1 Concentration of Solute The amount of solute







- Slides: 13

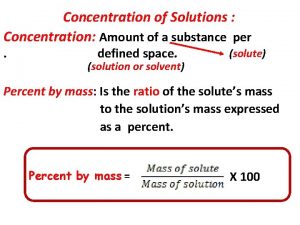
1 Concentration of Solute The amount of solute in a solution is given by its concentration. Molarity (M) = moles solute liters of solution

1. 0 L of water was used to make 1. 0 L of solution. Notice the water left over. 2

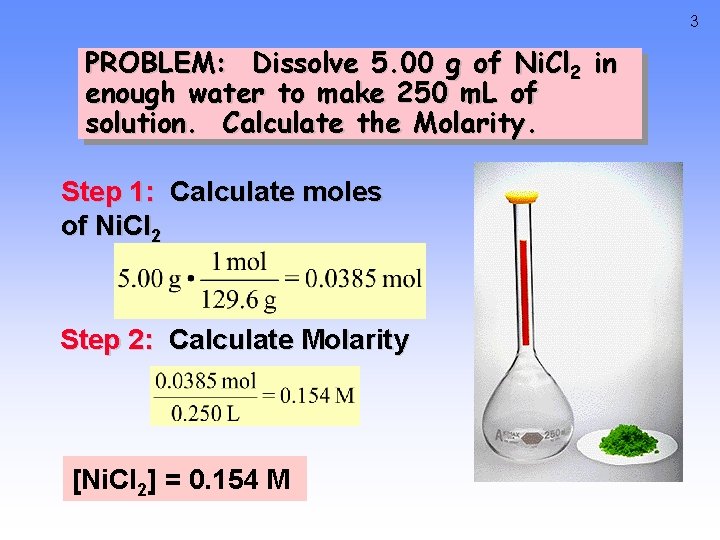
3 PROBLEM: Dissolve 5. 00 g of Ni. Cl 2 in enough water to make 250 m. L of solution. Calculate the Molarity. Step 1: Calculate moles of Ni. Cl 2 Step 2: Calculate Molarity [Ni. Cl 2] = 0. 154 M

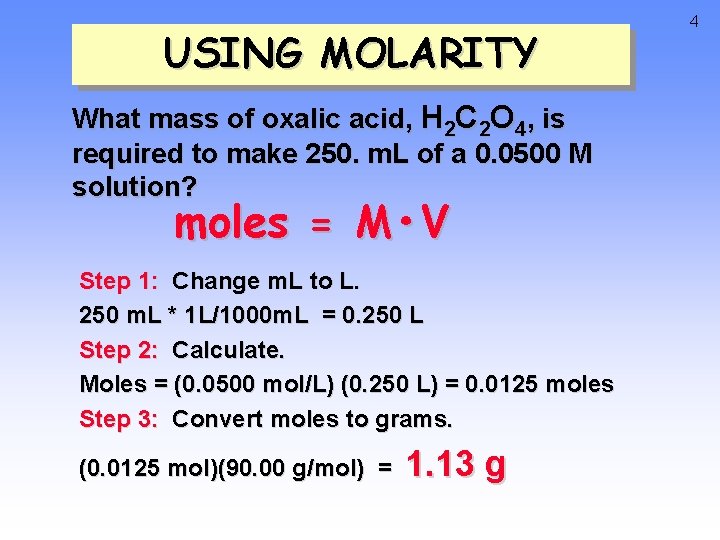
USING MOLARITY What mass of oxalic acid, H 2 C 2 O 4, is required to make 250. m. L of a 0. 0500 M solution? moles = M • V Step 1: Change m. L to L. 250 m. L * 1 L/1000 m. L = 0. 250 L Step 2: Calculate. Moles = (0. 0500 mol/L) (0. 250 L) = 0. 0125 moles Step 3: Convert moles to grams. (0. 0125 mol)(90. 00 g/mol) = 1. 13 g 4

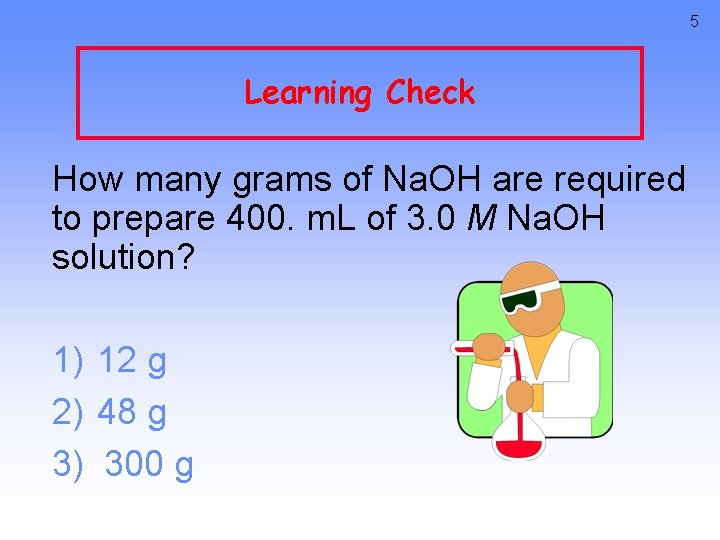
5 Learning Check How many grams of Na. OH are required to prepare 400. m. L of 3. 0 M Na. OH solution? 1) 12 g 2) 48 g 3) 300 g

Concentration Units An IDEAL SOLUTION is one where the properties depend only on the concentration of solute. Need conc. units to tell us the number of solute particles per solvent particle. The unit “molarity” does not do this! 6

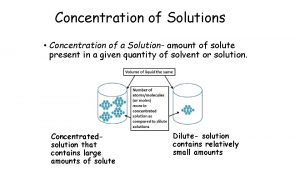
7 Preparing Solutions • Weigh out a solid solute and dissolve in a given quantity of solvent. • Dilute a concentrated solution to give one that is less concentrated.

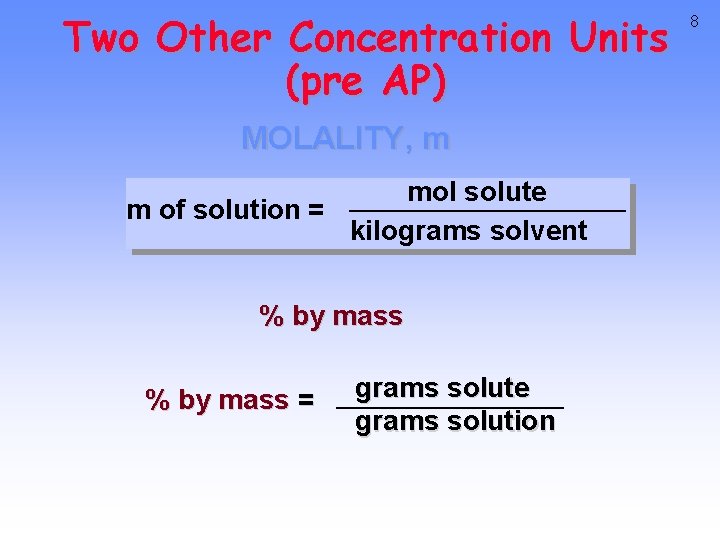
Two Other Concentration Units (pre AP) MOLALITY, m mol solute m of solution = kilograms solvent % by mass = grams solute grams solution 8

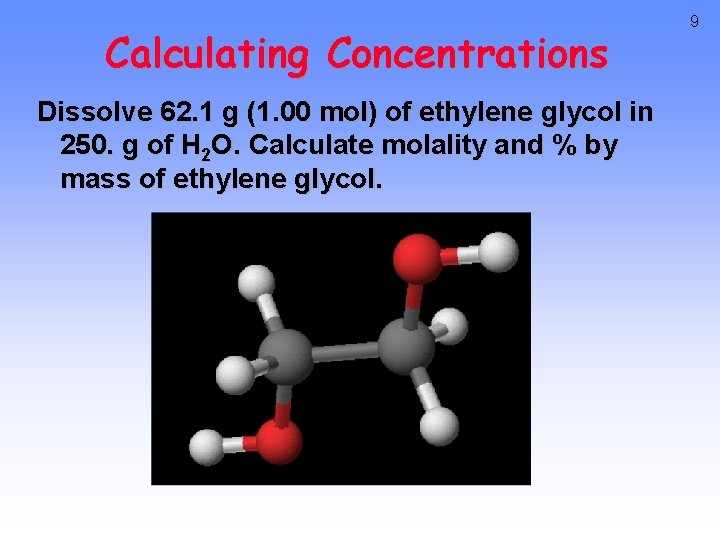
Calculating Concentrations Dissolve 62. 1 g (1. 00 mol) of ethylene glycol in 250. g of H 2 O. Calculate molality and % by mass of ethylene glycol. 9

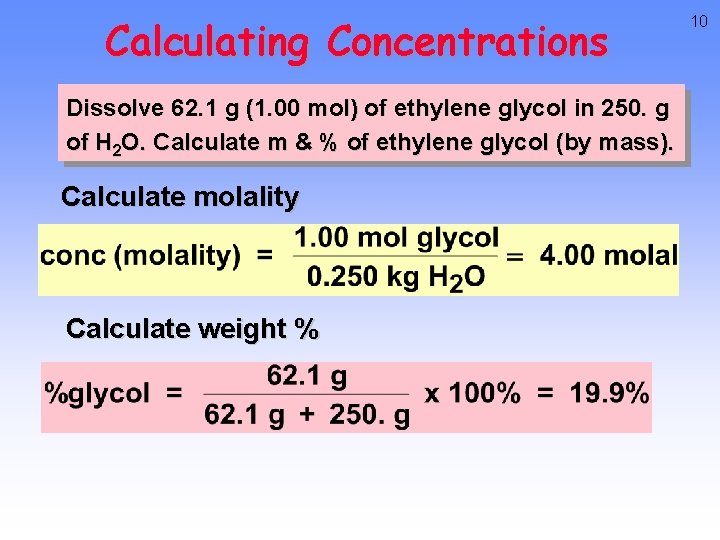
Calculating Concentrations Dissolve 62. 1 g (1. 00 mol) of ethylene glycol in 250. g of H 2 O. Calculate m & % of ethylene glycol (by mass). Calculate molality Calculate weight % 10

11 Learning Check A solution contains 15 g Na 2 CO 3 and 235 g of H 2 O? What is the mass % of the solution? 1) 15% Na 2 CO 3 2) 6. 4% Na 2 CO 3 3) 6. 0% Na 2 CO 3

12 Using mass % How many grams of Na. Cl are needed to prepare 250 g of a 10. 0% (by mass) Na. Cl solution?

13 Try this molality problem • 25. 0 g of Na. Cl is dissolved in 5000. m. L of water. Find the molality (m) of the resulting solution. m = mol solute / kg solvent 25 g Na. Cl 1 mol Na. Cl 58. 5 g Na. Cl = 0. 427 mol Na. Cl Since the density of water is 1 g/m. L, 5000 m. L = 5000 g, which is 5 kg 0. 427 mol Na. Cl 5 kg water = 0. 0854 m salt water
 A measure of the amount of solute dissolved in a solvent
A measure of the amount of solute dissolved in a solvent Estimate the solute concentration of the zucchini cells
Estimate the solute concentration of the zucchini cells Solute concentration
Solute concentration Movement of high concentration to low concentration
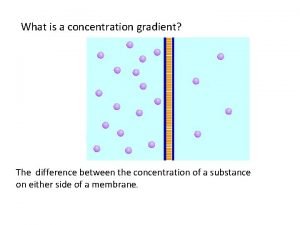
Movement of high concentration to low concentration Concentration gradient vs concentration difference
Concentration gradient vs concentration difference Cong thức tính động năng
Cong thức tính động năng Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Dot
Dot Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ Bổ thể
Bổ thể Phản ứng thế ankan
Phản ứng thế ankan Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan