Clinical Trial Centers Alliance offering specialized expertise to















![Testimonials Depression Study Sponsor Dr. [Andrew] Cutler and Patricia, [Florida Clinical Research Center] YOU Testimonials Depression Study Sponsor Dr. [Andrew] Cutler and Patricia, [Florida Clinical Research Center] YOU](https://slidetodoc.com/presentation_image_h/e29feb535860271bebfed6fb41014711/image-17.jpg)
![Testimonials ADHD Study Sponsor Dr. [Andrew] Cutler, Patti, and Florida Clinical Research Center staff Testimonials ADHD Study Sponsor Dr. [Andrew] Cutler, Patti, and Florida Clinical Research Center staff](https://slidetodoc.com/presentation_image_h/e29feb535860271bebfed6fb41014711/image-18.jpg)


- Slides: 24

Clinical Trial Centers Alliance offering specialized expertise to fit the unique needs of each client Bobbie Theodore Alliance Director clinicaltrials@btheodore. com www. clinicaltrialscenters. com

Executive Summary Our Alliance of experienced research sites and services can address portfolio clinical research activities. We propose flexible options to serve your clinical development needs as follows: A menu of services under one liaison and contract Expertise across a range of therapeutic areas, phase I through postmarketing Integrated services including clinical site conduct, with or without bundled CRO services such as protocol development, trial management, data management, statistical analysis, monitoring – any or all to meet your clinical program needs www. clinicaltrialcenters. com

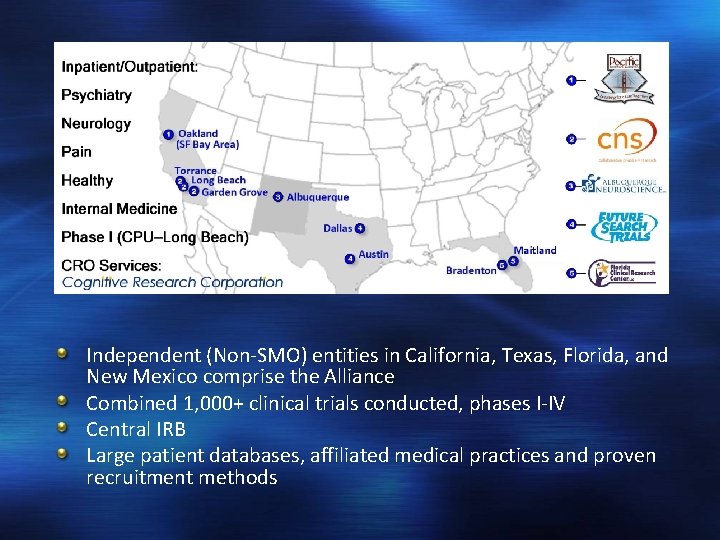
Independent (Non-SMO) entities in California, Texas, Florida, and New Mexico comprise the Alliance Combined 1, 000+ clinical trials conducted, phases I-IV Central IRB Large patient databases, affiliated medical practices and proven recruitment methods

Alliance Facilities - CALIFORNIA CNS, LLC Clinical Pharmacology Unit: 2600 Redondo Avenue, Suite 500, Long Beach, CA 90806 50+ bed CPU – targeted healthy subjects, CNS, stable psychiatry, neurology, pain, medical and bridging patient populations 20+ bed Licensed Psychiatric Hospital (JCAHO accredited) – acute psychiatry patient populations Outpatient offices – neurology, pain, and medical patient populations Principal Investigators: David Walling, Ph. D, (CEO) Psychologist Mark Leibowitz, MD, (Early Phase Medical Director) Internist Armen Goenjian, MD, (Medical Director) Psychiatrist Omidvar, MD, Neurologist Nirav Patel, MD, Neurologist Steven Reynolds, DO, Family Practitioner www. clinicaltrialcenters. com

Alliance Facilities - CALIFORNIA CNS, LLC Outpatient psychiatry offices: 12772 Valley View Street, Suite 3, Garden Grove, CA 92845 Principal Investigator: David Walling, Ph. D 19401 S. Vermont Avenue, Suite F-100, Torrance, CA 90502 Principal Investigator: Armen Goenjian, MD www. clinicaltrialcenters. com

Alliance Facilities - CALIFORNIA Pacific Research Partners, LLC 1611 Telegraph Avenue, Suite 1550, Oakland, CA 94612 (San Francisco Bay area) Principal Investigators: Ira Glick, MD, Psychiatrist and Anand Mehta, MD, Double Boarded in Internal Medicine and Endocrinology Inpatient –psychiatric patient population trials conducted at regional psychiatric facility Outpatient – psychiatry, cognition, MCI, fibromyalgia, pain, and general medical patient population trials www. clinicaltrialcenters. com

Alliance Facilities - TEXAS Future. Search Trials, LP 5508 Parkcrest Dr. , Ste. 300, Austin, TX 78731 Principal Investigators: John D. Hudson, MD, Double Boarded in Neurology and Sleep Medicine and Carmen Zegarra, MD, Psychiatrist Inpatient and Outpatient – child - adult psychiatry including phase I special patient population trials Inpatient - 6 bed sleep lab for PSG and sleep trials Outpatient – sleep, neurology, and pain trials 5445 La Sierra Drive, Suite 101, Dallas, TX 75231 Principal Investigator: Michael Downing, MD, Psychiatrist Inpatient – adult psychiatry population trials Outpatient – child – adult psychiatry, Alzheimer's, cognition, insomnia, and pain trials www. clinicaltrialcenters. com

Alliance Facilities - FLORIDA Florida Clinical Research Center, LLC 8043 Cooper Creek Blvd. , Suite 107, Bradenton, Florida 34201 Principal Investigators: Andrew J. Cutler, MD, (CEO) Double Boarded in Psychiatry and Internal Medicine, and Jose T. Zaglul, MD, Psychiatrist Inpatient – adult psychiatry, including targeted special phase I patient population trials Outpatient – child – adult psychiatry, cognition, insomnia, pain, and general medical patient population trials 2300 Maitland Center Pkwy, Ste. 230, Maitland, FL 32751 Principal Investigators: Martin S. Kane, MD, Board Certified Psychiatry and Neurology, Richard D. Knapp, DO, Board Certified Psychiatry and Addictions, and Joanne L. Northcutt, Ph. D, Child Health Psychologist Inpatient –psychiatry population trials, pediatric - adult Outpatient – child – adult psychiatry, cognition, insomnia, and pain patient population trials www. clinicaltrialcenters. com

Alliance Facilities – NEW MEXICO Albuquerque Neuroscience, Inc. 101 Hospital Loop NE, Suite 209, Albuquerque, NM 87109 Principal Investigators: Glenn Michael Dempsey, MD, Board Certified Psychiatry and Neurology, and Paula J. Lane, MD, Family Practice Outpatient – psychiatry, cognition, Alzheimer's, sleep disorders, pain, and general medical patient population trials www. clinicaltrialcenters. com

Staff Highlights Each Research Site Has Full-Time Dedicated And Highly Experienced Staff Investigators Board Certified in Psychiatry, Neurology, Sleep Medicine, Internal Medicine, Family Practice, Endocrinology, and Licensed Clinical Psychologists Protocol consultants to sponsors and CROs, and thought leaders in psychiatry, neurology, and sleep indications and phase I study designs Full-time certified psychometric clinical raters – M. D. , Ph. D. and M. A. -level with up to 20+ years rating experience Multiple full-time study coordinators including CCRCs, RNs, and LVNs Dedicated recruitment and outreach specialists with established referral networks in their communities Regulatory, QA, training and IT personnel www. clinicaltrialcenters. com

Specialized Capabilities and Experience Dedicated Clinical Pharmacology Unit (CPU) in Long Beach, CA Healthy subjects, and special patient populations, including Asian bridging Cardiac, telemetry and holter monitoring QTc and TQTc EEG and q. EEG evoked potentials Serial PK, serial ECG 1. 5 and 3 T MRI, f. MRI bold and 64 -Slice PET/CT system imaging Lumbar puncture and CSF collection PSG (polysomnography) Infusion, injection, oral, device, and transdermal patch delivery systems www. clinicaltrialcenters. com

Phase I-IV Trial Experience Psychiatry Addictions – smoking cessation, alcohol dependence, binge eating, opioid ADHD – adult and child, including classroom ADHD Anxiety – GAD, PTSD Bipolar – bipolar depression, mania, mixed Cognition – in schizophrenia, mild cognitive impairment Depression – MDD, refractory/treatment resistant, depression with sexual dysfunction Schizophrenia and schizoaffective disorders – acute, stable, cognitive dysfunction, negative symptoms Pediatric psychiatry Neurology Alzheimer’s – MCI, prodromal, mild, moderate, and severe Multiple Sclerosis – relapsing remitting Parkinson’s – early stage to advanced Post-stroke, Traumatic brain injury Sleep disorders – insomnia, narcolepsy, restless legs syndrome, shift worker, sleep apnea www. clinicaltrialcenters. com

Phase I-IV Trial Experience Pain Chronic pain Diabetic neuropathy Fibromyalgia Migraine Opioid pain Osteoarthritis Post herpetic neuralgia General Medical COPD Asthma Diabetes Hypercholesterolemia Hypertension Obesity OIC Respiratory Vaccines Women’s health www. clinicaltrialcenters. com

Recruitment and Retention Recruitment Full-time dedicated recruitment and outreach specialists on staff Large databases accumulated over 15+ years of research across all indications Affiliated PI physician private and group practice databases (psychiatry, neurology, family practice, specialty medical) Established relationships with network of physicians for additional patient referrals Outreach to and established relationships with residential facilities, board and cares, senior communities Participation in community events and support groups – provide free seminars, lunch and learns History of successful print, web, radio, and television advertising – recruitment departments have established relationships with media buyers for discounted advertising rates and preferred placement Ability to pre-qualify patients via IRB-approved pre-screen consent form Retention Inpatient facilities have private rooms, double occupancy for caregivers or loved ones as needed Site-provided patient transportation as needed Full-time dedicated staff for regular communication with patient and family, reminder and followup phone calls, and thorough pre-screening Many of the study patients are treated in the Investigator’s practices allowing for ease in transition, follow up, and retention www. clinicaltrialcenters. com

Affiliated Practices Affiliated Medical Practices CNS, CA is aligned with Drs. Goenjian, Omidvar, Patel and Reynolds large psychiatry, neurology and family group practices respectively in the LA and Orange County regions. Pacific Research Partners, CA has exclusive relationship with the largest mental health clinics in the San Francisco Bay Area Counties, and Dr. Mehta’s Bay Area Medical group practice. Future. Search Trials, Austin, TX is aligned with Dr. Hudson’s sleep medicine/neurology practice and sleep lab on premises of research facility. Future. Search Trials, Dallas, TX is aligned with Dr. Downing‘s psychiatry practice. Both Austin and Dallas sites have established community referral relationships with several residential facilities and mental health agencies. Florida Clinical Research Center, FL is aligned with Dr. Zaglul’s large child and adult mental health agency and residential facilities; and Drs. Knapp and Marraffino are aligned with The Center for Drug Free Living. Albuquerque Neuroscience, NM is aligned with local elderly facilities and community advocacy groups, group psychiatric and medical practices and a large multi-specialty regional organization www. clinicaltrialcenters. com

Alliance Advantages and Expertise Menu of site and study management services based on sponsor need Responsiveness and ease of single point of contact with sponsor relations team Dedicated regulatory/budget/contract staff for quick turnaround times including use of Central IRB Best clinical and operational practices shared across sites Thought leaders in CNS and Phase I indications with multiple publications, and advisory board memberships Clinical, budget, and protocol feedback during development process, if needed Established vendor relationships for seamless execution www. clinicaltrialcenters. com
![Testimonials Depression Study Sponsor Dr Andrew Cutler and Patricia Florida Clinical Research Center YOU Testimonials Depression Study Sponsor Dr. [Andrew] Cutler and Patricia, [Florida Clinical Research Center] YOU](https://slidetodoc.com/presentation_image_h/e29feb535860271bebfed6fb41014711/image-17.jpg)
Testimonials Depression Study Sponsor Dr. [Andrew] Cutler and Patricia, [Florida Clinical Research Center] YOU are NUMBER 1. My sincere appreciation and my congratulations to you for screening the first subject in the XXX depression study. The ice is broken and the ship has now sailed. Thank You Phase I Healthy Japanese Bridging Study Sponsor [Dr. Mark Leibowitz CNS] Congratulations on getting this study initiated so quickly! I heard that all eight subjects were successfully dosed today. Thank you for your hard work to achieve this milestone under such stringent timelines. We appreciate it greatly. Phase I Schizophrenia Study CRO I wanted to extend an extra special thank you to your [Dr. David Walling][CNS] team. Without your help today we could not have achieved the soft lock milestone. The entire team came together to meet our deliverable. Your staff stayed late on Wednesday (before coordinator’s jury duty), organized the team today and helped with last minute queries. Again, thanks for your continued hard work on the project and it’s been a pleasure to work with the team. Thanks. Schizophrenia Study CRO Dr. [Armen] Goenjian, [CNS], Congratulations!! Your site is the first that has reached the recruitment cap of 18 patients randomized in the XXX Suboptimal study. On behalf of the team, I would like to thank you and your site staff for the hard work and for being the first site to reach this goal!! Schizophrenia Study Sponsor Dr. Ira Glick, [Pacific Research Partners] We are planning on closing the screening of potential subjects for Study XXX at the end of December. While this study has been challenging to enroll, you and your site staff have done a tremendous job, both in terms of enrollment (16 subjects screened, 7 randomized) and data quality. www. clinicaltrialcenters. com
![Testimonials ADHD Study Sponsor Dr Andrew Cutler Patti and Florida Clinical Research Center staff Testimonials ADHD Study Sponsor Dr. [Andrew] Cutler, Patti, and Florida Clinical Research Center staff](https://slidetodoc.com/presentation_image_h/e29feb535860271bebfed6fb41014711/image-18.jpg)
Testimonials ADHD Study Sponsor Dr. [Andrew] Cutler, Patti, and Florida Clinical Research Center staff have been wonderful throughout their work on our studies. The team exceeded their enrollment goal and closed the study with a remarkably low screen failure and early discontinuation rate. In addition, and the quality of their work on both studies has been exceptional – their source and CRF data have been clean resulting in cost and time savings for our monitoring and data management activities. Dr. Cutler has also been incredibly helpful by collaborating with our team on patient recruitment strategies, the appropriate use of our study diagnostic tool, and sharing his thoughts about future development work with the compound. Alzheimer’s Study Sponsor Good afternoon Dr. [Omid] Omidvar, [CNS], I am writing to invite you to participate in a Phase IIb Alzheimer’s trial. You and your team were one of the top enrolling sites for the XXX Elderly MRD trial and we think that you and your team could be perfect for the trial. Multiple Sclerosis Study Sponsor Hi all, XXX (Senior Director of Clinical Operations with XXX) came by to meet with Dr. [Nirav] Patel, Anne Cabral, and the [CNS] team to acknowledge the outstanding job we all did on the XXX (MS) spasticity study! According to some of his internal metrics, we were performing at 300+% compared to other sites. That is amazing! Migraine Study Sponsor Dr. [John] Hudson and Future. Search Trials of Neurology] the XXX study team appreciates your efforts to reduce screen failures for this study. Currently, you have the lowest SF rate out of 40 US sites and the most randomized patients. XXX appreciates your efforts and expertise in this clinical trial. Insomnia Sleep Sponsor Dear Dr. [John] Hudson, [Future. Search Trials of Neurology], I hope you are doing well. I was reviewing the XXXsponsored trial enrollment numbers in my region, and was impressed with your enrollment numbers in the PSG study. I just wanted to say thank you so much for all of the hard work by you and your staff! www. clinicaltrialcenters. com

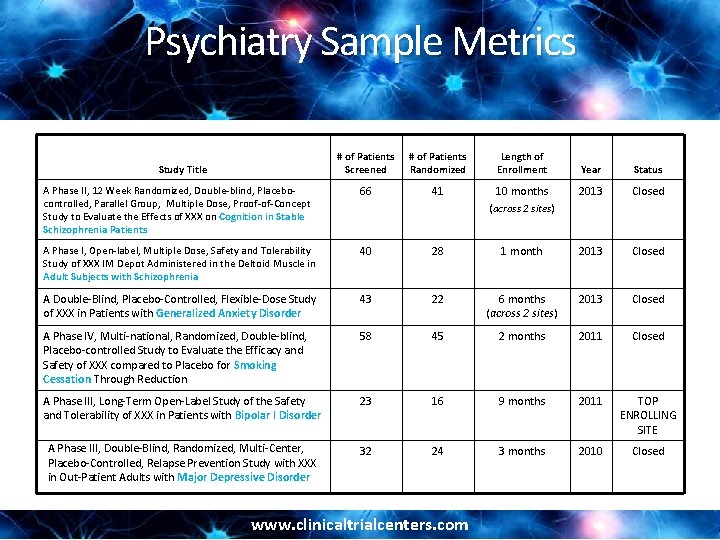
Psychiatry Sample Metrics # of Patients Screened # of Patients Randomized Length of Enrollment Year Status A Phase II, 12 Week Randomized, Double-blind, Placebocontrolled, Parallel Group, Multiple Dose, Proof-of-Concept Study to Evaluate the Effects of XXX on Cognition in Stable Schizophrenia Patients 66 41 10 months 2013 Closed A Phase I, Open-label, Multiple Dose, Safety and Tolerability Study of XXX IM Depot Administered in the Deltoid Muscle in Adult Subjects with Schizophrenia 40 28 1 month 2013 Closed A Double-Blind, Placebo-Controlled, Flexible-Dose Study of XXX in Patients with Generalized Anxiety Disorder 43 22 6 months (across 2 sites) 2013 Closed A Phase IV, Multi-national, Randomized, Double-blind, Placebo-controlled Study to Evaluate the Efficacy and Safety of XXX compared to Placebo for Smoking Cessation Through Reduction 58 45 2 months 2011 Closed A Phase III, Long-Term Open-Label Study of the Safety and Tolerability of XXX in Patients with Bipolar I Disorder 23 16 9 months 2011 TOP ENROLLING SITE A Phase III, Double-Blind, Randomized, Multi-Center, Placebo-Controlled, Relapse Prevention Study with XXX in Out-Patient Adults with Major Depressive Disorder 32 24 3 months 2010 Closed Study Title (across 2 sites) www. clinicaltrialcenters. com

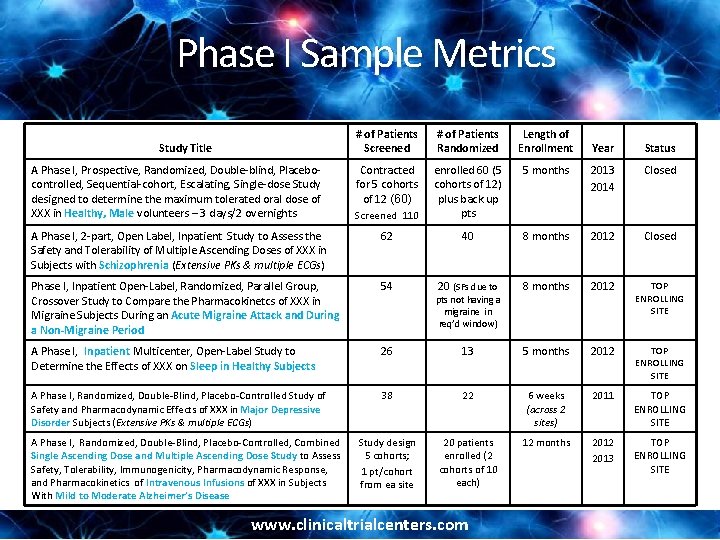
Phase I Sample Metrics # of Patients Screened # of Patients Randomized Length of Enrollment Contracted for 5 cohorts of 12 (60) Screened 110 enrolled 60 (5 cohorts of 12) plus back up pts A Phase I, 2 -part, Open Label, Inpatient Study to Assess the Safety and Tolerability of Multiple Ascending Doses of XXX in Subjects with Schizophrenia (Extensive PKs & multiple ECGs) 62 Phase I, Inpatient Open-Label, Randomized, Parallel Group, Crossover Study to Compare the Pharmacokinetcs of XXX in Migraine Subjects During an Acute Migraine Attack and During a Non-Migraine Period Study Title Year Status 5 months 2013 2014 Closed 40 8 months 2012 Closed 54 20 (SFs due to pts not having a migraine in req’d window) 8 months 2012 TOP ENROLLING SITE A Phase I, Inpatient Multicenter, Open-Label Study to Determine the Effects of XXX on Sleep in Healthy Subjects 26 13 5 months 2012 TOP ENROLLING SITE A Phase I, Randomized, Double-Blind, Placebo-Controlled Study of Safety and Pharmacodynamic Effects of XXX in Major Depressive Disorder Subjects (Extensive PKs & multiple ECGs) 38 22 6 weeks (across 2 sites) 2011 TOP ENROLLING SITE Study design 5 cohorts; 1 pt/cohort from ea site 20 patients enrolled (2 cohorts of 10 each) 12 months 2012 2013 TOP ENROLLING SITE A Phase I, Prospective, Randomized, Double-blind, Placebocontrolled, Sequential-cohort, Escalating, Single-dose Study designed to determine the maximum tolerated oral dose of XXX in Healthy, Male volunteers – 3 days/2 overnights A Phase I, Randomized, Double-Blind, Placebo-Controlled, Combined Single Ascending Dose and Multiple Ascending Dose Study to Assess Safety, Tolerability, Immunogenicity, Pharmacodynamic Response, and Pharmacokinetics of Intravenous Infusions of XXX in Subjects With Mild to Moderate Alzheimer’s Disease www. clinicaltrialcenters. com

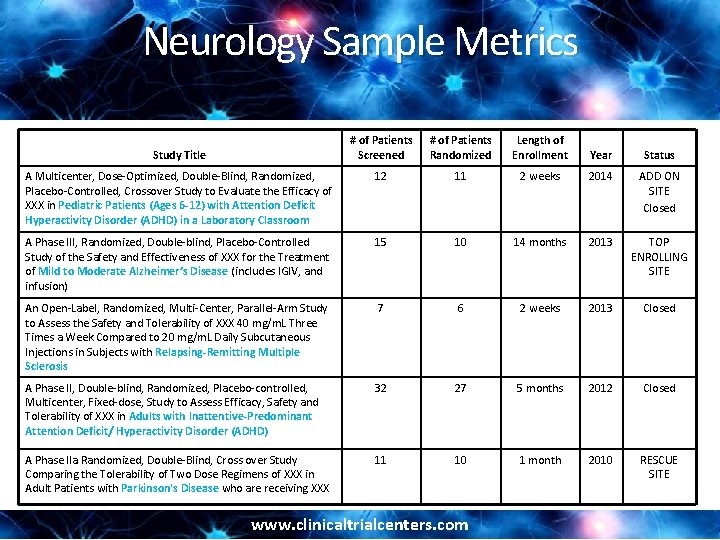
Neurology Sample Metrics # of Patients Screened # of Patients Randomized Length of Enrollment Year Status A Multicenter, Dose-Optimized, Double-Blind, Randomized, Placebo-Controlled, Crossover Study to Evaluate the Efficacy of XXX in Pediatric Patients (Ages 6 -12) with Attention Deficit Hyperactivity Disorder (ADHD) in a Laboratory Classroom 12 11 2 weeks 2014 ADD ON SITE Closed A Phase III, Randomized, Double-blind, Placebo-Controlled Study of the Safety and Effectiveness of XXX for the Treatment of Mild to Moderate Alzheimer’s Disease (includes IGIV, and infusion) 15 10 14 months 2013 TOP ENROLLING SITE An Open-Label, Randomized, Multi-Center, Parallel-Arm Study to Assess the Safety and Tolerability of XXX 40 mg/m. L Three Times a Week Compared to 20 mg/m. L Daily Subcutaneous Injections in Subjects with Relapsing-Remitting Multiple Sclerosis 7 6 2 weeks 2013 Closed A Phase II, Double-blind, Randomized, Placebo-controlled, Multicenter, Fixed-dose, Study to Assess Efficacy, Safety and Tolerability of XXX in Adults with Inattentive-Predominant Attention Deficit/ Hyperactivity Disorder (ADHD) 32 27 5 months 2012 Closed A Phase IIa Randomized, Double-Blind, Cross over Study Comparing the Tolerability of Two Dose Regimens of XXX in Adult Patients with Parkinson's Disease who are receiving XXX 11 10 1 month 2010 RESCUE SITE Study Title www. clinicaltrialcenters. com

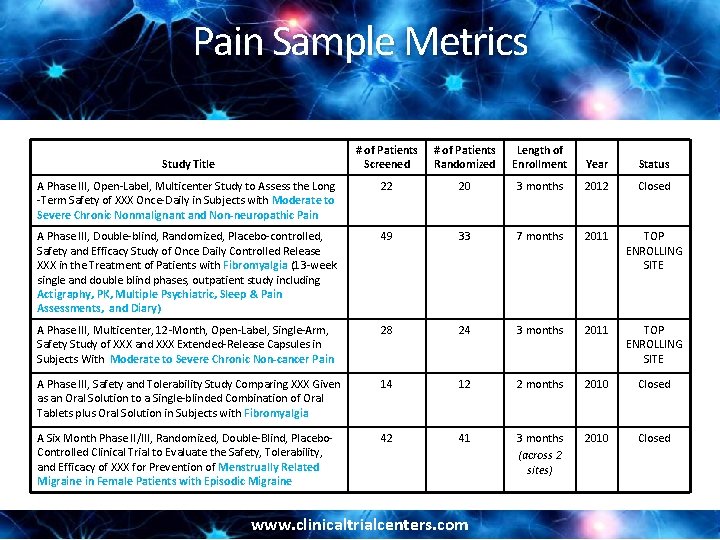
Pain Sample Metrics # of Patients Screened # of Patients Randomized Length of Enrollment Year Status A Phase III, Open-Label, Multicenter Study to Assess the Long -Term Safety of XXX Once-Daily in Subjects with Moderate to Severe Chronic Nonmalignant and Non-neuropathic Pain 22 20 3 months 2012 Closed A Phase III, Double-blind, Randomized, Placebo-controlled, Safety and Efficacy Study of Once Daily Controlled Release XXX in the Treatment of Patients with Fibromyalgia (13 -week single and double blind phases, outpatient study including Actigraphy, PK, Multiple Psychiatric, Sleep & Pain Assessments, and Diary) 49 33 7 months 2011 TOP ENROLLING SITE A Phase III, Multicenter, 12 -Month, Open-Label, Single-Arm, Safety Study of XXX and XXX Extended-Release Capsules in Subjects With Moderate to Severe Chronic Non-cancer Pain 28 24 3 months 2011 TOP ENROLLING SITE A Phase III, Safety and Tolerability Study Comparing XXX Given as an Oral Solution to a Single-blinded Combination of Oral Tablets plus Oral Solution in Subjects with Fibromyalgia 14 12 2 months 2010 Closed A Six Month Phase II/III, Randomized, Double-Blind, Placebo. Controlled Clinical Trial to Evaluate the Safety, Tolerability, and Efficacy of XXX for Prevention of Menstrually Related Migraine in Female Patients with Episodic Migraine 42 41 3 months (across 2 sites) 2010 Closed Study Title www. clinicaltrialcenters. com

Curriculum Vitae Click on PI names to view CVs: David P. Walling, Ph. D. CEO and PI – CNS, LLC Armen K. Goenjian, M. D. , D. F. A. P. A. , F. A. C. G. S. PI and Partner – CNS, LLC Omidvar, M. D. PI – CNS, LLC Nirav S. Patel, M. D. PI – CNS, LLC Steven Reynolds, D. O. PI – CNS, LLC Mark Leibowitz, M. D. – PI – CNS, LLC Ira D. Glick, M. D. PI – Pacific Research Partners, LLC Anand Mehta, M. D. PI – Pacific Research Partners, LLC John Douglas Hudson, M. D. PI– Future. Search Trials of Neurology, LP Michael Downing, M. D. PI– Future. Search Trials of Dallas, LP Andrew J. Cutler, M. D. CEO and PI – Florida Clinical Research Center, LLC Jose T. Zaglul, M. D. PI – Florida Clinical Research Center, LLC Richard D. Knapp, D. O. PI – Florida Clinical Research Center, LLC Joanne Northcutt, Ph. D. PI – Florida Clinical Research Center, LLC Martin Kane, M. D. PI – Florida Clinical Research Center, LLC Glenn Michael Dempsey, M. D. PI – Albuquerque Neuroscience, Inc Paula J. Lane, M. D. PI – Albuquerque Neuroscience, Inc www. clinicaltrialcenters. com

Contact For further information on Alliance services contact: Bobbie Theodore (916) 939 -6696 clinicaltrials@btheodore. com www. clinicaltrialcenters. com
 Clinical trial centers alliance
Clinical trial centers alliance Safety centers of expertise
Safety centers of expertise Nida clinical trial network
Nida clinical trial network Rsna ctp anonymizer
Rsna ctp anonymizer Clinical trial matching service
Clinical trial matching service Master clinical trial agreement
Master clinical trial agreement Clinicaltrials.gov api
Clinicaltrials.gov api Mosaico clinical trial
Mosaico clinical trial Companion diagnostic regulation
Companion diagnostic regulation Morpheus clinical trial
Morpheus clinical trial Accelerated clinical trial agreement acta
Accelerated clinical trial agreement acta Clinical trial financial management
Clinical trial financial management Protocol registration system
Protocol registration system Clinical trial exports
Clinical trial exports Clinical trial budget example
Clinical trial budget example Trofinetide
Trofinetide Drug development timeline
Drug development timeline Ivr iwr for clinical trial
Ivr iwr for clinical trial Cynchia
Cynchia Clinical trial worksheet
Clinical trial worksheet Novel clinical drug trial design
Novel clinical drug trial design Accelerated clinical trial agreement acta
Accelerated clinical trial agreement acta Expertise oriented evaluation approach
Expertise oriented evaluation approach Jm audit expertise
Jm audit expertise Andriessen expertise
Andriessen expertise