SCORAD III Randomised noninferiority phase III trial of





- Slides: 14

SCORAD III: Randomised non-inferiority phase III trial of single-dose radiotherapy (RT) compared to multifraction RT in patients with metastatic spinal canal compression. Peter J Hoskin, Kirsten Hopkins, Vivek Misra, Tanya Holt, Rhona Mc. Menemin, Danny Dubois, Fiona Mc. Kinna, Bernadette Foran, Krishnaswamy Madhavan, Carol Mac. Gregor, Andrew Bates, Noelle O’Rourke, Jason Lester, Tim Sevitt, Daniel Roos, Sanjay Dixit, Gillian Brown, Seonaid Arnott, Sharon Shibu Thomas, Sharon Forsyth, Sandy Beare, Krystyna Reczko, Andre Lopes – Statistician International clinical trials day 14 th May 2018

Background • Spinal cord compression (SCC) happens when pressure on the spinal cord stops the nerves working normally • Common complication of metastatic cancer • Most patients receive radiotherapy (RT) to improve neurological function and mobility and to relieve pain • Currently, no standard RT schedule • SCORAD aim: to compare multifraction radiotherapy (20 Gy/5 F, ie, 5 visits) against single fraction radiotherapy (8 Gy/1 F, ie, 1 visit)

SCORAD trial: 8 Gy/1 F vs 20 Gy/5 F • Non-inferiority design in palliative setting: • In SCC, survival is very poor • Looking to reduce treatment and number of clinic visits • Primary endpoint: • Ambulatory status response at 8 weeks (from randomisation) • Uses 4 point scale : 1 = good status 4 = worst status • Maximum allowable difference for non-inferiority in the 8 Gy/1 F was -11% • Large sample size: 700 patients.

Outcome measures Advanced cancer patients: • Difficult to distinguish symptoms of the disease from effects of treatment • Timing of the assessments matter as the health deteriorates rapidly and survival is short • Assessments of physical functioning outcomes and quality of life were essential • Self-reporting measures required the need for simple and sensitive questions Other outcome measures • Ambulatory status response at 1, 4 and 12 weeks • Overall survival • Bladder and bowel function at 1, 4, 8 and 12 weeks • Adverse events (RTOG or CTCAE v 4. 02) • Quality of life (EORTC QLQ-C 30) • Further treatment and MSCC retreatment

Patient characteristics Baseline characteristics Recruitment: Feb 2008 - Apr 2016 Multicentre: 42 UK and 5 Australian sites 20 Gy/5 f N=341 8 Gy/1 f N=345 70 (33 to 95) 70 (23 to 96) 248 (73%) 255 (74%) 152 (45%) 66 (19%) 40 (12%) 38 (11%) 12 (4%) 6 (2%) 4 (1%) 23 (7%) 152 (44%) 66 (19%) 39 (11%) 35 (10%) 11 (3%) 9 (3%) 7 (2%) 26 (8%) 156 (46%) 159 (46%) 311 (91%) 30 (9%) 77 (23%) 146 (43%) 90 (26%) 28 (8%) 303 (88%) 42 (12%) 76 (22%) 152 (44%) 91 (26%) 26 (8%) Age, years Median (range) Sex Male Site of primary cancer Prostate Lung Breast GI Renal Skin Bladder Gynae, head & neck, Sarcoma, unspecified Extent of metastases Nonskeletal mets present Number of SCC sites Single Multiple Ambulatory status Grade 1: Ambulatory without walking aids Grade 2: Ambulatory with walking aids Grade 3: Unable to ambulate Grade 4: No motor power

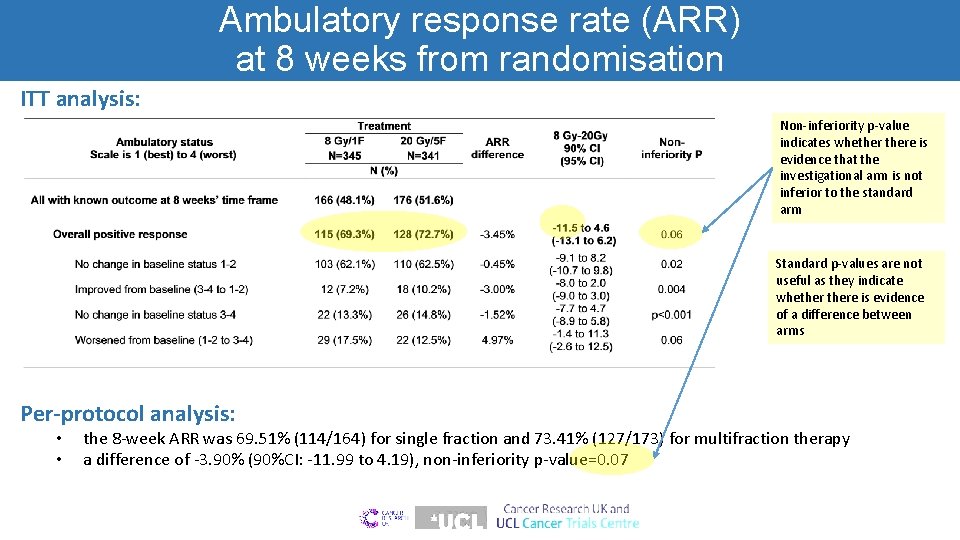
Ambulatory response rate (ARR) at 8 weeks from randomisation ITT analysis: Non-inferiority p-value indicates whethere is evidence that the investigational arm is not inferior to the standard arm Standard p-values are not useful as they indicate whethere is evidence of a difference between arms Per-protocol analysis: • • the 8 -week ARR was 69. 51% (114/164) for single fraction and 73. 41% (127/173) for multifraction therapy a difference of -3. 90% (90%CI: -11. 99 to 4. 19), non-inferiority p-value=0. 07

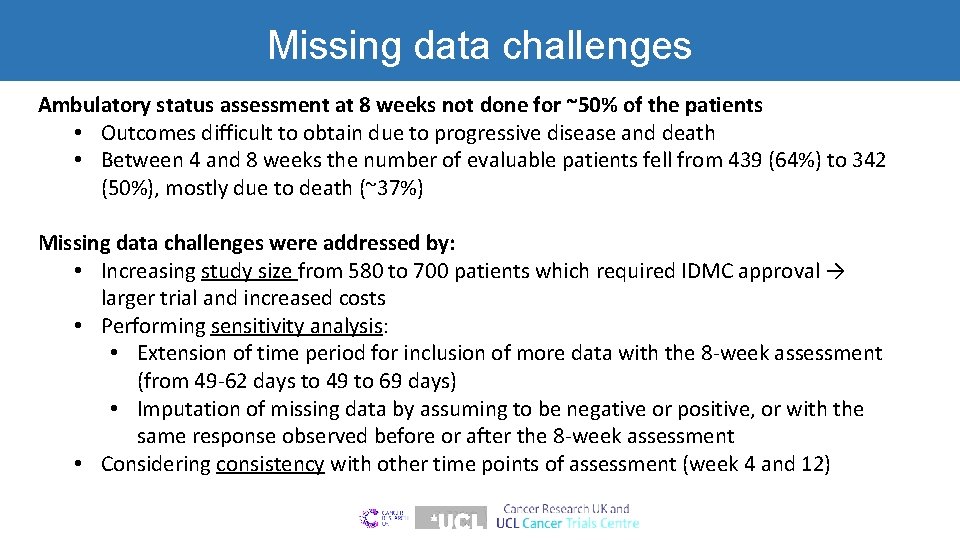
Missing data challenges Ambulatory status assessment at 8 weeks not done for ~50% of the patients • Outcomes difficult to obtain due to progressive disease and death • Between 4 and 8 weeks the number of evaluable patients fell from 439 (64%) to 342 (50%), mostly due to death (~37%) Missing data challenges were addressed by: • Increasing study size from 580 to 700 patients which required IDMC approval → larger trial and increased costs • Performing sensitivity analysis: • Extension of time period for inclusion of more data with the 8 -week assessment (from 49 -62 days to 49 to 69 days) • Imputation of missing data by assuming to be negative or positive, or with the same response observed before or after the 8 -week assessment • Considering consistency with other time points of assessment (week 4 and 12)

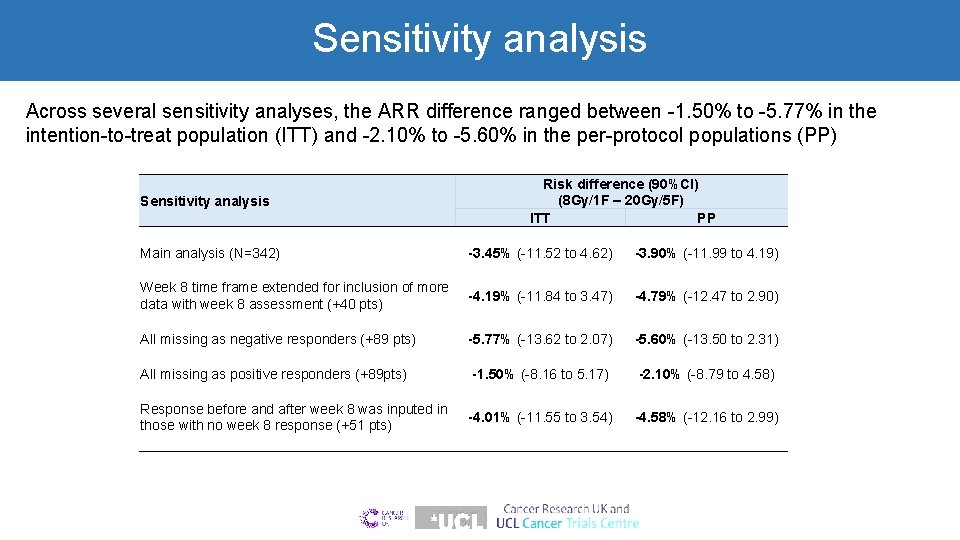
Sensitivity analysis Across several sensitivity analyses, the ARR difference ranged between -1. 50% to -5. 77% in the intention-to-treat population (ITT) and -2. 10% to -5. 60% in the per-protocol populations (PP) Sensitivity analysis Risk difference (90%CI) (8 Gy/1 F – 20 Gy/5 F) ITT PP Main analysis (N=342) -3. 45% (-11. 52 to 4. 62) -3. 90% (-11. 99 to 4. 19) Week 8 time frame extended for inclusion of more data with week 8 assessment (+40 pts) -4. 19% (-11. 84 to 3. 47) -4. 79% (-12. 47 to 2. 90) All missing as negative responders (+89 pts) -5. 77% (-13. 62 to 2. 07) -5. 60% (-13. 50 to 2. 31) All missing as positive responders (+89 pts) -1. 50% (-8. 16 to 5. 17) -2. 10% (-8. 79 to 4. 58) Response before and after week 8 was inputed in -4. 01% (-11. 55 to 3. 54) those with no week 8 response (+51 pts) -4. 58% (-12. 16 to 2. 99)

ARR at other time points Main result are just below the non-inferiority margin. What do we do? Answer: Look at other time points for consistency. 1 Non-inferiority test p-value (difference in proportions test p-value)

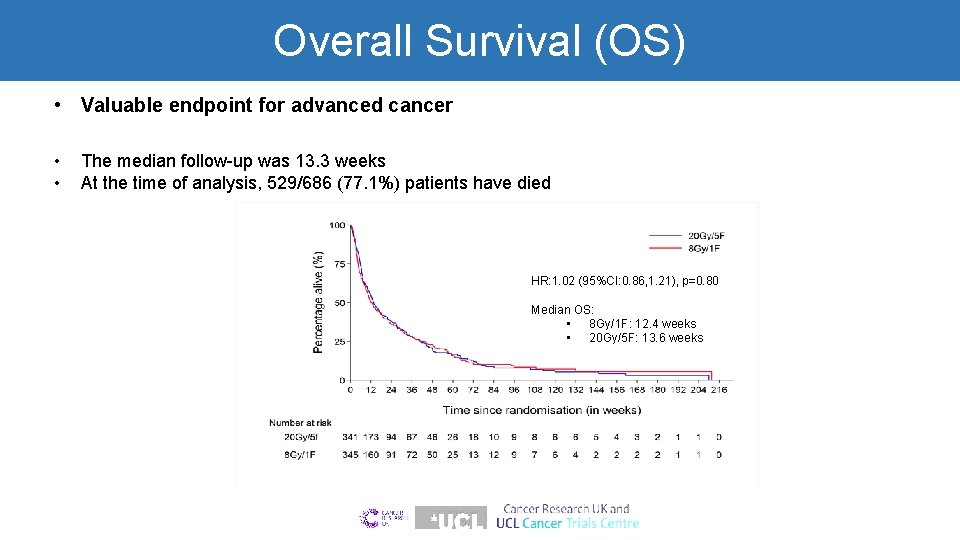
Overall Survival (OS) • Valuable endpoint for advanced cancer • • The median follow-up was 13. 3 weeks At the time of analysis, 529/686 (77. 1%) patients have died HR: 1. 02 (95%CI: 0. 86, 1. 21), p=0. 80 Median OS: • 8 Gy/1 F: 12. 4 weeks • 20 Gy/5 F: 13. 6 weeks

Adverse events (AEs) Adverse events Radiation reaction Pain Diarrhoea Dysphagia Constipation Sore throat Fatigue Urinary Any adverse event • • Grade 1 & 2 N=686 8 Gy/1 F N=345 20 Gy/5 F N=341 11. 6% 10. 1% 14. 2% 6. 7% 6. 4% 3. 8% 48. 7% 2. 9% 19. 4% 9. 7% 10. 6% 9. 4% 3. 5% 9. 7% 55. 4% 2. 9% 51. 9% Grade 3 & 4 N=686 8 Gy/1 F 20 Gy/5 F N=345 N=341 5. 2% 0. 6% 0. 9% 0. 3% 2. 6% 1. 8% 8. 1% 1. 2% 9. 7% 0. 3% 56. 9% 20. 6% 20. 5% Grade 5 AE: o 3 patients in the single fraction and 5 patients in the multifraction; none of the deaths were due to cancer and they were unrelated to radiotherapy Abnormal bladder function: o More common in 8 Gy/1 F arm from week 1 o At 8 weeks, 34/166 (20. 0%) 20 Gy/5 F vs 47/151 (31. 1%) 8 Gy/1 F had an abnormal bladder function

Are other results consistent with non-inferiority for single fraction? Abnormal bowel function: • No evidence of a significant difference at weeks 1, 4, 8 and 12 Supportive care for SCC: • Similar rates of use of analgesics (92. 2% 8 Gy/1 F vs 91. 5% 20 Gy/5 F) and any other type of treatment (97. 4% 8 Gy/1 F vs 97. 1% 20 Gy/5 F) Further treatment: • Similar rates of further treatment within 12 months (30. 1% 8 Gy/1 F vs 32. 3% 20 Gy/5 F) Changes in Qo. L score for a one-week increase in time were similar between trial groups (20 Gy/5 F vs 8 Gy/1 F): • Global health status: 0. 53 vs 0. 55 (p=0. 95) • Physical functioning: -0. 33 vs -0. 54 (p=0. 42) • Emotional functioning: 0. 41 vs 0. 27 (p=0. 59) • Pain: -2. 28 vs -2. 28 (p>0. 99)

Conclusions • SCORAD is the largest ever trial in metastatic spinal canal compression • Single dose of radiotherapy is as effective as multiple doses for treating MSCC with no impact on OS • Single dose RT recommended in this setting: • Patient convenience considering short survival time, or their carers • Significant reduction in hospital visits • Better use of RT resources at hospitals • Cost-effective

Acknowledgements • CR UK & UCL Cancer Trials Centre Cancer Research UK (C 2422/A 11408) • Trans Tasman Radiation Oncology Group (TROG) • SCORAD Trial Management Group • The 47 centers who recruited patients • IDMC: John Yarnold, Pat Price, Lucy Kilburn • TSC: Nick Reed, Andrew Clamp, Fergus Macbeth, Richard Stephens • All the patients and their families who took part
 Scorad
Scorad Rcbd layout
Rcbd layout Crd rcbd
Crd rcbd Phase 4 trial
Phase 4 trial Mobile phase and stationary phase
Mobile phase and stationary phase Which detector used in hplc
Which detector used in hplc Stationary and mobile phase
Stationary and mobile phase In a triangle connected source feeding a y connected load
In a triangle connected source feeding a y connected load Normal phase vs reverse phase chromatography
Normal phase vs reverse phase chromatography Normal phase vs reverse phase chromatography
Normal phase vs reverse phase chromatography Broad phase vs narrow phase
Broad phase vs narrow phase Tswett pronunciation
Tswett pronunciation Line current and phase current
Line current and phase current Ipfp phase iii
Ipfp phase iii Hamlet act iii scene ii
Hamlet act iii scene ii



























