HIV Vaccine efficacy studies and the MOSAICO clinical

















- Slides: 27

HIV Vaccine efficacy studies and the MOSAICO clinical trial Sabrina Spinosa Guzman Janssen Protocol Chair HVTN satellite 21 st July 2019 Martin Freeman, Not What It's Cracked Up to Be Diagnosed with AIDS in 1990, Martin lives in San Francisco where he continues to create new pieces.

Disclosure I have the following conflicts of interest to declare: • I am an employee of Janssen Vaccines & Prevention B. V. , a pharmaceutical company of Johnson & Johnson • I hold equity shares in Johnson & Johnson

Towards a global HIV vaccine

Considerations for efficacy evaluation Clade diversity Transmission mode Use of Pr. EP

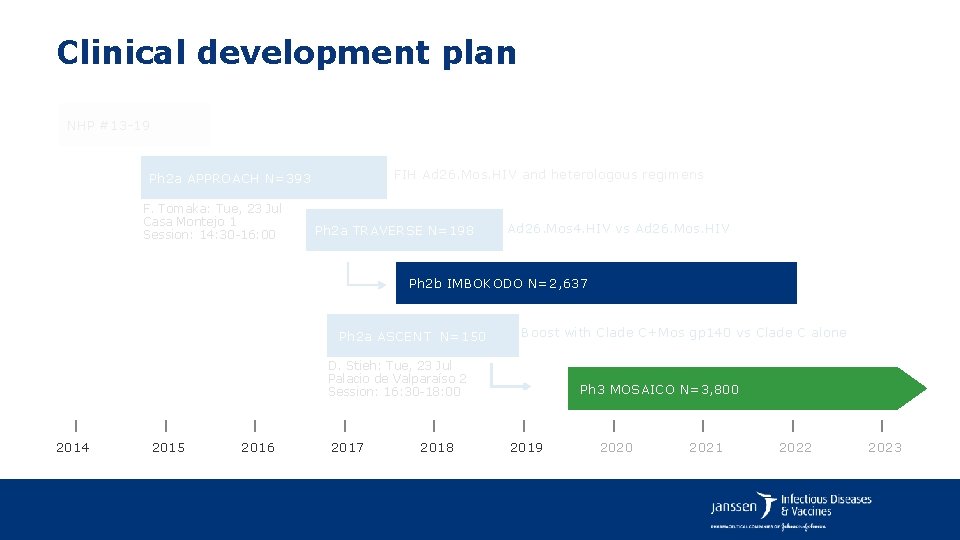
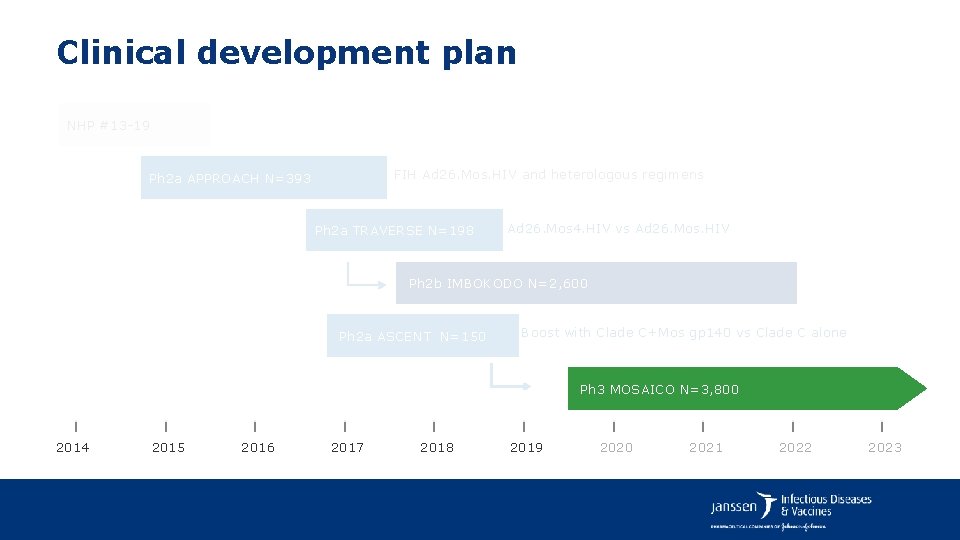
Clinical development plan NHP #13 -19 FIH Ad 26. Mos. HIV and heterologous regimens Ph 2 a APPROACH N=393 F. Tomaka: Tue, 23 Jul Casa Montejo 1 Session: 14: 30 -16: 00 Ph 2 a TRAVERSE N=198 Ad 26. Mos 4. HIV vs Ad 26. Mos. HIV Ph 2 b IMBOKODO N=2, 637 Ph 2 a ASCENT N=150 Boost with Clade C+Mos gp 140 vs Clade C alone D. Stieh: Tue, 23 Jul Palacio de Valparaíso 2 Session: 16: 30 -18: 00 2014 2015 2016 2017 2018 Ph 3 MOSAICO N=3, 800 2019 2020 2021 2022 2023

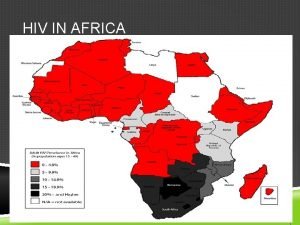
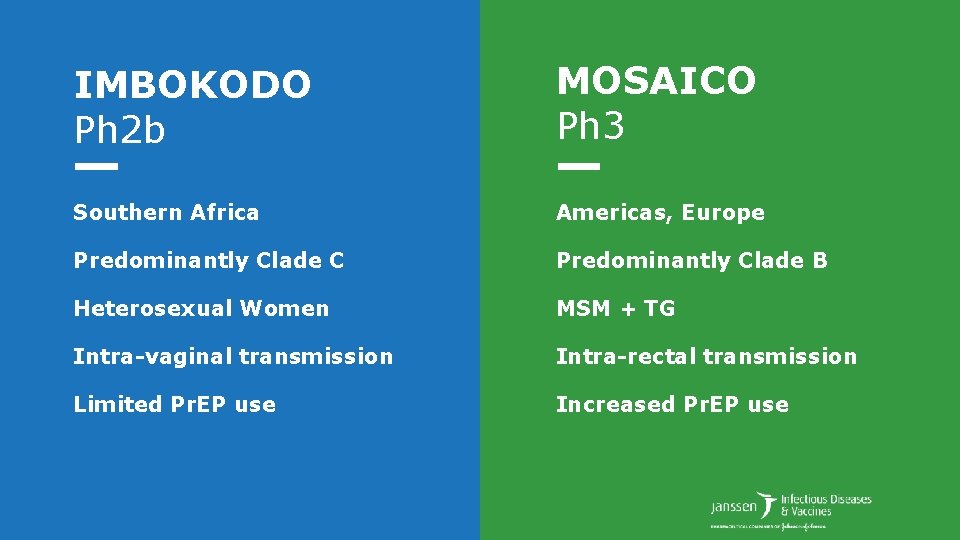
IMBOKODO Ph 2 b MOSAICO Ph 3 Southern Africa Americas, Europe Predominantly Clade C Predominantly Clade B Heterosexual Women MSM + TG Intra-vaginal transmission Intra-rectal transmission Limited Pr. EP use Increased Pr. EP use

IMBOKODO HPX 2008/HVTN 705 A phase 2 b multicenter, randomized, parallel group, placebo-controlled, double -blind clinical trial.

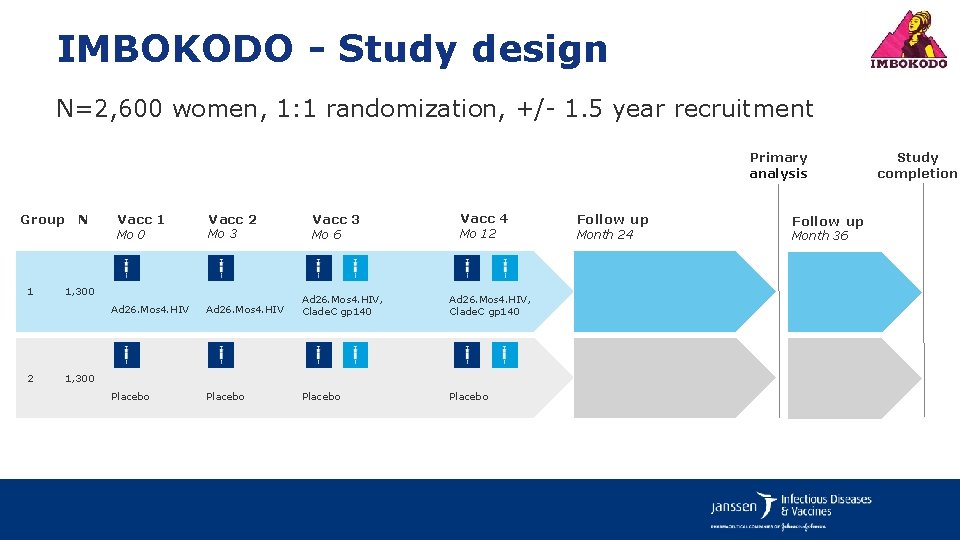
IMBOKODO - Study design N=2, 600 women, 1: 1 randomization, +/- 1. 5 year recruitment Primary analysis Group N 1 2 Vacc 1 Mo 0 Vacc 2 Mo 3 1, 300 Vacc 3 Mo 6 Vacc 4 Mo 12 Ad 26. Mos 4. HIV, Clade. C gp 140 Placebo 1, 300 Follow up Month 24 Follow up Month 36 Study completion

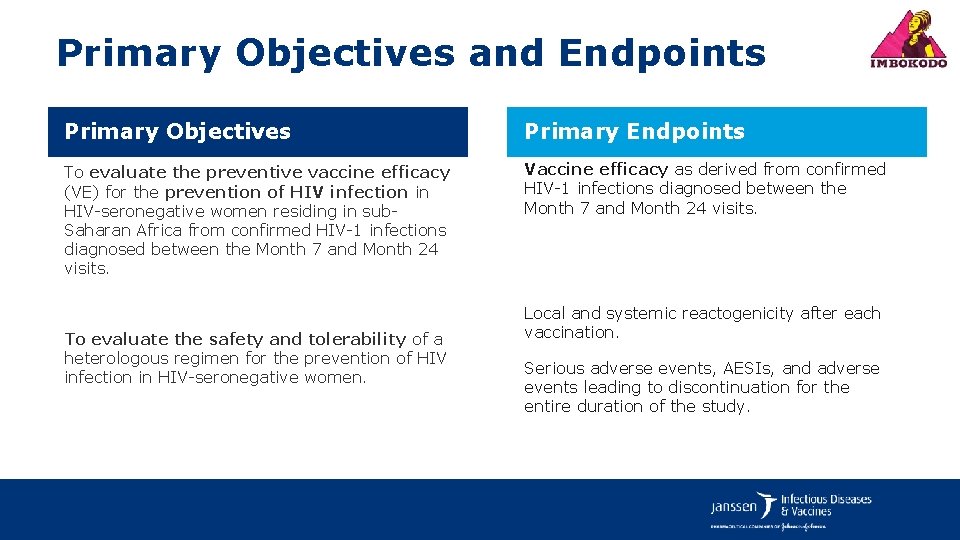
Primary Objectives and Endpoints Primary Objectives Primary Endpoints To evaluate the preventive vaccine efficacy (VE) for the prevention of HIV infection in HIV-seronegative women residing in sub. Saharan Africa from confirmed HIV-1 infections diagnosed between the Month 7 and Month 24 visits. Vaccine efficacy as derived from confirmed HIV-1 infections diagnosed between the Month 7 and Month 24 visits. To evaluate the safety and tolerability of a heterologous regimen for the prevention of HIV infection in HIV-seronegative women. Local and systemic reactogenicity after each vaccination. Serious adverse events, AESIs, and adverse events leading to discontinuation for the entire duration of the study.

Clinical development plan NHP #13 -19 FIH Ad 26. Mos. HIV and heterologous regimens Ph 2 a APPROACH N=393 Ph 2 a TRAVERSE N=198 Ad 26. Mos 4. HIV vs Ad 26. Mos. HIV Ph 2 b IMBOKODO N=2, 600 Ph 2 a ASCENT N=150 Boost with Clade C+Mos gp 140 vs Clade C alone Ph 3 MOSAICO N=3, 800 2014 2015 2016 2017 2018 2019 2020 2021 2022 2023

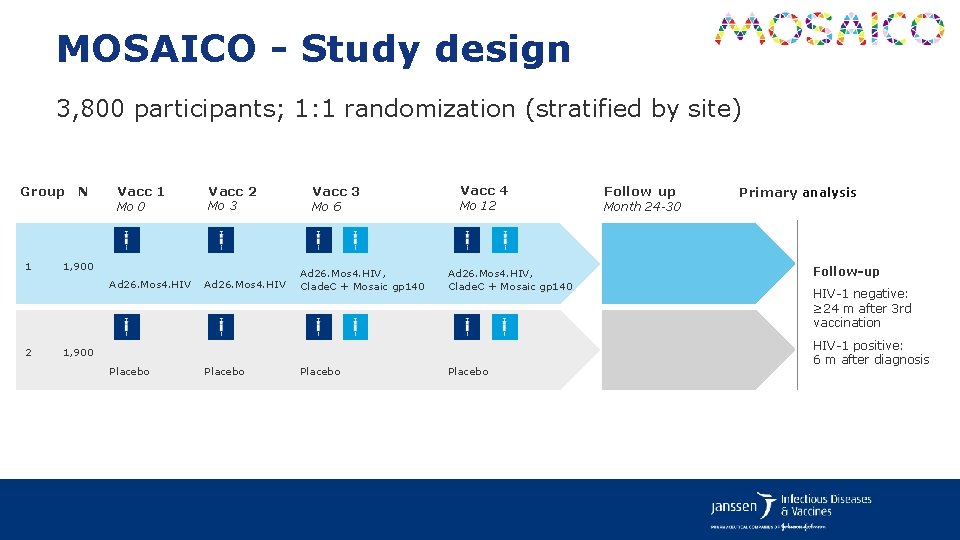
MOSAICO HPX 3002/HVTN 706 A Multi-center, Randomized, Double-blind, Placebo-controlled Phase 3 Efficacy Study of a Heterologous Vaccine Regimen of Ad 26. Mos 4. HIV and Adjuvanted Clade C gp 140 and Mosaic gp 140 to Prevent HIV-1 Infection Among Cis-gender Men and Transgender Individuals who Have Sex with Cis-gender Men and/or Transgender Individuals.

MOSAICO - Study design 3, 800 participants; 1: 1 randomization (stratified by site) Group N 1 Vacc 1 Mo 0 Mo 3 1, 900 Ad 26. Mos 4. HIV 2 Vacc 2 Ad 26. Mos 4. HIV Vacc 3 Mo 6 Ad 26. Mos 4. HIV, Clade. C + Mosaic gp 140 Vacc 4 Mo 12 Ad 26. Mos 4. HIV, Clade. C + Mosaic gp 140 1, 900 Placebo Follow up Month 24 -30 Primary analysis Follow‑up HIV-1 negative: ≥ 24 m after 3 rd vaccination HIV-1 positive: 6 m after diagnosis

Primary Objective and Endpoint Primary Objective Primary Endpoint To evaluate the vaccine efficacy (VE) for the prevention of HIV-1 infection in HIV-1 seronegative cis-gender men and transgender individuals having sex with cis-gender men and/or transgender individuals. Confirmed HIV-1 infections diagnosed between Month 7 and Month x (with 24≤x≤ 30 months) visits in the perprotocol (PP) population.

Secondary Objectives To evaluate the safety and reactogenicity. To evaluate VE at other timepoints and in other analysis populations. To evaluate the immune responses elicited by the vaccine regimen. To evaluate VE by and adjusting for potential (baseline) confounders.

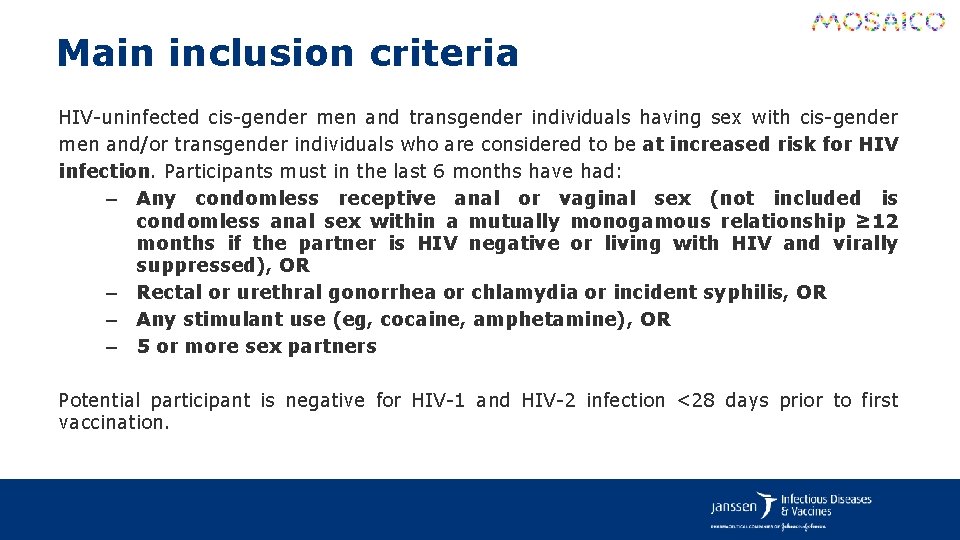
Main inclusion criteria HIV-uninfected cis-gender men and transgender individuals having sex with cis-gender men and/or transgender individuals who are considered to be at increased risk for HIV infection. Participants must in the last 6 months have had: – Any condomless receptive anal or vaginal sex (not included is condomless anal sex within a mutually monogamous relationship ≥ 12 months if the partner is HIV negative or living with HIV and virally suppressed), OR – Rectal or urethral gonorrhea or chlamydia or incident syphilis, OR – Any stimulant use (eg, cocaine, amphetamine), OR – 5 or more sex partners Potential participant is negative for HIV-1 and HIV-2 infection <28 days prior to first vaccination.

Main exclusion criteria Potential participant shares needles during injection of drugs or any other substance. Potential participant choosing to use Pr. EP – Note: Once participants received the first vaccination, they can decide at any time to initiate Pr. EP, while remaining in the study. – The use of long acting Pr. EP is disallowed from 24 months prior to Day 1.

HIV prevention in the study All participants will be offered comprehensive prevention methods: § § § Risk reduction counseling Condom and lubricant provision Pre-Exposure prophylaxis counseling, referral, and linkage Post Exposure prophylaxis counseling referral, and linkage STI screening, access to treatment and referral The trial will allow some comparison of the Vaccine + Standard Prevention package vs. Standard Prevention package alone

Global site distribution Europe Americas USA: 24 Poland: 3 Mexico: 3 Peru: 5 Spain: 6 Brazil: 9 Argentina: 4 Italy: 3

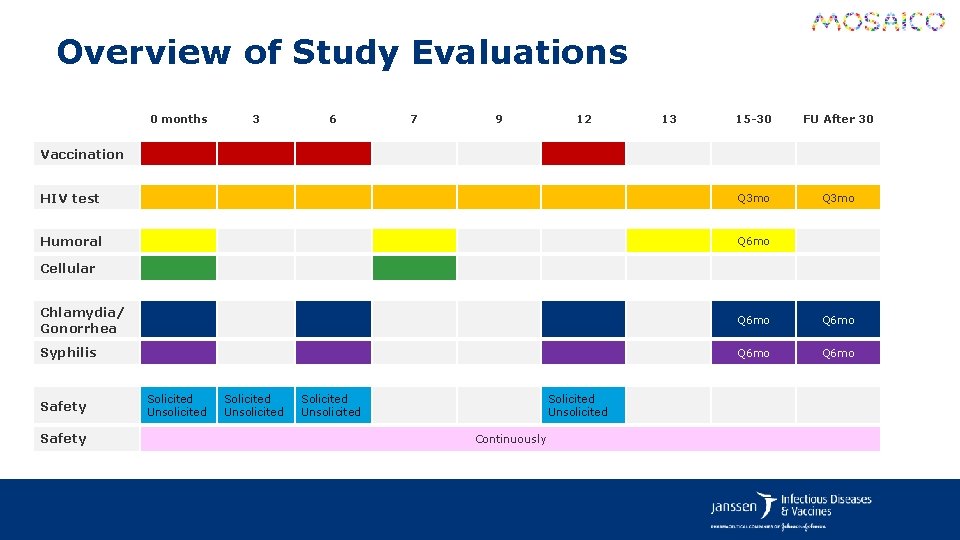
Overview of Study Evaluations 0 months 3 6 7 9 12 13 15 -30 FU After 30 HIV test Q 3 mo Humoral Q 6 mo Vaccination Cellular Chlamydia/ Gonorrhea Q 6 mo Syphilis Q 6 mo Safety Solicited Unsolicited Continuously

Efficacy Evaluations § An HIV test will be performed approximately every 3 months. Testing will be performed according to a sponsor-approved HIV testing algorithm that differentiates VISP from true HIV infection. § Flexibility is given for additional HIV tests as unscheduled visits. Participants should refrain from performing any HIV testing outside of the study protocol. § Participant with a confirmed positive HIV test during the study, will remain in the study but NO further vaccinations will be administered. A blinded endpoint adjudication process will be in place.

Participant Reported Outcomes § Sexual Activity Questionnaire Assess determinants of HIV acquisition throughout the study as well as information on HIV testing outside the study. § Questionnaire on the Use of Oral Pr. EP Information on use of and adherence to Pr. EP. § Social Impact Questionnaire Problems experienced with personal relationships, employment, education, health care, housing, health, disability or life insurance, travel, and immigration. § Vaccine regimen acceptability

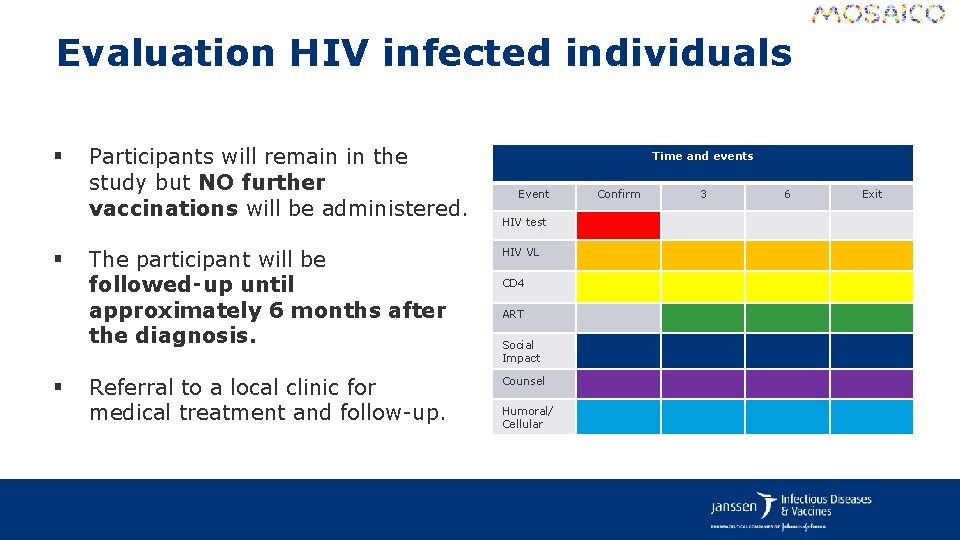
Evaluation HIV infected individuals § § § Participants will remain in the study but NO further vaccinations will be administered. Time and events Event HIV test The participant will be followed‑up until approximately 6 months after the diagnosis. HIV VL Referral to a local clinic for medical treatment and follow-up. Counsel CD 4 ART Social Impact Humoral/ Cellular Confirm 3 6 Exit

IMBOKODO Ph 2 b MOSAICO Ph 3 Southern Africa Americas, Europe Predominantly Clade C Predominantly Clade B Heterosexual Women MSM + TG Intra-vaginal transmission Intra-rectal transmission Limited Pr. EP use Increased Pr. EP use Fully enrolled Q 2 2019 Start Q 3 2019

HPX 3002/HVTN 706 Protocol Team Acknowledgements Janssen Team Sabrina Spinosa Guzman, Protocol Leadership Chair Ludo Lavreys, Study Responsible Physician Vicky Cárdenas, Study Responsible Scientist Frank Tomaka, Franchise Clinical Leader Maria Grazia Pau, Compound Development Team Leader Carla Truyers, Study Statistician Steven Nijs, Clinical Team Statistical Lead Daniel Stieh, Biomarkers Lead Zelda Euler, Senior Scientist Wolf Ribbens, Senior Associate Scientist Raphaele Roten, Medical Safety Officer Lorenz Scheppler, Global Regulatory Affairs Olive Yuan, Associate Director Data Management Caroline Hodin, Global Data Manager Specialist Wouter Vandermeiren, Senior Global Data Manager Chris Mc. Shane, GCDO Clinical Program Leader Karen Buleza, GCDO Clinical Trial Leader Johan De Decker, Senior Clinical Trial Manager Cornelia Linthicum, Senior Clinical Trial Manager Eveline Hoste, Associate Director Reg Medical Writing Anick Vandingenen, Associate Dir. Reg Medical Writing Corina Ramers-Verhoeven, Global Communication Leader, Vaccines R&D

HPX 3002/HVTN 706 Protocol Team Acknowledgements HVTN Team Larry Corey, Principal Investigator Jim Kublin, Principal Staff Scientist Susan Buchbinder, HVTN Chair Philipp Mann, Protocol Team Leader Megan Jones, Clinical Safety Specialist Robert De La Grecca, Regional Medical Liaison India Tindale, Clinical Trials Manager Niles Eaton, Director of Site Operations Laurie Rinn, Regulatory Associate Mariel Franklin, Regulatory Project Manager Stephaun Wallace, Sen Community Engagement Project Manager Aziel Gangerdine, Director of Communication Steven Wakefield, Director of External Relations DAIDS Julia Hutter, Medical Officer Lab John Hural, Associate Director of Laboratory Operations Mike Stirewalt, Quality Assurance Program Manager Katheryn Dougherty, Quality Assurance Associate Jennifer Hanke, Protocol Operations Coordinator Lisa Sanders, Protocol Operations Coordinator SCHARP Jessica Andriesen, Associate Director of Data Operations Lisa Bunts, Data Operations Project Manager Lauren Young, Lab Data Manager Nada Aboulhosn, Research Project Manager Kate Ostbye, Sr. Manager, Programming Julie Stofel, Manager, Clinical Programming Craig Chin, Prin. Clinical Programmer Brad Fischer, Sr. Clinical Programmer Abby Isaacs, Statistical Research Associate Alex Luedtke, Study Statistician Marco Carone, Study Statistician

HPX 3002/HVTN 706 Acknowledgements National Institute of Allergy and Infectious Diseases U. S. Army Medical Research and Development Command

Acknowledgements Janssen COMPOUND DEVELOPMENT TEAM DAIDS, NIAID Iedo Beeksma Antoine El Khoury Ad Knaapen Steven Nijs Valerie Oriol-Mathieu Maria Grazia Pau Lorenz Scheppler Daniel Stieh Frank Tomaka John Trott Frank Wegmann Mo Weijtens Carl Dieffenbach Julia Hutter Mary Marovich Tina Tong . . . and their teams SENIOR MANAGEMENT Jerry Sadoff Stefan Thoelen Macaya Douoguih Jenny Hendriks Hanneke Schuitemaker Johan van Hoof Mathai Mammen Paul Stoffels Communities and advocates for their valuable input All the investigators, their staffs and study participants, external consultants and funders of the clinical development program
 Vaccine efficacy
Vaccine efficacy Vaccine efficacy
Vaccine efficacy Mosaico clinical trial
Mosaico clinical trial Measuring personality in organisational behaviour
Measuring personality in organisational behaviour What is role efficacy
What is role efficacy Potency vs efficacy
Potency vs efficacy Potency vs efficacy
Potency vs efficacy Nebido efficacy
Nebido efficacy Optimal self-confidence
Optimal self-confidence Self-efficacy theory
Self-efficacy theory Collective teacher efficacy
Collective teacher efficacy Collective teacher efficacy
Collective teacher efficacy Collective teacher efficacy
Collective teacher efficacy Albert bandura self efficacy
Albert bandura self efficacy Drug efficacy
Drug efficacy Luminous efficacy comparison chart
Luminous efficacy comparison chart Drug efficacy
Drug efficacy Efficacy therapy
Efficacy therapy Efficacy potency
Efficacy potency Paradigm shift from women studies to gender studies
Paradigm shift from women studies to gender studies Mosaico justiniano
Mosaico justiniano Portador
Portador Estructura
Estructura Escultor romano famoso
Escultor romano famoso El arte del mosaico en bizancio
El arte del mosaico en bizancio Cartogramma a punti
Cartogramma a punti Gli strumenti della geografia
Gli strumenti della geografia Pacto mosaico
Pacto mosaico