ESONCO10180218 1 Novel Clinical Trial Designs to Explore













- Slides: 24

ES/ONCO/1018/0218 1

Novel Clinical Trial Designs to Explore Immunotherapy Combinations Jun Wang, MD, Ph. D Senior Medical Director, Cancer Immunotherapy, Genentech/Roche

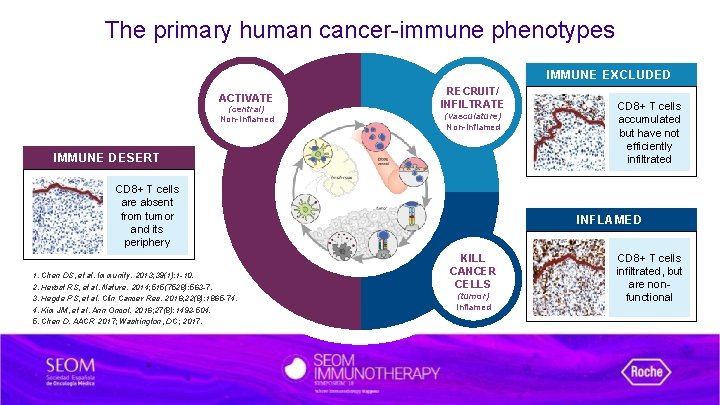
The primary human cancer-immune phenotypes IMMUNE EXCLUDED ACTIVATE (central) Non-Inflamed RECRUIT/ INFILTRATE (vasculature) Non-Inflamed IMMUNE DESERT CD 8+ T cells are absent from tumor and its periphery 1. Chen DS, et al. Immunity. 2013; 39(1): 1 -10. 2. Herbst RS, et al. Nature. 2014; 515(7528): 563 -7. 3. Hegde PS, et al. Clin Cancer Res. 2016; 22(8): 1865 -74. 4. Kim JM, et al. Ann Oncol. 2016; 27(8): 1492 -504. 5. Chen D. AACR 2017; Washington, DC; 2017. CD 8+ T cells accumulated but have not efficiently infiltrated INFLAMED KILL CANCER CELLS (tumor) Inflamed CD 8+ T cells infiltrated, but are nonfunctional

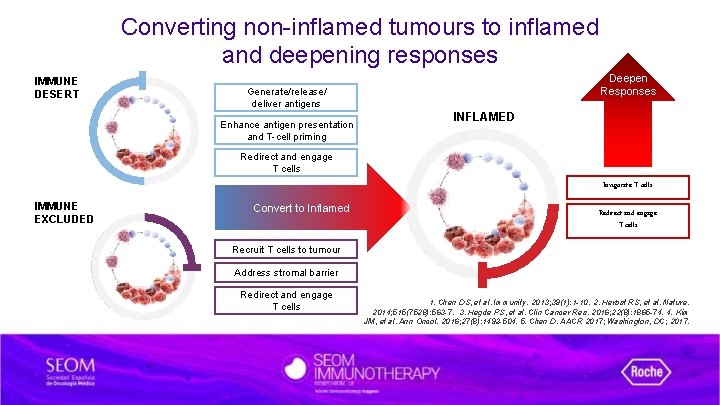
Converting non-inflamed tumours to inflamed and deepening responses IMMUNE DESERT Deepen Responses Generate/release/ deliver antigens Enhance antigen presentation and T-cell priming INFLAMED Redirect and engage T cells Invigorate T cells IMMUNE EXCLUDED Convert to Inflamed Redirect and engage T cells Recruit T cells to tumour Address stromal barrier Redirect and engage T cells 1. Chen DS, et al. Immunity. 2013; 39(1): 1 -10. 2. Herbst RS, et al. Nature. 2014; 515(7528): 563 -7. 3. Hegde PS, et al. Clin Cancer Res. 2016; 22(8): 1865 -74. 4. Kim JM, et al. Ann Oncol. 2016; 27(8): 1492 -504. 5. Chen D. AACR 2017; Washington, DC; 2017.

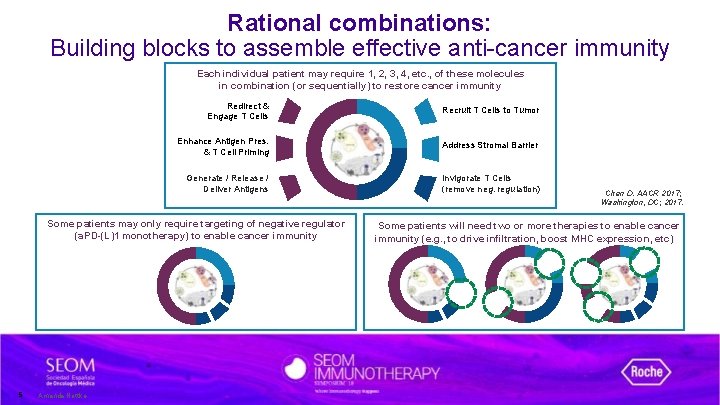
Rational combinations: Building blocks to assemble effective anti-cancer immunity Each individual patient may require 1, 2, 3, 4, etc. , of these molecules in combination (or sequentially) to restore cancer immunity Redirect & Engage T Cells Recruit T Cells to Tumor Enhance Antigen Pres. & T Cell Priming Address Stromal Barrier Generate / Release / Deliver Antigens Invigorate T Cells (remove neg. regulation) Some patients may only require targeting of negative regulator (a. PD-(L)1 monotherapy) to enable cancer immunity 5 Amanda Nottke. Chen D. AACR 2017; Washington, DC; 2017. Some patients will need two or more therapies to enable cancer immunity (e. g. , to drive infiltration, boost MHC expression, etc)

Problem: Combination possibilities and trials are growing exponentially to the point of not being sustainable Number of Combination Trials Initiated Number of Combination Trials 300 250 226 200 150 100 50 0 7 6 7 2006 2007 2008 Source: Beacon IO Combination 6 356 PROJECTED 350 11 2009 16 2010 19 2011 35 48 2012 2013 92 2014 2015 2016 Adapted from Vanessa Lucey of CRI by Gergely Jarmy. Chen DS, et al. Immunity. 2013; 39(1): 1 -10

Problem: Combination possibilities and trials are growing exponentially to the point of not being sustainable Number of Combination Trials Initiated Number of Combination Trials 300 250 226 200 150 100 50 0 7 6 7 2006 2007 2008 Source: Beacon IO Combination 7 356 >1100 PROJECTED 350 11 2009 16 2010 19 2011 35 48 2012 2013 92 2014 2015 2016 Adapted from Vanessa Lucey of CRI by Gergely Jarmy. Chen DS, et al. Immunity. 2013; 39(1): 1 -10

Problem: Combination possibilities and trials are growing exponentially to the point of not being sustainable Number of Combination Trials Initiated Number of Combination Trials 300 250 100 0 Is this Sustainable? 226 200 50 7 6 2007 7 2008 Source: Beacon IO Combination 8 356 >1100 PROJECTED 350 11 2009 16 2010 19 2011 35 48 2012 2013 92 2014 2015 2016 Adapted from Vanessa Lucey of CRI by Gergely Jarmy. Chen DS, et al. Immunity. 2013; 39(1): 1 -10

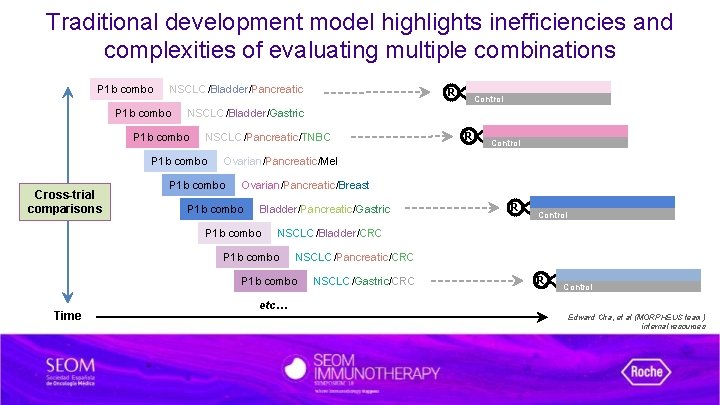
Traditional development model highlights inefficiencies and complexities of evaluating multiple combinations P 1 b combo NSCLC/Bladder/Pancreatic P 1 b combo NSCLC/Pancreatic/TNBC P 1 b combo R Control Ovarian/Pancreatic/Mel P 1 b combo Ovarian/Pancreatic/Breast P 1 b combo Bladder/Pancreatic/Gastric P 1 b combo R Control NSCLC/Bladder/CRC P 1 b combo NSCLC/Pancreatic/CRC P 1 b combo Time Control NSCLC/Bladder/Gastric P 1 b combo Cross-trial comparisons R NSCLC/Gastric/CRC R Control etc… Edward Cha, et al (MORPHEUS team) internal resources

What is next in cancer immunotherapy? Outline Clinical development challenges for CIT combos : • Shear magnitude of combination potentials (patient numbers – experimental & control arms) • Designs (single arm vs basket vs umbrella, comparator– historical vs. concurrent) • Patient characteristics (I/E criteria, testing all-comers vs. potential biomarker enriched) • Endpoints (ORR, Do. R, DCR/CBR, PFS, OS; pseudoprogression) • Dose/schedule/duration of individual components • Stats (adaptive designs, ability to account for NPH & ‘tail of the curve’ in early studies) • Understanding the contribution of individual components • Toxicity insights (cross trial oversight) Innovative trial designs: Potential solution for some these development challenges Edward Cha, et al (MORPHEUS team) internal resources 10

What is next in cancer immunotherapy? Outline Clinical development challenges for CIT combos: • Shear magnitude of combination potentials (patient numbers – experimental & control arms) • Designs (single arm vs basket vs umbrella, comparator– historical vs. concurrent) • Patient characteristics (I/E criteria, testing all-comers vs. potential biomarker enriched) • Endpoints (ORR, Do. R, DCR/CBR, PFS, OS; pseudoprogression) • Dose/schedule/duration of individual components • Stats (adaptive designs, ability to account for NPH & ‘tail of the curve’ in early studies) • Understanding the contribution of individual components • Toxicity insights (cross trial oversight) Innovative trial designs: Potential solution for some these development challenges Edward Cha, et al (MORPHEUS team) internal resources 11

Traditional development model highlights inefficiencies and complexities of evaluating multiple combinations P 1 b combo NSCLC/Bladder/Pancreatic P 1 b combo R Multiple cohorts of patients receiving control / comparator regimens Control NSCLC/Bladder/Gastric P 1 b combo NSCLC/Pancreatic/TNBC P 1 b combo R Control Ovarian/Pancreatic/Mel P 1 b combo Ovarian/Pancreatic/Breast There is a need to approach clinical trials differently Cross-trial comparisons P 1 b combo Bladder/Pancreatic/Gastric P 1 b combo Potential drifts in patient population, evolving standards, and access to novel post-trial therapies Time R Control NSCLC/Bladder/CRC P 1 b combo NSCLC/Pancreatic/CRC P 1 b combo NSCLC/Gastric/CRC R Control etc… Edward Cha, et al (MORPHEUS team) internal resources

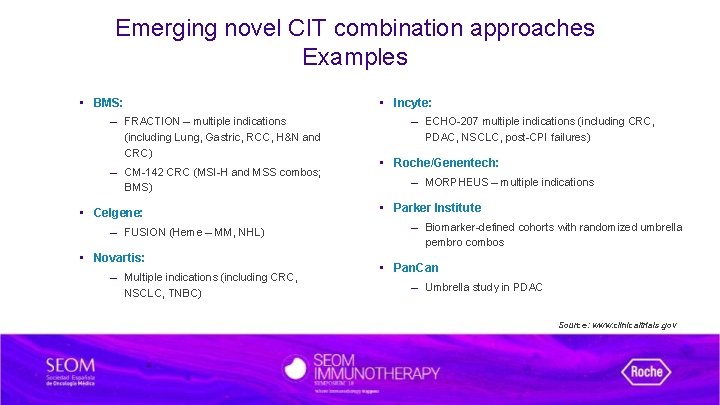
Emerging novel CIT combination approaches Examples • BMS: – FRACTION – multiple indications (including Lung, Gastric, RCC, H&N and CRC) – CM-142 CRC (MSI-H and MSS combos; BMS) • Celgene: – FUSION (Heme – MM, NHL) • Novartis: – Multiple indications (including CRC, NSCLC, TNBC) • Incyte: – ECHO-207 multiple indications (including CRC, PDAC, NSCLC, post-CPI failures) • Roche/Genentech: – MORPHEUS – multiple indications • Parker Institute – Biomarker-defined cohorts with randomized umbrella pembro combos • Pan. Can – Umbrella study in PDAC Source: www. clinicaltrials. gov

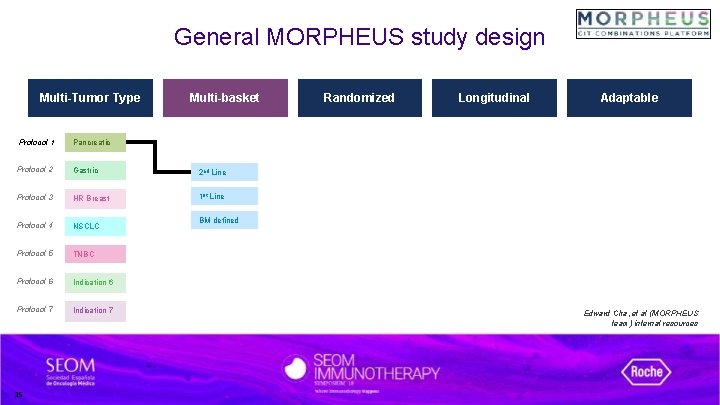
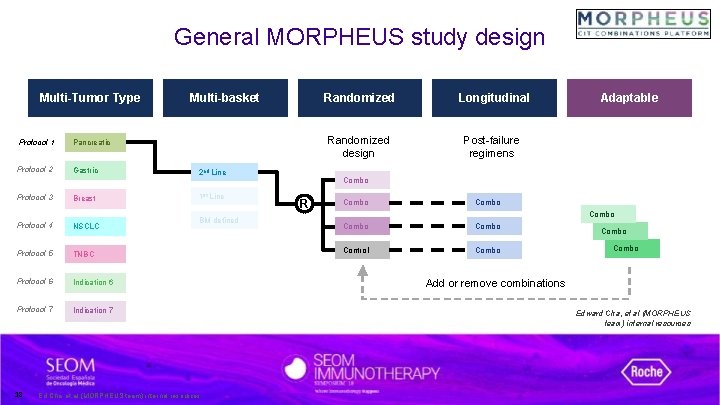
General MORPHEUS study design Development platform designed to 1) raise decision confidence, 2) speed time-to-results, and 3) improve efficiency in accelerating combinations with transformational potential. Multi-Tumor Type Protocol 1 Pancreatic Protocol 2 Gastric Protocol 3 HR Breast Protocol 4 NSCLC Protocol 5 TNBC Protocol 6 Indication 6 Protocol 7 Indication 7 Multi-basket Randomized Longitudinal Adaptable Edward Cha, et al (MORPHEUS team) internal resources

General MORPHEUS study design Multi-Tumor Type Multi-basket Protocol 1 Pancreatic Protocol 2 Gastric 2 nd Line Protocol 3 HR Breast 1 st Line Protocol 4 NSCLC Protocol 5 TNBC Protocol 6 Indication 6 Protocol 7 Indication 7 15 Randomized Longitudinal Adaptable BM defined Edward Cha, et al (MORPHEUS team) internal resources

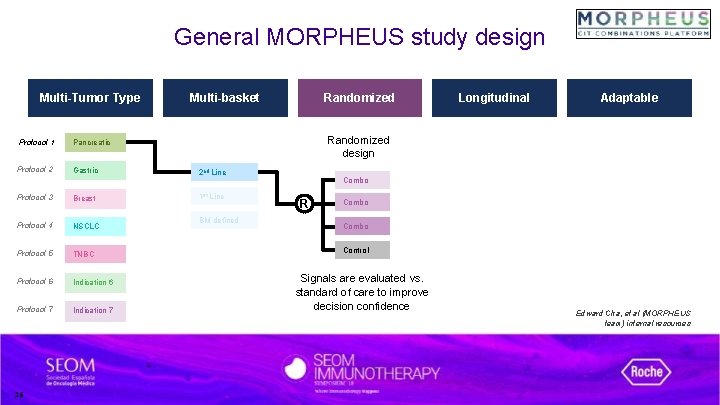
General MORPHEUS study design Multi-Tumor Type Protocol 1 Pancreatic Protocol 2 Gastric Protocol 3 Breast Protocol 4 NSCLC Protocol 5 TNBC Protocol 6 Indication 6 Protocol 7 Indication 7 16 Multi-basket Randomized Longitudinal Adaptable Randomized design 2 nd Line 1 st Line BM defined Combo R Combo Control Signals are evaluated vs. standard of care to improve decision confidence Edward Cha, et al (MORPHEUS team) internal resources

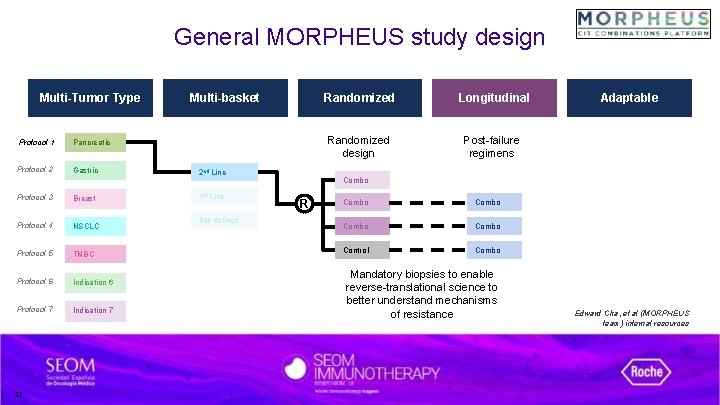
General MORPHEUS study design Multi-Tumor Type Protocol 1 Pancreatic Protocol 2 Gastric Protocol 3 Breast Protocol 4 NSCLC Protocol 5 TNBC Protocol 6 Indication 6 Protocol 7 Indication 7 17 Multi-basket 2 nd Line 1 st Line BM defined Randomized Longitudinal Randomized design Post-failure regimens Adaptable Combo R Combo Control Combo Mandatory biopsies to enable reverse-translational science to better understand mechanisms of resistance Edward Cha, et al (MORPHEUS team) internal resources

General MORPHEUS study design Multi-Tumor Type Protocol 1 Pancreatic Protocol 2 Gastric Protocol 3 Breast Protocol 4 NSCLC Protocol 5 TNBC Protocol 6 Indication 6 Protocol 7 Indication 7 18 Multi-basket 2 nd Line 1 st Line BM defined Ed Cha, et al (MORPHEUS team) internal resources Randomized Longitudinal Randomized design Post-failure regimens Adaptable Combo R Combo Control Combo Add or remove combinations Edward Cha, et al (MORPHEUS team) internal resources

Adaptive & modular designs 2 nd Line 1 st Line Weighted randomization can be utilized to keep control arms enrollment rate to <35% Control R Combo BM-defined Combo Combo Edward Cha, et al (MORPHEUS team) internal resources 19

Overview of the MORPHEUS Platform Studies’ Trial Design R CIT, cancer immunotherapy; PD, progressive disease; R, randomization. a Mandatory serial biopsy arms are currently applicable only to experimental arms with clinical activity in the specific MORPHEUS studies Oh D-Y, et al. J Clin Oncol. 2018; 36(suppl): abstr TPS 4134.

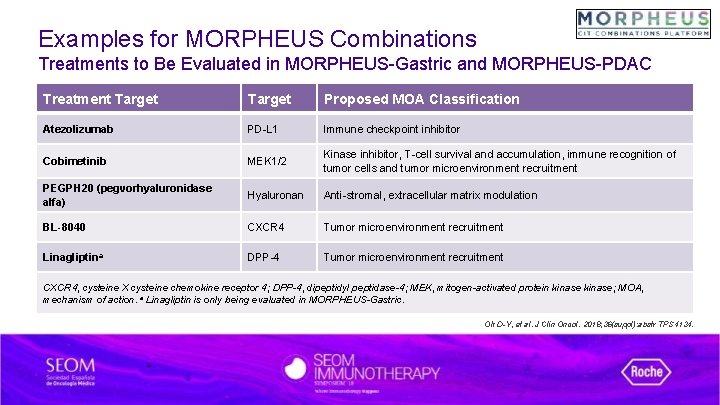
Examples for MORPHEUS Combinations Treatments to Be Evaluated in MORPHEUS-Gastric and MORPHEUS-PDAC Treatment Target Proposed MOA Classification Atezolizumab PD-L 1 Immune checkpoint inhibitor Cobimetinib MEK 1/2 Kinase inhibitor, T-cell survival and accumulation, immune recognition of tumor cells and tumor microenvironment recruitment PEGPH 20 (pegvorhyaluronidase alfa) Hyaluronan Anti-stromal, extracellular matrix modulation BL-8040 CXCR 4 Tumor microenvironment recruitment Linagliptina DPP-4 Tumor microenvironment recruitment CXCR 4, cysteine X cysteine chemokine receptor 4; DPP-4, dipeptidyl peptidase-4; MEK, mitogen-activated protein kinase; MOA, mechanism of action. a Linagliptin is only being evaluated in MORPHEUS-Gastric. Oh D-Y, et al. J Clin Oncol. 2018; 36(suppl): abstr TPS 4134.

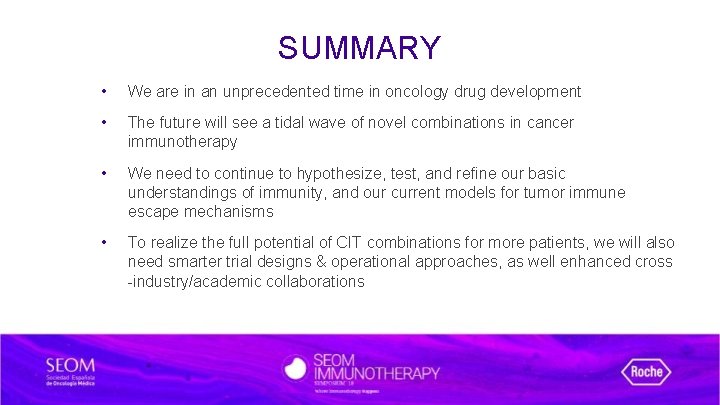
SUMMARY • We are in an unprecedented time in oncology drug development • The future will see a tidal wave of novel combinations in cancer immunotherapy • We need to continue to hypothesize, test, and refine our basic understandings of immunity, and our current models for tumor immune escape mechanisms • To realize the full potential of CIT combinations for more patients, we will also need smarter trial designs & operational approaches, as well enhanced cross -industry/academic collaborations

Thank You! Genentech/Roche thanks all the patients, families and physicians who have participated in our clinical trials to advance our scientific understanding of cancer immunotherapies 23

 Novel clinical drug trial design
Novel clinical drug trial design Clinical trial budget example
Clinical trial budget example Accelerated clinical trial agreement acta
Accelerated clinical trial agreement acta Nida clinical trials network
Nida clinical trials network Fsfv clinical trial
Fsfv clinical trial Cynchia
Cynchia Master clinical trial agreements
Master clinical trial agreements Clinicaltrials.gov api
Clinicaltrials.gov api Mosaico janssen
Mosaico janssen Ivd clinical trial design
Ivd clinical trial design Rsna ctp anonymizer
Rsna ctp anonymizer Clinical trial matching service
Clinical trial matching service Clinical trial financial management
Clinical trial financial management Protocol registration system
Protocol registration system Clinical trial exports
Clinical trial exports Morpheus bms
Morpheus bms Trofinetide clinical trial
Trofinetide clinical trial Ui division of sponsored programs
Ui division of sponsored programs Drug development timeline
Drug development timeline Clinical trial centers alliance
Clinical trial centers alliance Clinical trial worksheet
Clinical trial worksheet Explore your future
Explore your future The most extraordinary country to explore
The most extraordinary country to explore What country did henry hudson explore for
What country did henry hudson explore for Fcat explorer games
Fcat explorer games














































