4 6 Real solution activity of solute and

- Slides: 18

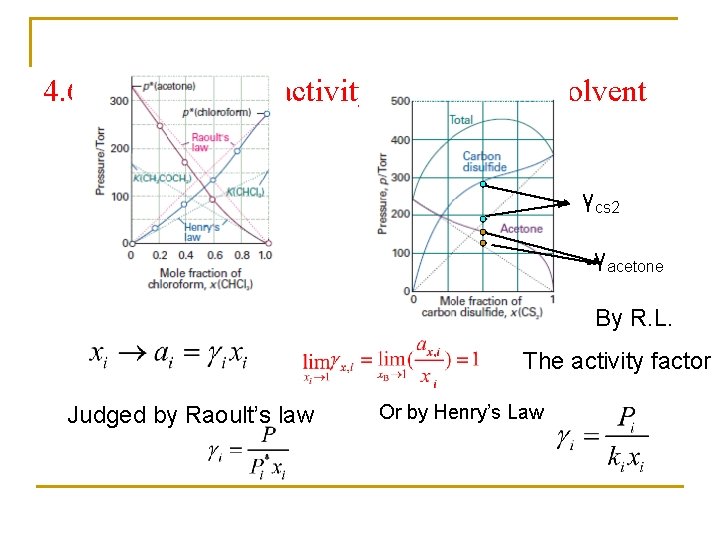
4. 6 Real solution: activity of solute and solvent γcs 2 γacetone By R. L. The activity factor Judged by Raoult’s law Or by Henry’s Law

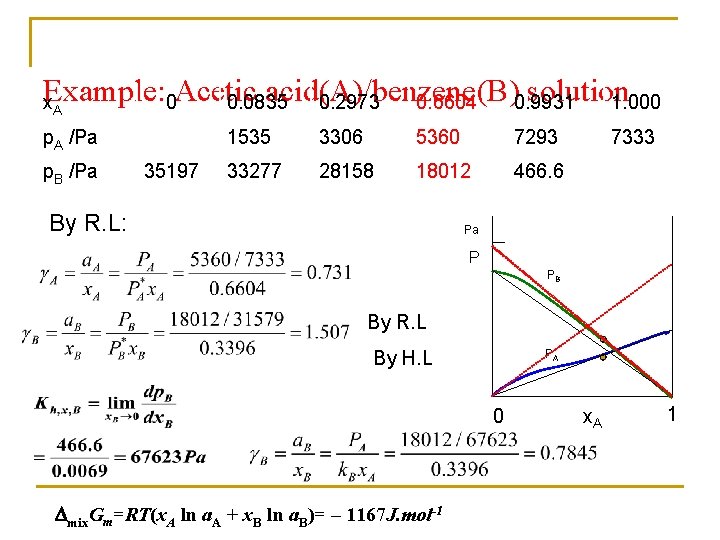
Example: 0 Acetic acid(A)/benzene(B) solution 0. 0835 0. 2973 0. 6604 0. 9931 1. 000 x. A p. A /Pa p. B /Pa 35197 1535 3306 5360 7293 33277 28158 18012 466. 6 By R. L: 7333 Pa P PB By R. L PA By H. L 0 mix. Gm=RT(x. A ln a. A + x. B ln a. B)= – 1167 J. mol-1 x. A 1

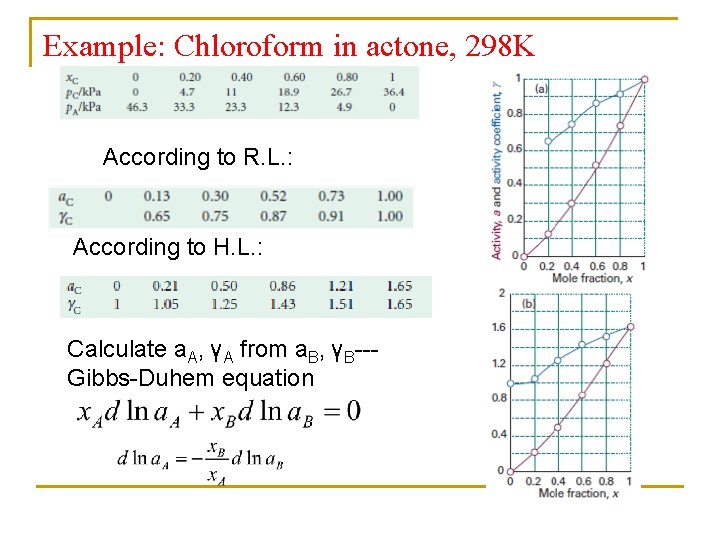
Example: Chloroform in actone, 298 K According to R. L. : According to H. L. : Calculate a. A, γA from a. B, γB--Gibbs-Duhem equation

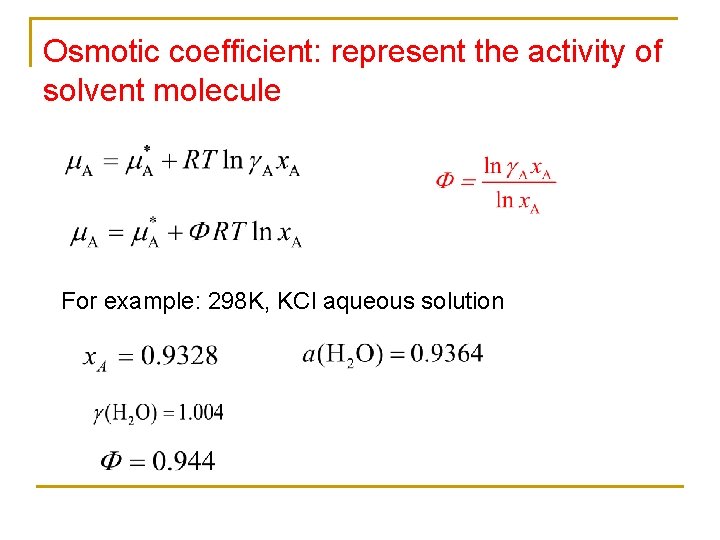
Osmotic coefficient: represent the activity of solvent molecule For example: 298 K, KCl aqueous solution

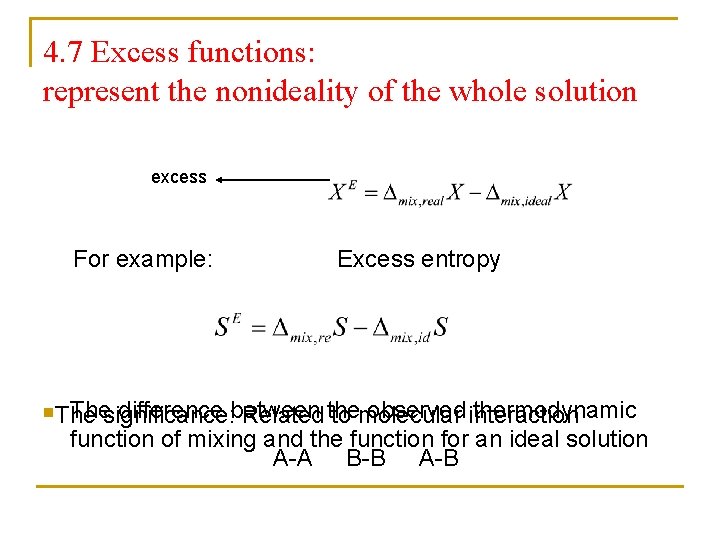
4. 7 Excess functions: represent the nonideality of the whole solution excess For example: Excess entropy n. Thesignificance: difference between observed interaction thermodynamic Related the to molecular function of mixing and the function for an ideal solution A-A B-B A-B

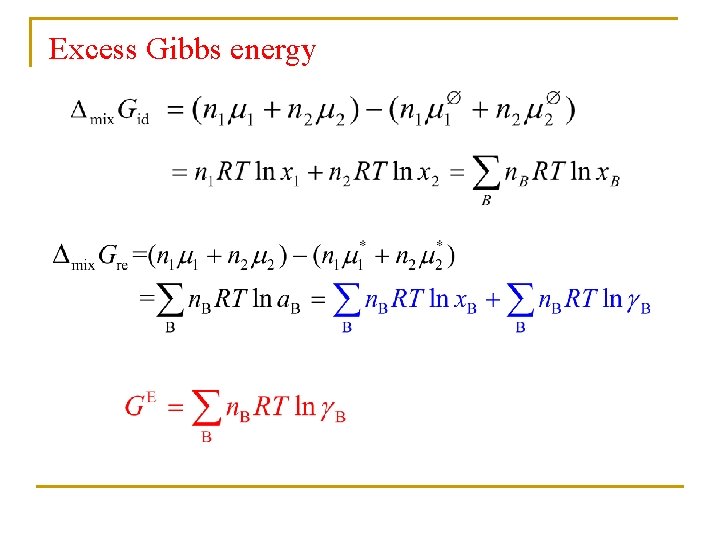
Excess Gibbs energy

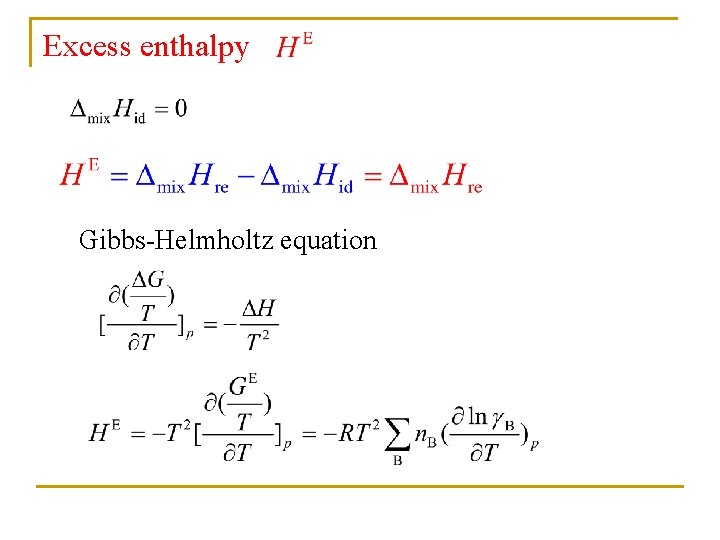
Excess enthalpy Gibbs-Helmholtz equation

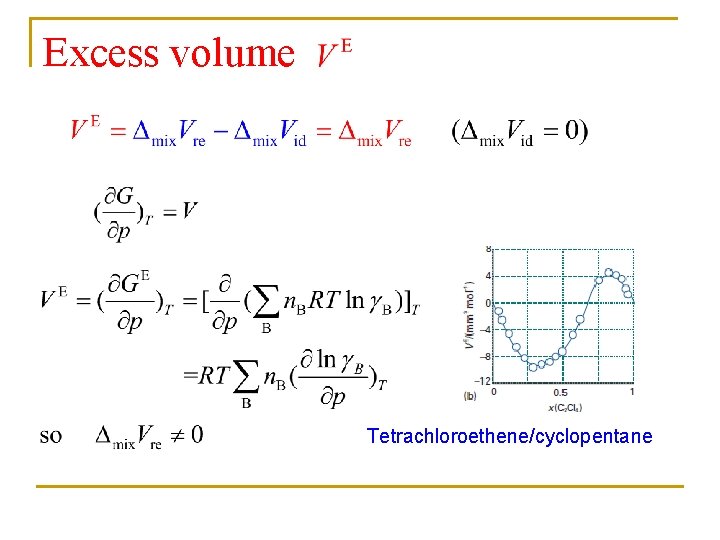
Excess volume Tetrachloroethene/cyclopentane

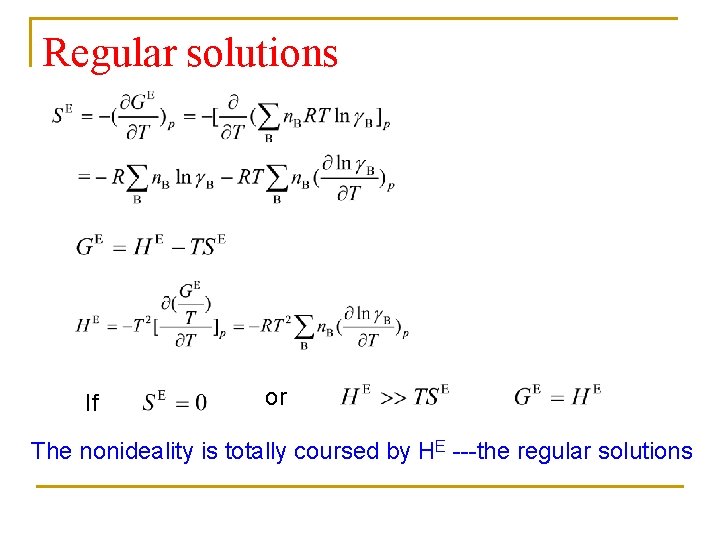
Regular solutions If or The nonideality is totally coursed by HE ---the regular solutions

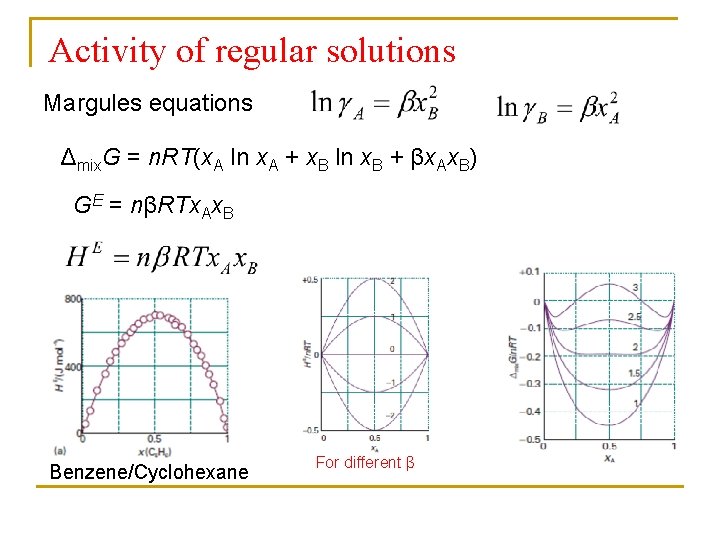
Activity of regular solutions Margules equations Δmix. G = n. RT(x. A ln x. A + x. B ln x. B + βx. Ax. B) GE = nβRTx. Ax. B Benzene/Cyclohexane For different β

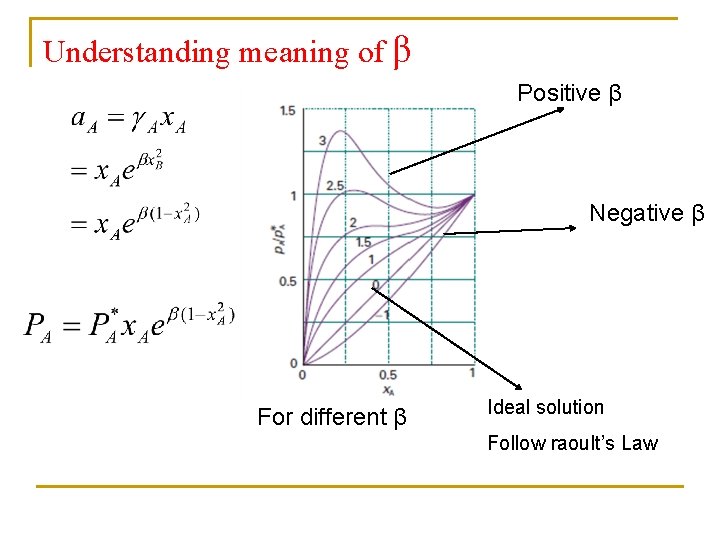
Understanding meaning of β Positive β Negative β For different β Ideal solution Follow raoult’s Law

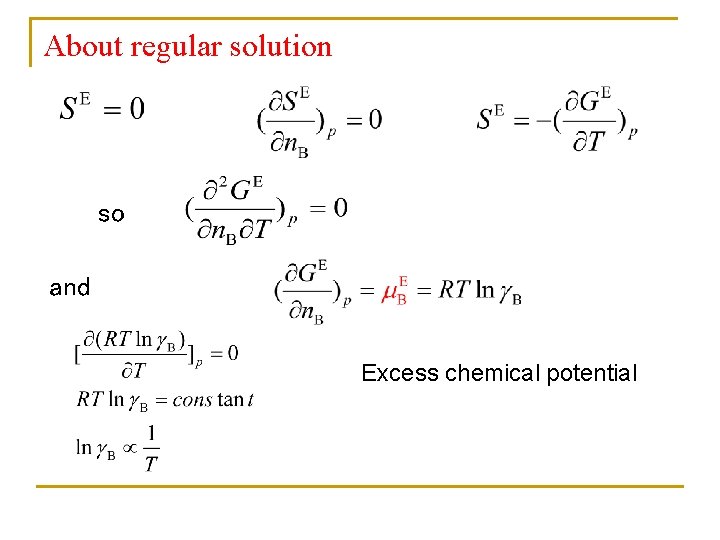
About regular solution so and Excess chemical potential

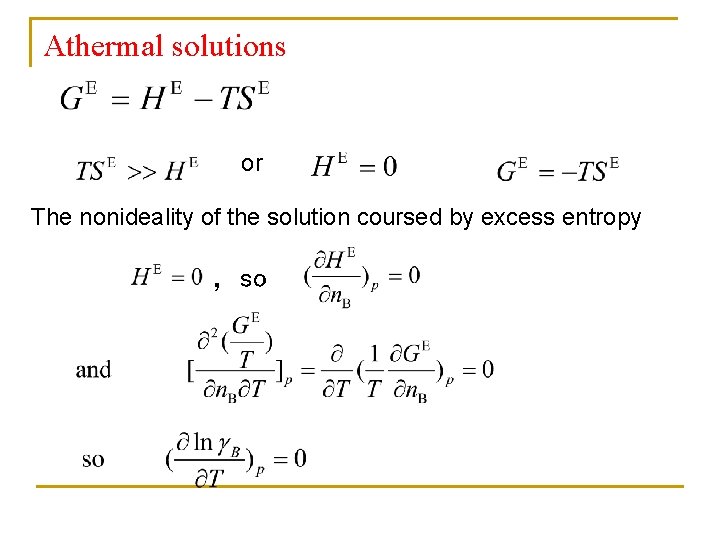
Athermal solutions or The nonideality of the solution coursed by excess entropy ,so

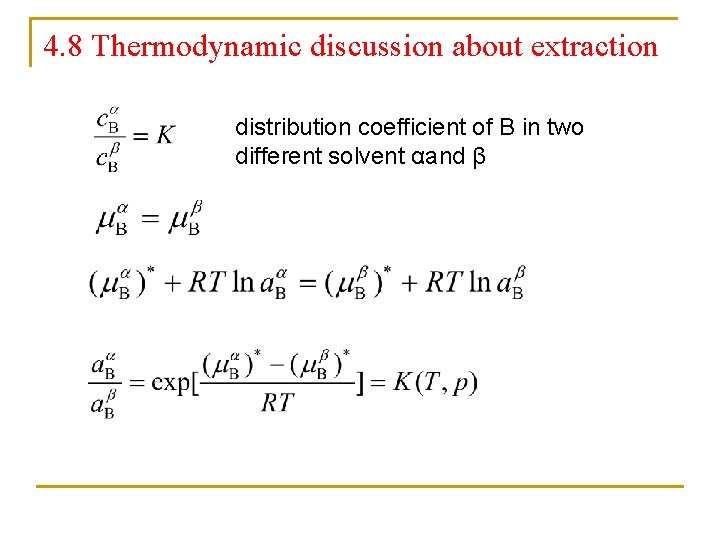
4. 8 Thermodynamic discussion about extraction distribution coefficient of B in two different solvent αand β

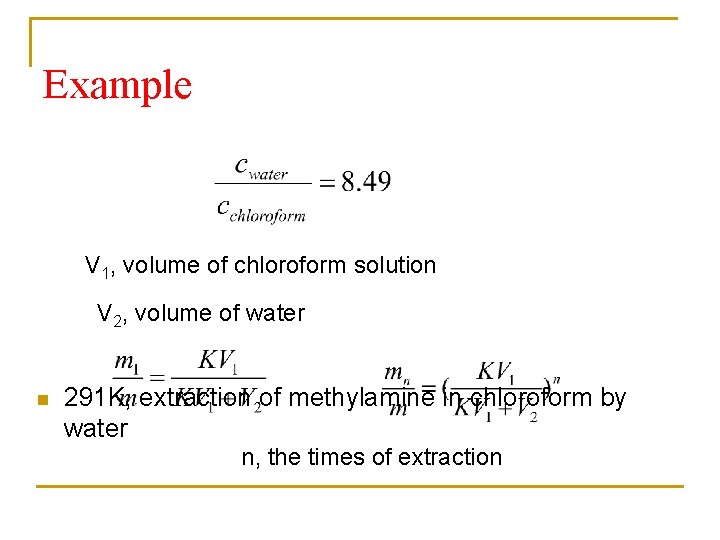
Example V 1, volume of chloroform solution V 2, volume of water n 291 K, extraction of methylamine in chloroform by water n, the times of extraction

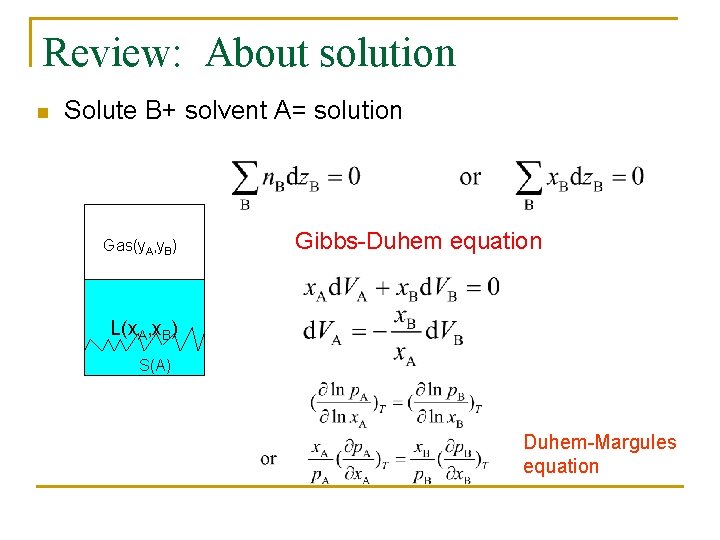
Review: About solution n Solute B+ solvent A= solution Gas(y. A, y. B) Gibbs-Duhem equation L(x. A, x. B) S(A) Duhem-Margules equation

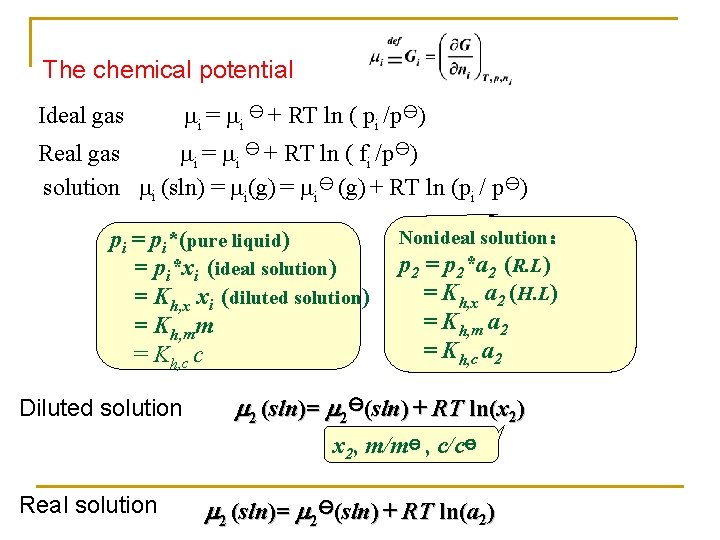
The chemical potential Ideal gas i = i y + RT ln ( pi /py) Real gas i = i y + RT ln ( fi /py) solution i (sln) = i(g) = iy (g) + RT ln (pi / py) pi = pi*(pure liquid) = pi*xi (ideal solution) = Kh, x xi (diluted solution) = Kh, mm = Kh, c c Diluted solution Nonideal solution: p 2 = p 2*a 2 (R. L) = Kh, x a 2 (H. L) = Kh, m a 2 = Kh, c a 2 2 (sln)= 2 y(sln) + RT ln(x 2) x 2, m/my , c/cy Real solution 2 (sln)= 2 y(sln) + RT ln(a 2)

Homework Discussion: n Group I: Thermodynamics in Dialysis technique Group II: Osmosis phenomenon in biological systems Group III: If the solvent of an diluted solution follow Raoult’s Law, the solute would follow the Henry’s Law. n