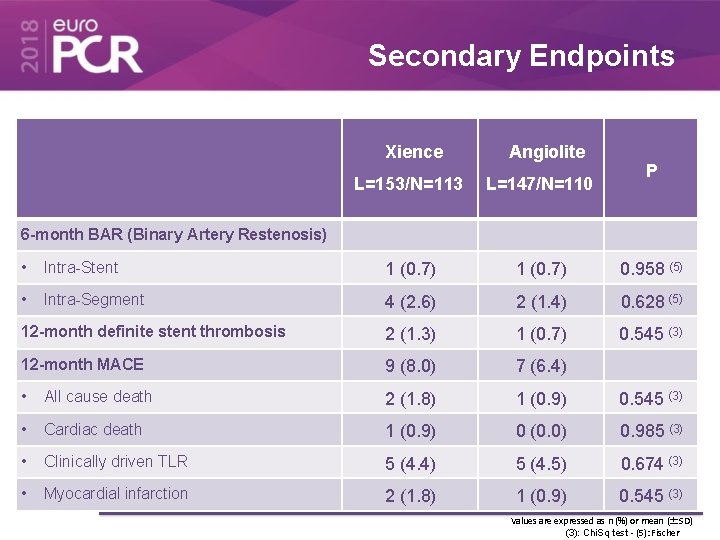
Secondary Endpoints Xience Angiolite L=153/N=113 L=147/N=110 P 6 -month BAR (Binary Artery Restenosis) • Intra-Stent 1 (0. 7) 0. 958 (5) • Intra-Segment 4 (2. 6) 2 (1. 4) 0. 628 (5) 12 -month definite stent thrombosis 2 (1. 3) 1 (0. 7) 0. 545 (3) 12 -month MACE 9 (8. 0) 7 (6. 4) • All cause death 2 (1. 8) 1 (0. 9) 0. 545 (3) • Cardiac death 1 (0. 9) 0 (0. 0) 0. 985 (3) • Clinically driven TLR 5 (4. 4) 5 (4. 5) 0. 674 (3) • Myocardial infarction 2 (1. 8) 1 (0. 9) 0. 545 (3) Values are expressed as n (%) or mean (±SD) (3): Chi. Sq test - (5): Fischer

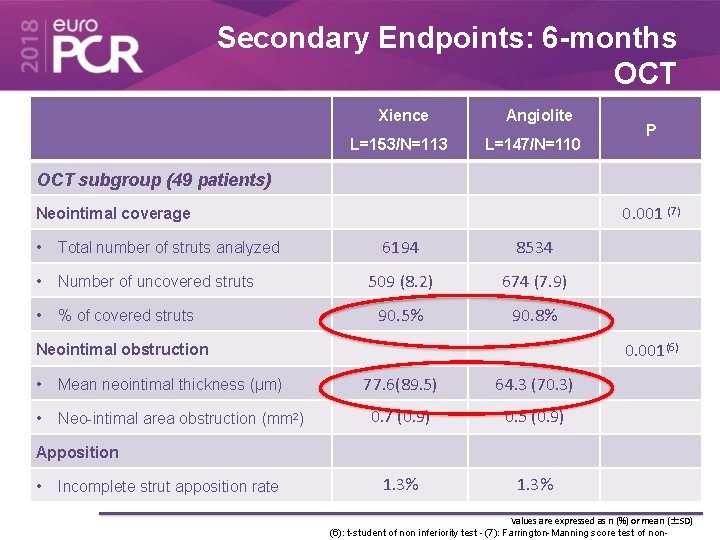
Secondary Endpoints: 6 -months OCT Xience L=153/N=113 Angiolite L=147/N=110 P OCT subgroup (49 patients) 0. 001 (7) Neointimal coverage • Total number of struts analyzed • Number of uncovered struts • % of covered struts 6194 8534 509 (8. 2) 674 (7. 9) 90. 5% 90. 8% 0. 001(6) Neointimal obstruction • Mean neointimal thickness (µm) • Neo-intimal area obstruction (mm 2) 77. 6(89. 5) 64. 3 (70. 3) 0. 7 (0. 9) 0. 5 (0. 9) 1. 3% Apposition • Incomplete strut apposition rate Values are expressed as n (%) or mean (±SD) (6): t-student of non inferiority test - (7): Farrington-Manning score test of non-

Conclusions • Angiolite® DES demonstrates high efficacy and safety: – Very low LLL and binary restenosis – Very low rates of cardiovascular death, myocardial infarction, stent thrombosis and target vessel revascularization • Thanks to specific stent design, biostable polymer and Sirolimus released within 60 days, Angiolite® DES also demonstrates a favorable early healing profile and longer-term efficacy.

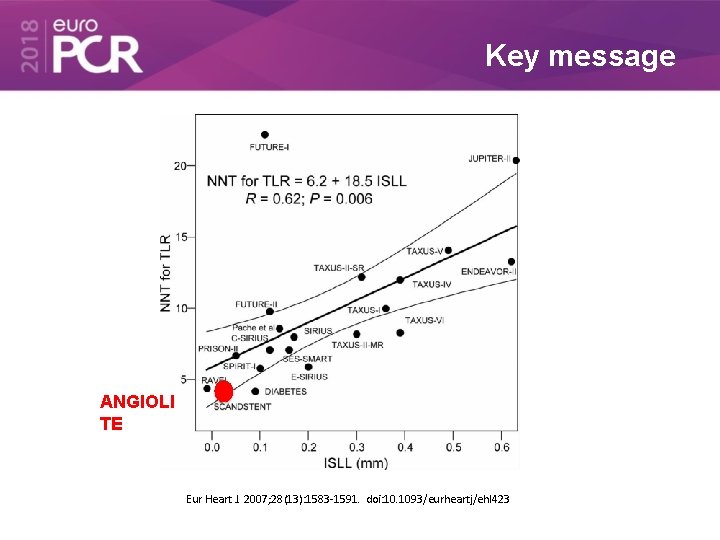
Key message ANGIOLI TE Eur Heart J. 2007; 28(13): 1583 -1591. doi: 10. 1093/eurheartj/ehl 423

Take-home message The “ANGIOLITE” RCT • • Angiolite® DES is an innovative DES with optimized technical features Angiolite® DES is non inferior to Xience from safety and efficacy standpoints Angiolite® DES demonstrates a favorable early healing profile and longer-term efficacy Angiolite® DES is among the best of the new DES generation

The 24 -month results are expected to be presented at Euro. PCR 2019 Thank you for your attention


Q&A

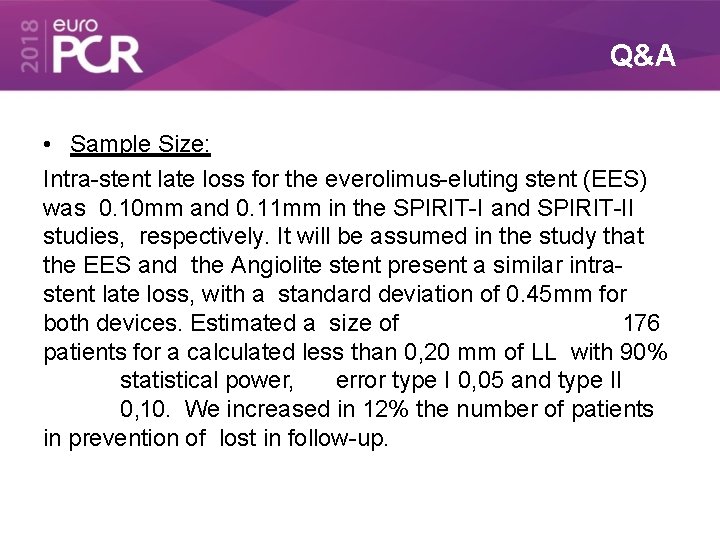
Q&A • Sample Size: Intra-stent late loss for the everolimus-eluting stent (EES) was 0. 10 mm and 0. 11 mm in the SPIRIT-I and SPIRIT-II studies, respectively. It will be assumed in the study that the EES and the Angiolite stent present a similar intrastent late loss, with a standard deviation of 0. 45 mm for both devices. Estimated a size of 176 patients for a calculated less than 0, 20 mm of LL with 90% statistical power, error type I 0, 05 and type II 0, 10. We increased in 12% the number of patients in prevention of lost in follow-up.
 Advantage of randomized controlled trial
Advantage of randomized controlled trial Clinical trial timeline
Clinical trial timeline Cynchia
Cynchia Clinical trial centers alliance
Clinical trial centers alliance Clinical trial worksheet
Clinical trial worksheet Novel clinical drug trial design
Novel clinical drug trial design Accelerated clinical trial agreement acta
Accelerated clinical trial agreement acta Nida clinical trials network
Nida clinical trials network Rsna ctp anonymizer
Rsna ctp anonymizer Iwr clinical trial
Iwr clinical trial Master clinical trial agreement
Master clinical trial agreement Clinical trial api
Clinical trial api Mosaico clinical trial
Mosaico clinical trial Companion diagnostic regulation
Companion diagnostic regulation Morpheus clinical trial
Morpheus clinical trial Accelerated clinical trial agreement acta
Accelerated clinical trial agreement acta Clinical trial matching service
Clinical trial matching service Clinical trial financial management
Clinical trial financial management Prs clinical trial
Prs clinical trial Clinical trial exports
Clinical trial exports Clinical trial budget example
Clinical trial budget example Trofinetide
Trofinetide Rbal
Rbal Randomized complete block design
Randomized complete block design Randomized polynomial time
Randomized polynomial time Expected running time
Expected running time Randomized skip list
Randomized skip list Crd iii
Crd iii Completely randomized design definition
Completely randomized design definition