Alcohols and ethers Preparation properties and reactions of








- Slides: 24

Alcohols and ethers

• Preparation, properties and reactions of alcohols • (Preparation from haloalkanes, alkenes and reduction of carbonyl compounds using Li. Al. H 4) • Physical properties of alcohols related to bonding • Dehydration, reaction with metals, reaction with carboxylic acids and acid chlorides • Naming and general structure of ethers • Boiling point related to bonding • Preparation using haloalkanes with alkoxides • Chemical and physical properties linked to molecular size and uses

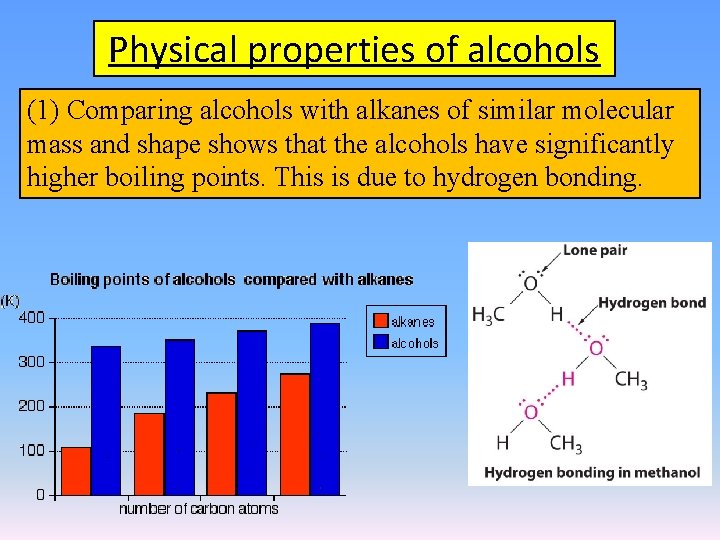
Physical properties of alcohols (1) Comparing alcohols with alkanes of similar molecular mass and shape shows that the alcohols have significantly higher boiling points. This is due to hydrogen bonding.

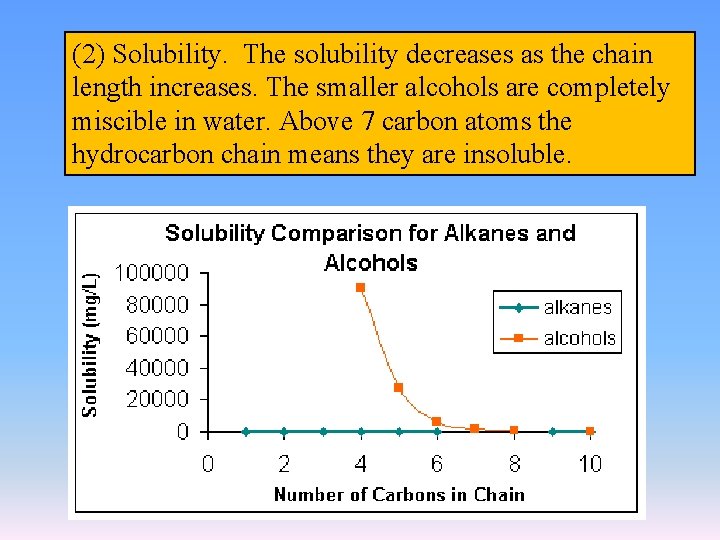
(2) Solubility. The solubility decreases as the chain length increases. The smaller alcohols are completely miscible in water. Above 7 carbon atoms the hydrocarbon chain means they are insoluble.

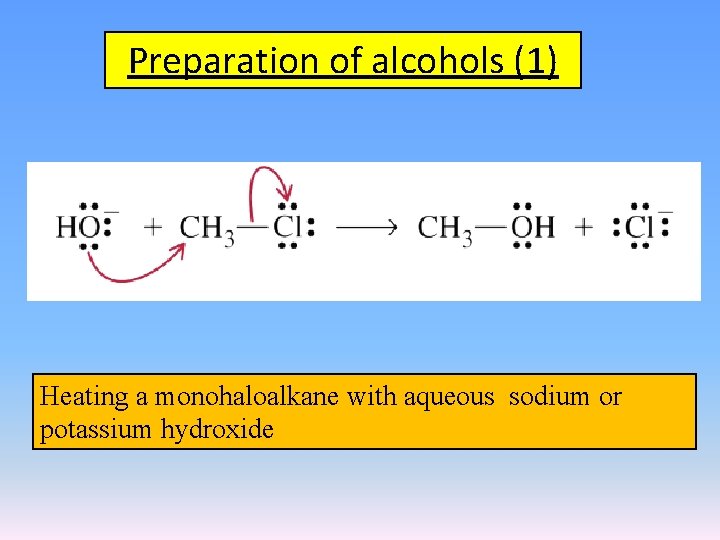
Preparation of alcohols (1) Heating a monohaloalkane with aqueous sodium or potassium hydroxide

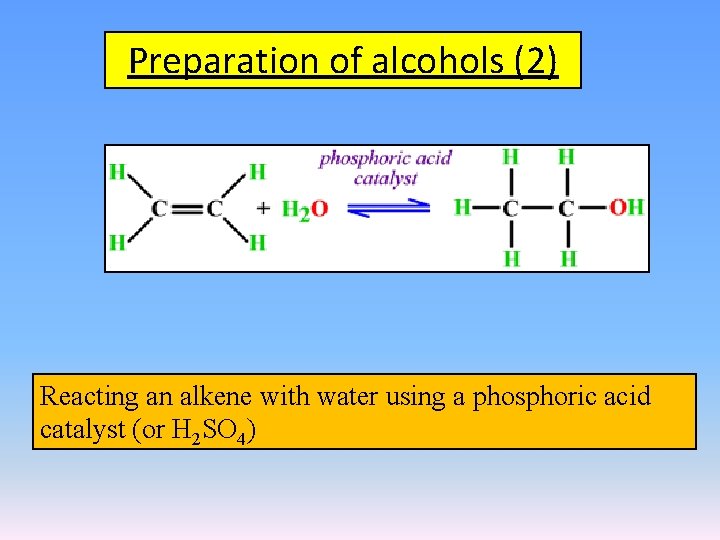
Preparation of alcohols (2) Reacting an alkene with water using a phosphoric acid catalyst (or H 2 SO 4)

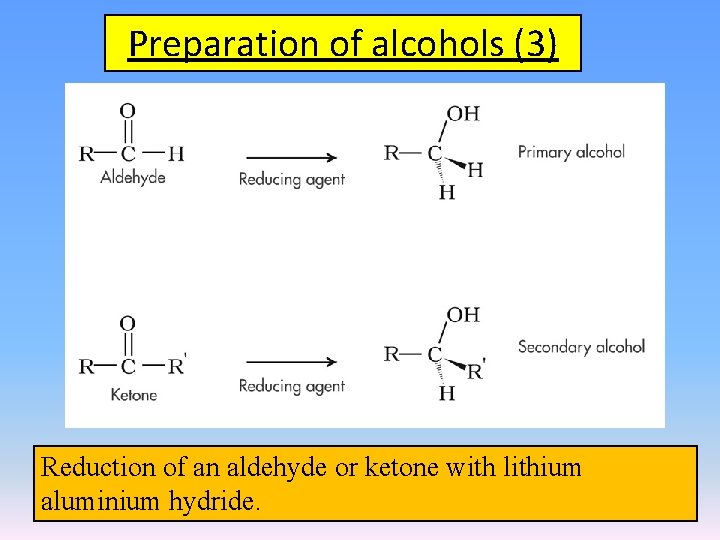
Preparation of alcohols (3) Reduction of an aldehyde or ketone with lithium aluminium hydride.

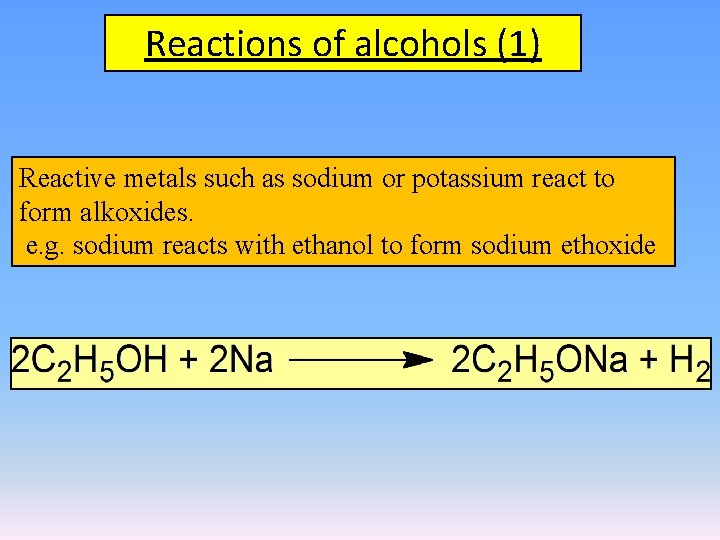
Reactions of alcohols (1) Reactive metals such as sodium or potassium react to form alkoxides. e. g. sodium reacts with ethanol to form sodium ethoxide

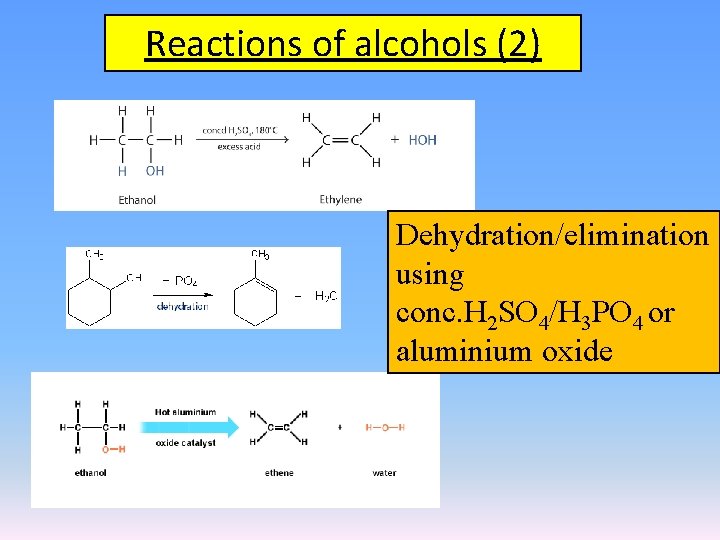
Reactions of alcohols (2) Dehydration/elimination using conc. H 2 SO 4/H 3 PO 4 or aluminium oxide

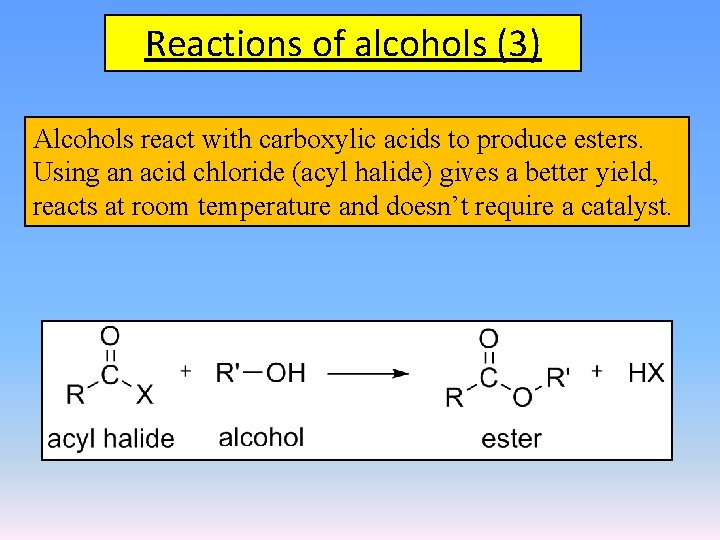
Reactions of alcohols (3) Alcohols react with carboxylic acids to produce esters. Using an acid chloride (acyl halide) gives a better yield, reacts at room temperature and doesn’t require a catalyst.


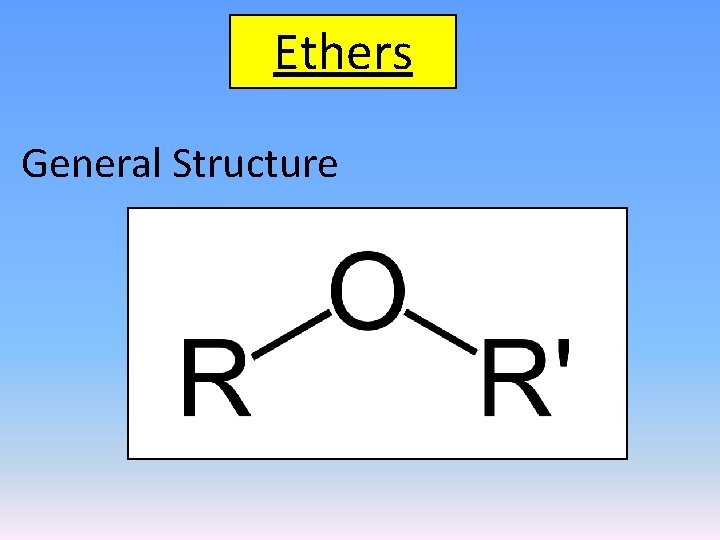
Ethers General Structure

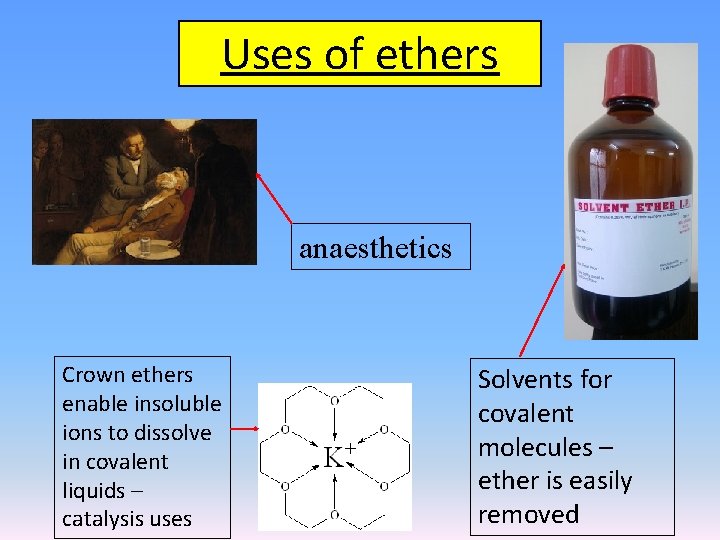
Uses of ethers anaesthetics Crown ethers enable insoluble ions to dissolve in covalent liquids – catalysis uses Solvents for covalent molecules – ether is easily removed

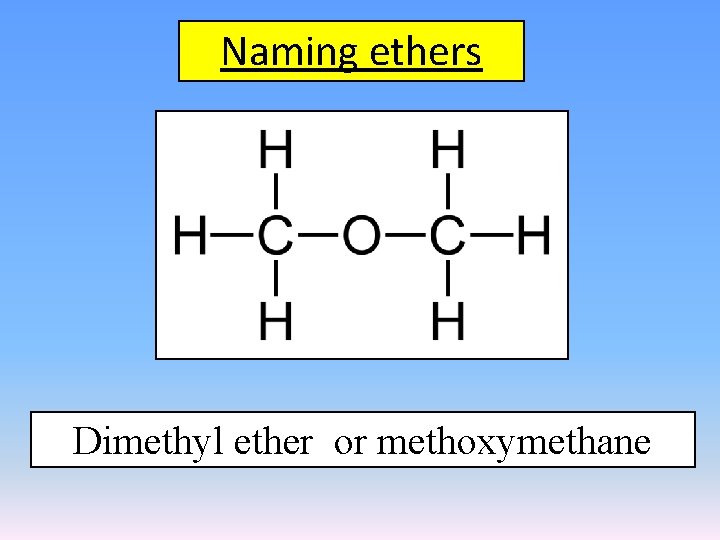
Naming ethers Dimethyl ether or methoxymethane

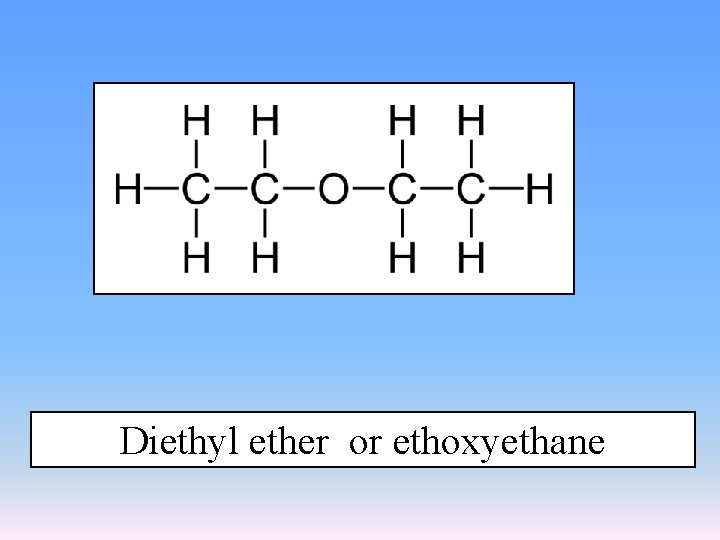
Diethyl ether or ethoxyethane

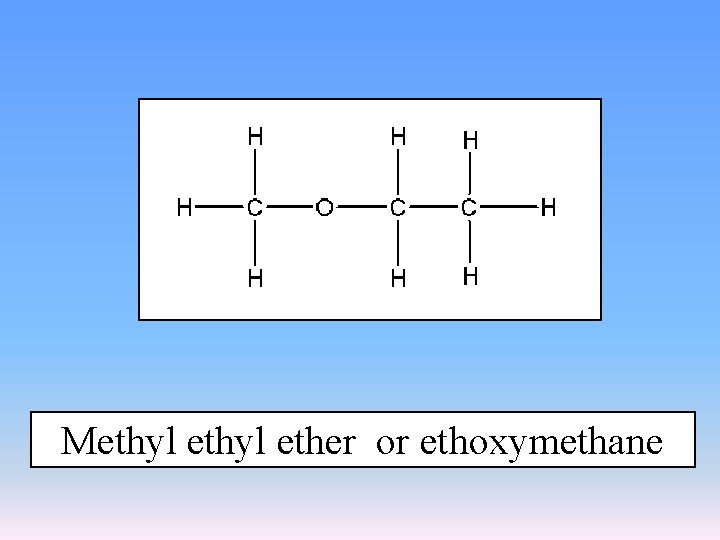
Methyl ether or ethoxymethane

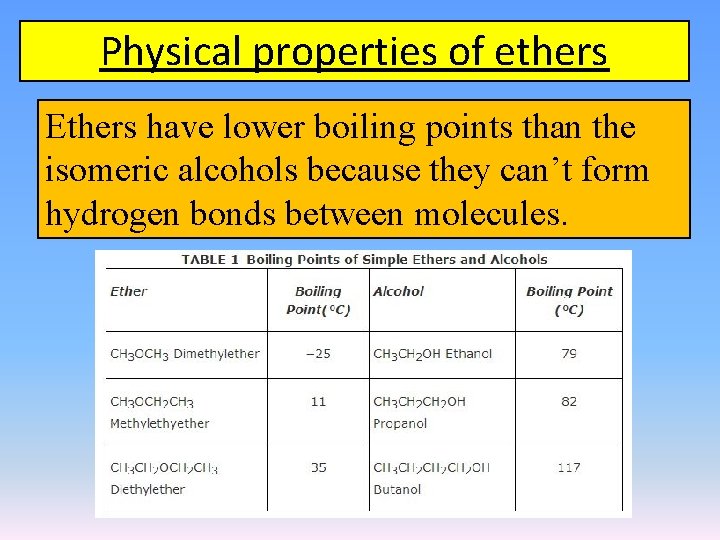
Physical properties of ethers Ethers have lower boiling points than the isomeric alcohols because they can’t form hydrogen bonds between molecules.


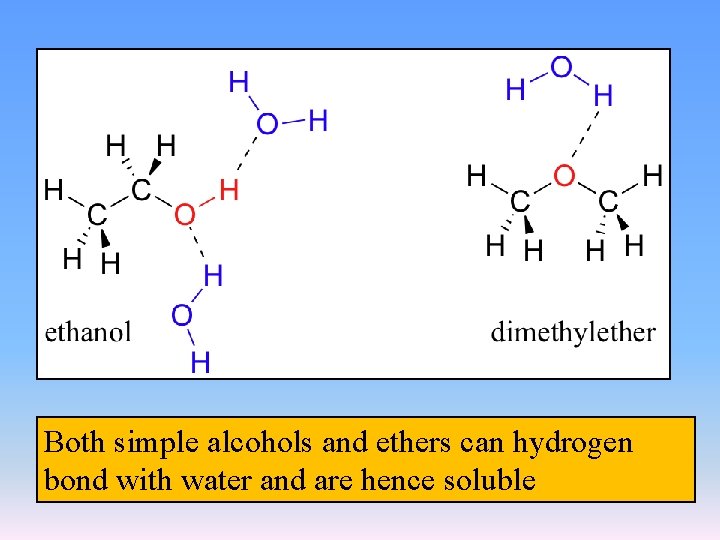
Both simple alcohols and ethers can hydrogen bond with water and are hence soluble

Ethers are volatile and highly flammable https: //www. angelo. edu/faculty/kboudrea/de mos/ether_trough. htm

Ethers are excellent solvents due to low reactivity and ability to dissolve many organic compounds.

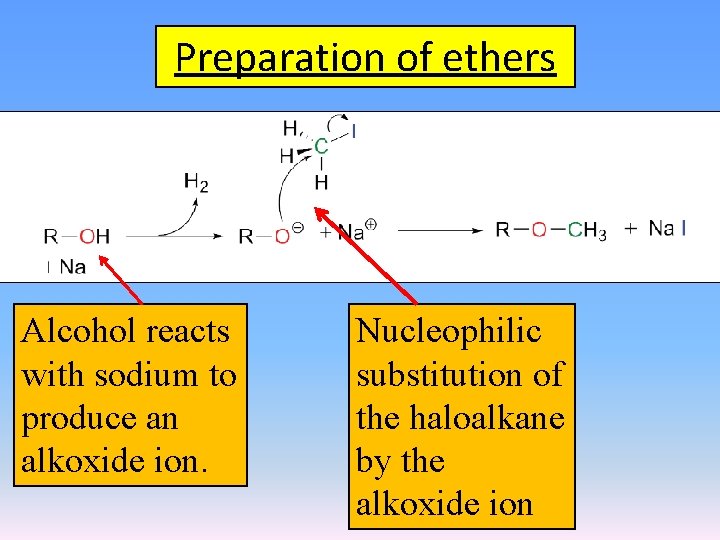
Preparation of ethers Alcohol reacts with sodium to produce an alkoxide ion. Nucleophilic substitution of the haloalkane by the alkoxide ion


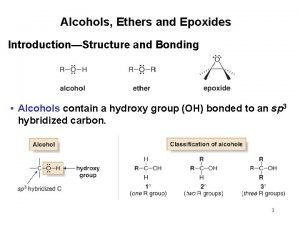
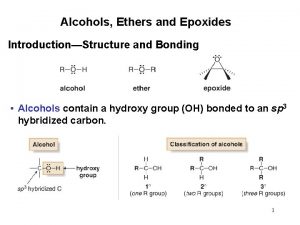
 Alcohols phenols thiols and ethers
Alcohols phenols thiols and ethers Naming ether
Naming ether Reactions of aldehydes and ketones chemsheets answers
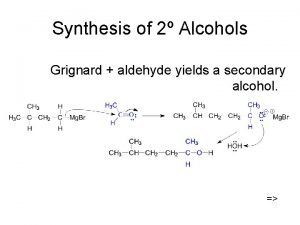
Reactions of aldehydes and ketones chemsheets answers Ethylene oxide + grignard reagent
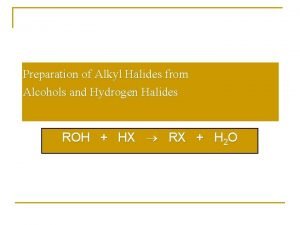
Ethylene oxide + grignard reagent Preparation of alkyl halides from alcohols
Preparation of alkyl halides from alcohols Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Examples of redox reaction
Examples of redox reaction Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Butaanzuur
Butaanzuur Naming phenols
Naming phenols Ether naming
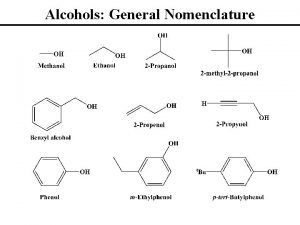
Ether naming Alcohols nomenclature
Alcohols nomenclature Ethers boiling point
Ethers boiling point David klein organic chemistry 3rd edition
David klein organic chemistry 3rd edition Acidic cleavage of ethers
Acidic cleavage of ethers Epoxide plus grignard
Epoxide plus grignard Acidic cleavage of ethers
Acidic cleavage of ethers Oxidation of primary alcohols
Oxidation of primary alcohols Cppp cholesterol
Cppp cholesterol Epoxide reaction with grignard reagent
Epoxide reaction with grignard reagent Lucas reagent is
Lucas reagent is Lucas' reagent
Lucas' reagent Na2cr2o7 mechanism
Na2cr2o7 mechanism