1617 Semester 2 Physical Chemistry TKK2446 Instructor Rama

















- Slides: 48

16/17 Semester 2 Physical Chemistry (TKK-2446) Instructor: Rama Oktavian Email: Oktavian. rama 2@gmail. com Office Hr. : M. 10 – 12, T. 10 – 12, W. 09 – 10, F. 10 – 15

Outlines 1. Gas: Properties 2. Gas laws: Boyle and Charles law 3. Ideal gas law 4. Equation of state

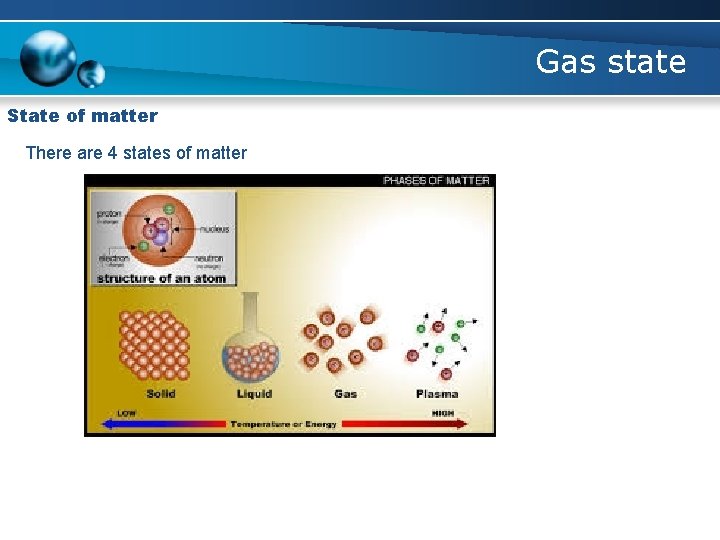
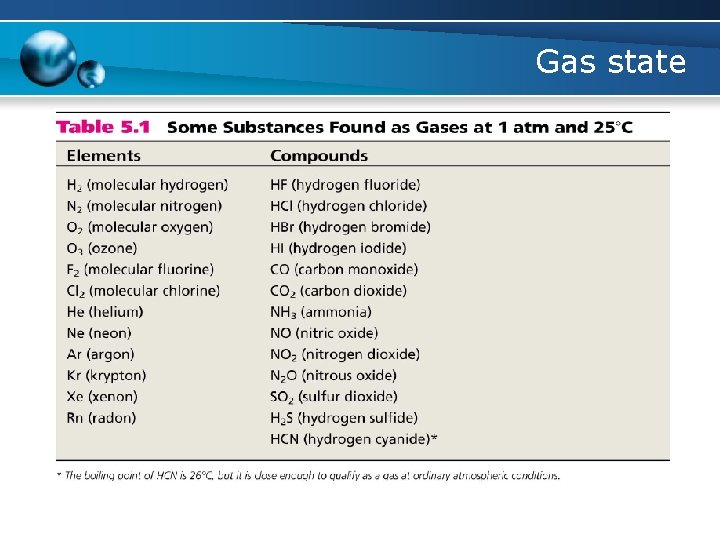
Gas state State of matter There are 4 states of matter

Gas state State of matter Properties of solid retains a fixed volume and shape rigid - particles locked into place not easily compressible little free space between particles Microscopic view of a solid does not flow easily rigid - particles cannot move/slide past one another

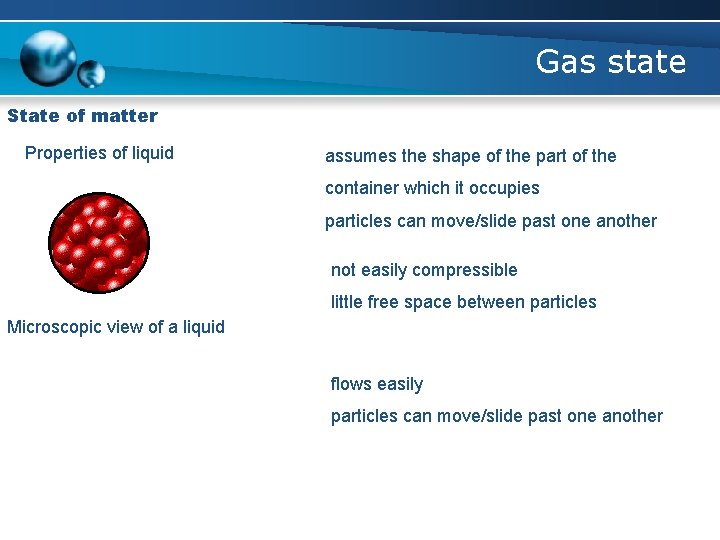
Gas state State of matter Properties of liquid assumes the shape of the part of the container which it occupies particles can move/slide past one another not easily compressible little free space between particles Microscopic view of a liquid flows easily particles can move/slide past one another

Gas state State of matter Properties of gas Microscopic properties assumes the shape and volume of its container particles can move past one another compressible lots of free space between particles Microscopic view of a gas flows easily particles can move past one another

Gas state State of matter Properties of gas Macroscopic properties Properties that can be observed and measured • Properties of bulk gases • Observable Microscopic view of a gas How to make relation between those macroscopic properties of gas? ? – Pressure, volume, mass, temperature. . The general form of an equation of state is p=f(T, V, n)

Gas state

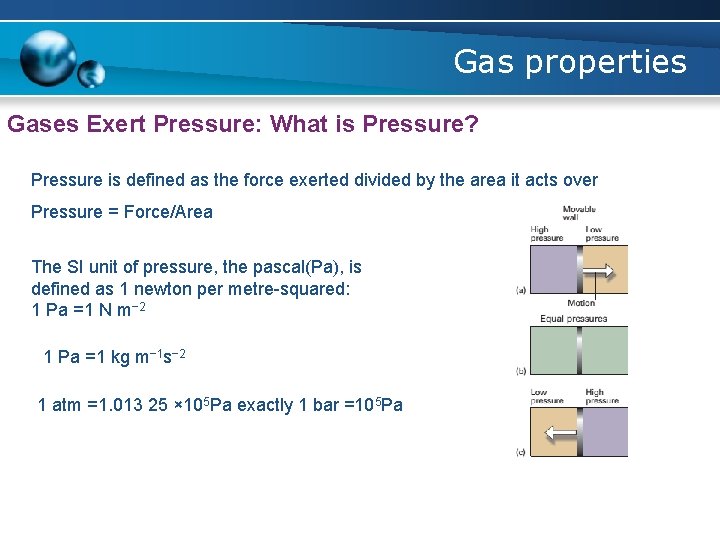
Gas properties Gases Exert Pressure: What is Pressure? Pressure is defined as the force exerted divided by the area it acts over Pressure = Force/Area The SI unit of pressure, the pascal(Pa), is defined as 1 newton per metre-squared: 1 Pa =1 N m− 2 1 Pa =1 kg m− 1 s− 2 1 atm =1. 013 25 × 105 Pa exactly 1 bar =105 Pa

Gas properties Gases Exert Pressure: What is Pressure? Pressure is defined as the force exerted divided by the area it acts over Pressure = Force/Area Self-test 1. 1 Calculate the pressure (in pascals and atmospheres) exerted by a mass of 1. 0 kg pressing through the point of a pin of area 1. 0 × 10− 2 mm 2 at the surface of the Earth.

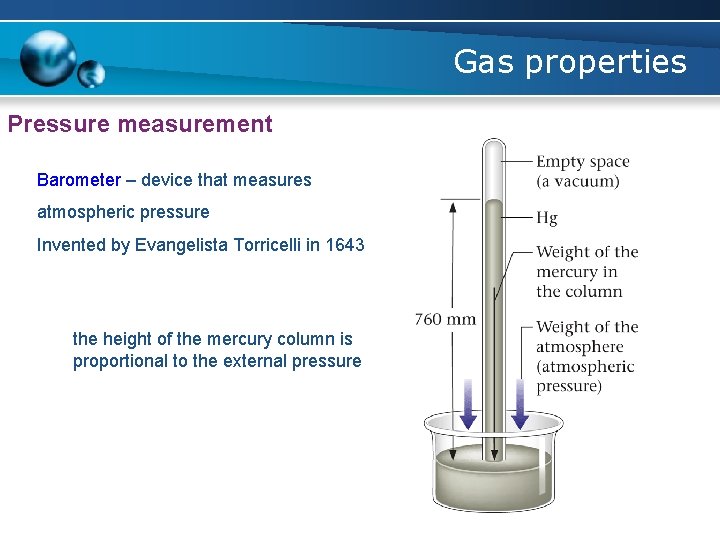
Gas properties Pressure measurement Barometer – device that measures atmospheric pressure Invented by Evangelista Torricelli in 1643 the height of the mercury column is proportional to the external pressure

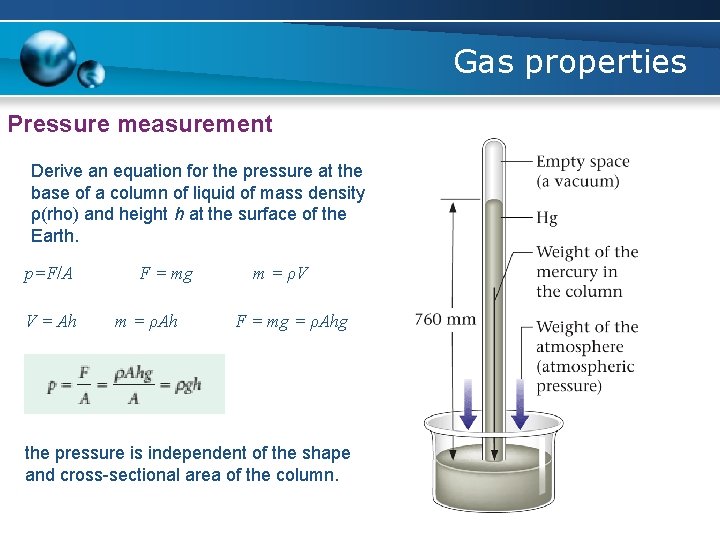
Gas properties Pressure measurement Derive an equation for the pressure at the base of a column of liquid of mass density ρ(rho) and height h at the surface of the Earth. p=F/A V = Ah F = mg m = ρAh m = ρV F = mg = ρAhg the pressure is independent of the shape and cross-sectional area of the column.

Gas properties Pressure measurement

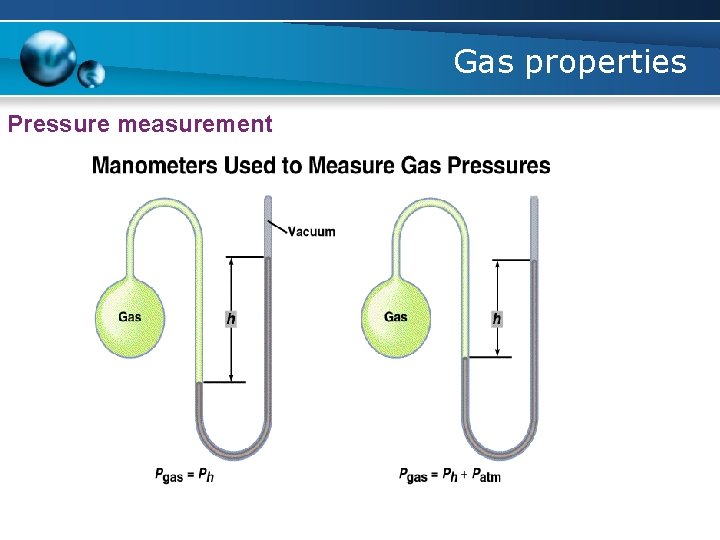
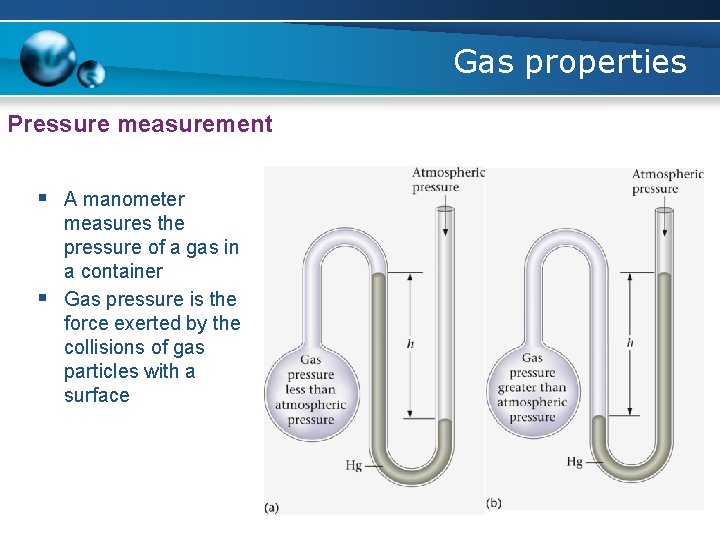
Gas properties Pressure measurement § A manometer § measures the pressure of a gas in a container Gas pressure is the force exerted by the collisions of gas particles with a surface

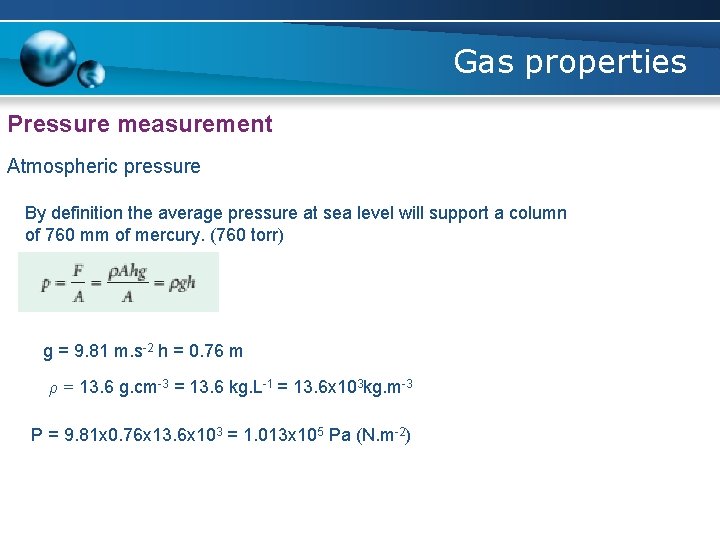
Gas properties Pressure measurement Atmospheric pressure By definition the average pressure at sea level will support a column of 760 mm of mercury. (760 torr) g = 9. 81 m. s-2 h = 0. 76 m ρ = 13. 6 g. cm-3 = 13. 6 kg. L-1 = 13. 6 x 103 kg. m-3 P = 9. 81 x 0. 76 x 13. 6 x 103 = 1. 013 x 105 Pa (N. m-2)

Gas properties Pressure measurement Atmospheric pressure problem If we made a barometer out of water, what would be the height of the water column if the pressure is 745 torr?

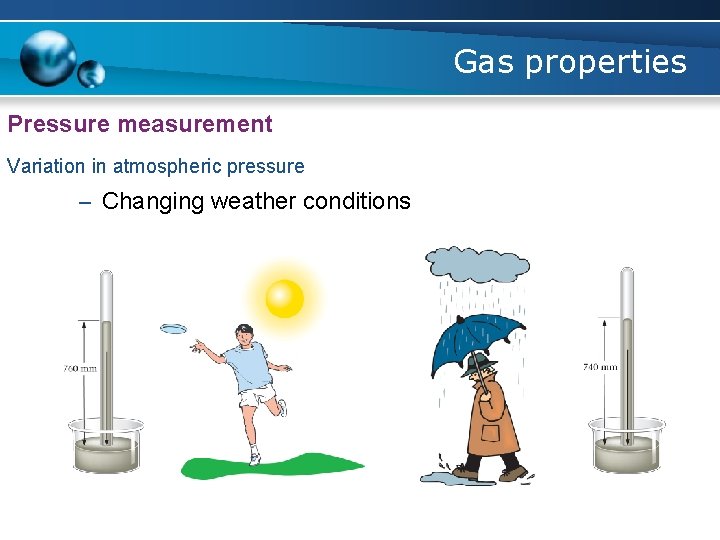
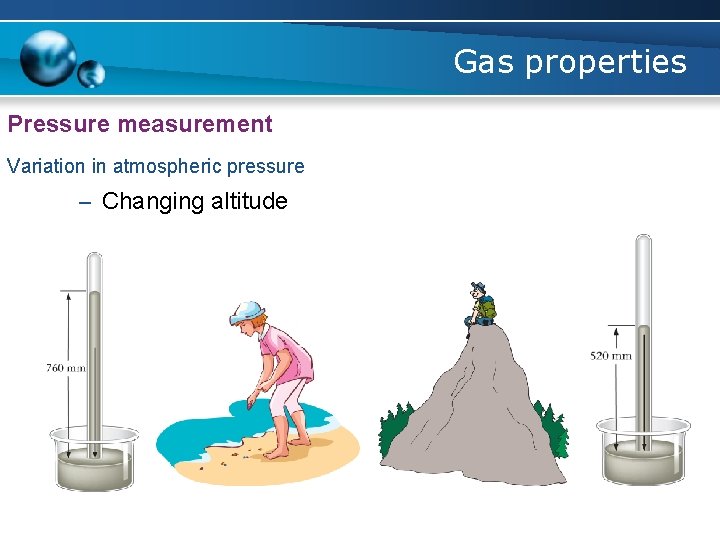
Gas properties Pressure measurement Variation in atmospheric pressure – Changing weather conditions

Gas properties Pressure measurement Variation in atmospheric pressure – Changing altitude

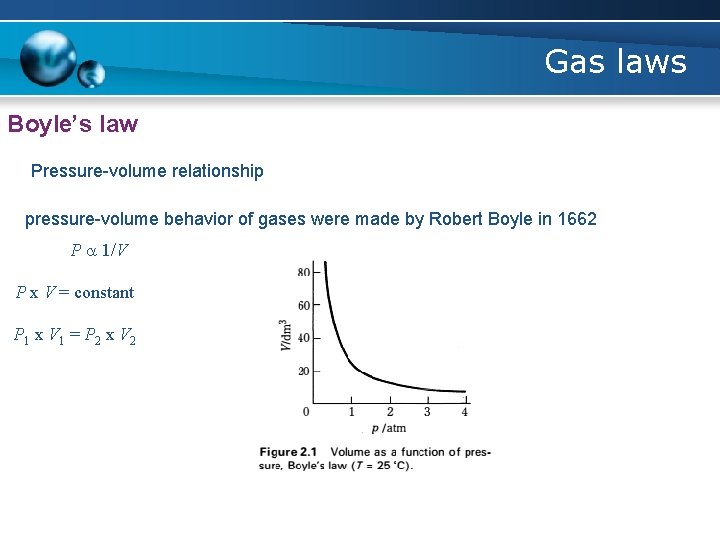
Gas laws Boyle’s law • Boyle’s Law is one of the laws in physics that concern the behaviour of gases • When a gas is under pressure it takes up less space: • The higher the pressure, the smaller the volume • Boyles Law tells us about the relationship between the volume of a gas and its pressure at a constant temperature • The law states that pressure is inversely proportional to the volume

Gas laws Boyle’s law Pressure-volume relationship pressure-volume behavior of gases were made by Robert Boyle in 1662 P a 1/V P x V = constant P 1 x V 1 = P 2 x V 2

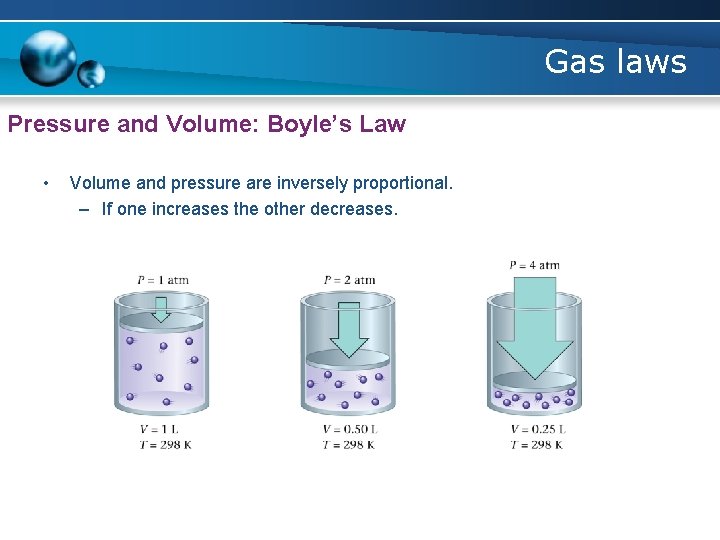
Gas laws Pressure and Volume: Boyle’s Law • Volume and pressure are inversely proportional. – If one increases the other decreases.

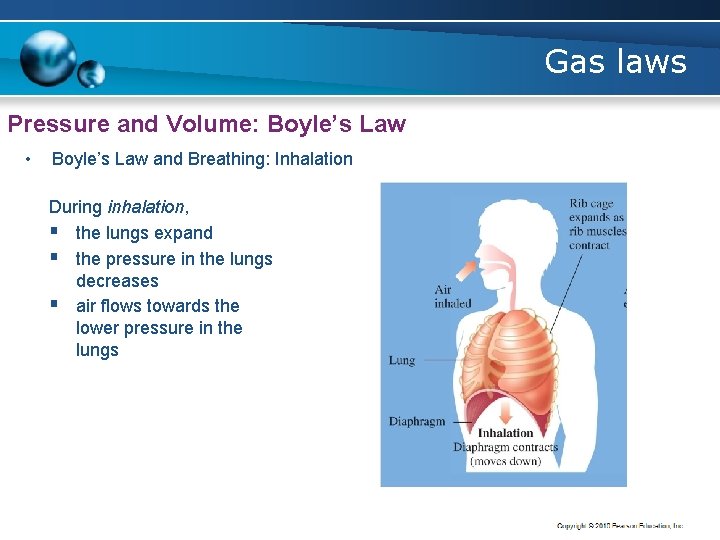
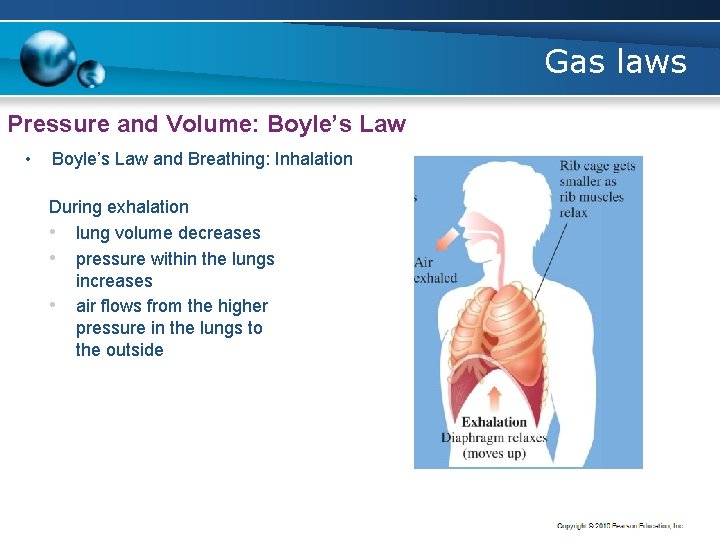
Gas laws Pressure and Volume: Boyle’s Law • Boyle’s Law and Breathing: Inhalation During inhalation, § the lungs expand § the pressure in the lungs decreases § air flows towards the lower pressure in the lungs

Gas laws Pressure and Volume: Boyle’s Law • Boyle’s Law and Breathing: Inhalation During exhalation • lung volume decreases • pressure within the lungs increases • air flows from the higher pressure in the lungs to the outside

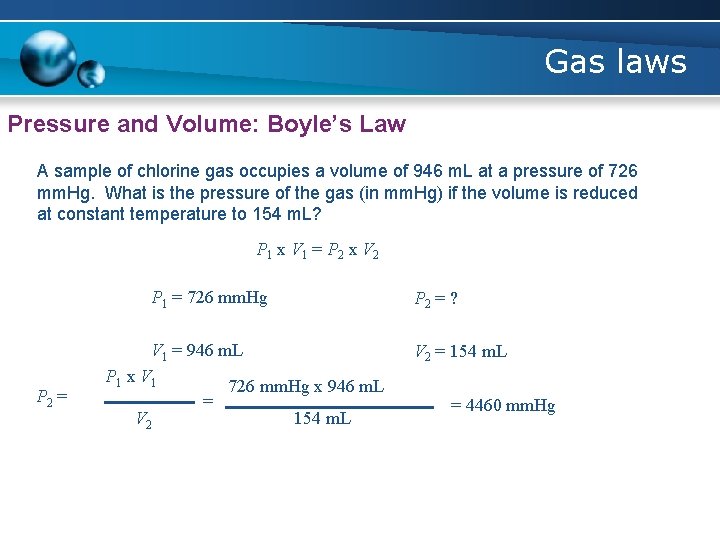
Gas laws Pressure and Volume: Boyle’s Law A sample of chlorine gas occupies a volume of 946 m. L at a pressure of 726 mm. Hg. What is the pressure of the gas (in mm. Hg) if the volume is reduced at constant temperature to 154 m. L? P 1 x V 1 = P 2 x V 2 P 1 = 726 mm. Hg P 2 = V 1 = 946 m. L P 1 x V 1 726 mm. Hg x 946 m. L = V 2 154 m. L P 2 = ? V 2 = 154 m. L = 4460 mm. Hg

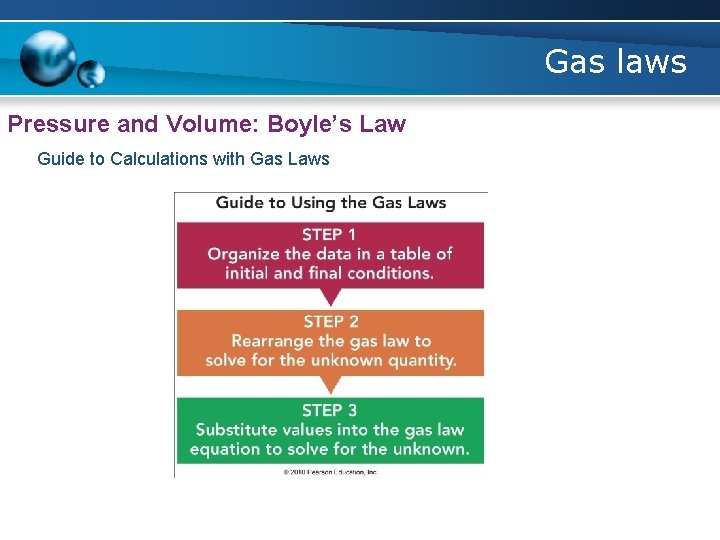
Gas laws Pressure and Volume: Boyle’s Law Guide to Calculations with Gas Laws

Gas laws Pressure and Volume: Boyle’s Law Problem A deep sea diver is working at a depth where the pressure is 3. 0 atmospheres. He is breathing out air bubbles. The volume of each air bubble is 2 cm 2. At the surface the pressure is 1 atmosphere. What is the volume of each bubble when it reaches the surface?

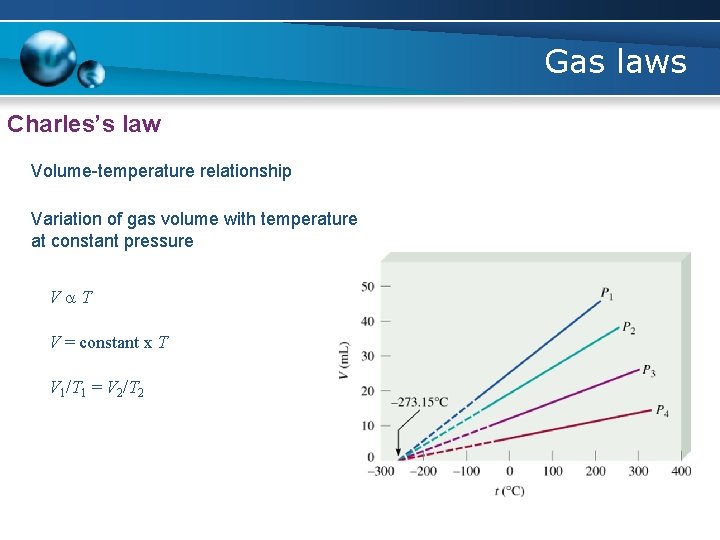
Gas laws Charles’s law • French chemist Jacques Charles discovered that the volume of a gas at constant pressure changes with temperature. • As the temperature of the gas increases, so does its volume, and as its temperature decreases, so does its volume. • The law says that at constant pressure, the volume of a fixed number of particles of gas is directly proportional to the absolute (Kelvin) temperature

Gas laws Charles’s law Volume-temperature relationship Variation of gas volume with temperature at constant pressure Va. T V = constant x T V 1/T 1 = V 2/T 2

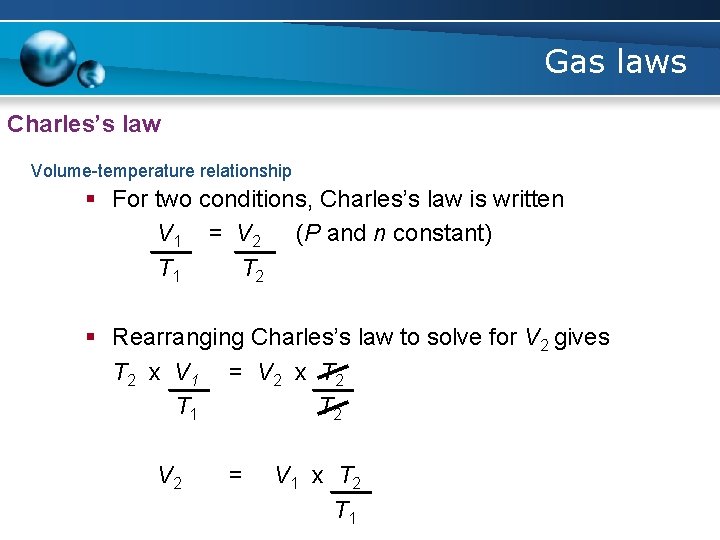
Gas laws Charles’s law Volume-temperature relationship § For two conditions, Charles’s law is written V 1 = V 2 (P and n constant) T 1 T 2 § Rearranging Charles’s law to solve for V 2 gives T 2 x V 1 = V 2 x T 2 T 1 T 2 V 2 = V 1 x T 2 T 1

Gas laws Charles’s law Example problem A balloon has a volume of 785 m. L at 21 °C. If the temperature drops to 0 °C, what is the new volume of the balloon (P constant)? STEP 1 Set up data table: Conditions 1 Conditions 2 V 1 = 785 m. L T 1 = 21 °C = 294 K V 2 = ? T 2 = 0 °C = 273 K Know Predict V decreases T decreases Be sure to use the Kelvin (K) temperature in gas calculations.

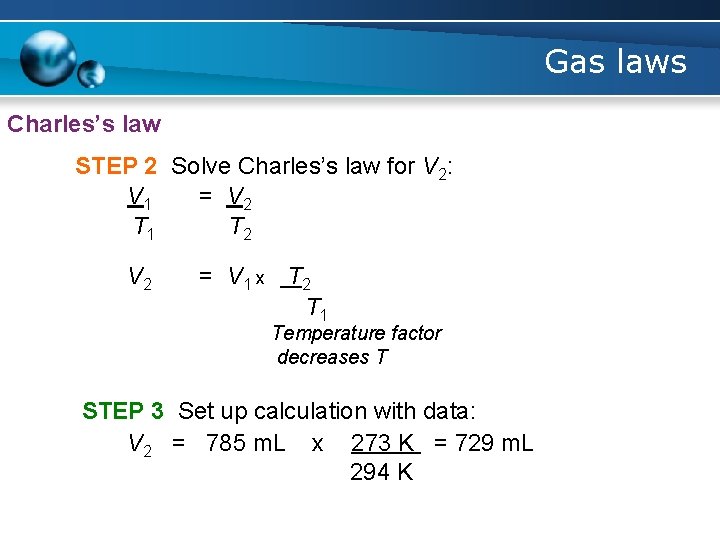
Gas laws Charles’s law STEP 2 Solve Charles’s law for V 2: V 1 = V 2 T 1 T 2 V 2 = V 1 x T 2 T 1 Temperature factor decreases T STEP 3 Set up calculation with data: V 2 = 785 m. L x 273 K = 729 m. L 294 K

Gas laws Charles’s law A sample of oxygen gas has a volume of 420 m. L at a temperature of 18 °C. At what temperature (in °C) will the volume of the oxygen be 640 m. L (P and n constant)? 1) 443 °C 2) 170 °C 3) – 82 °C

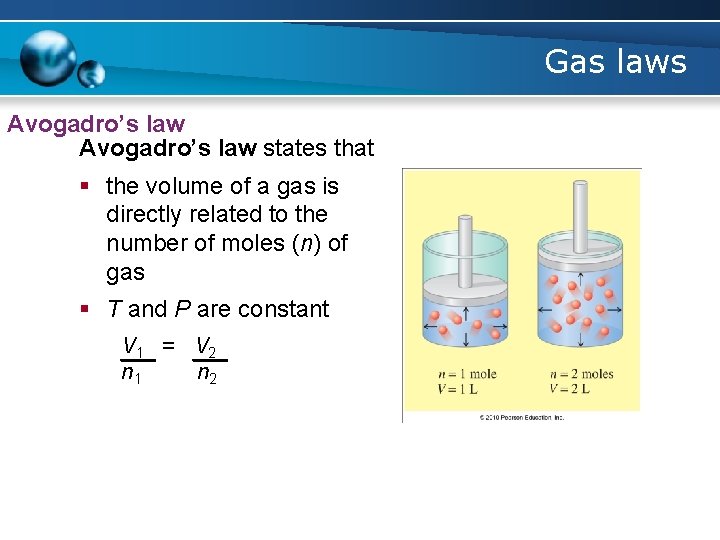
Gas laws Avogadro’s law states that § the volume of a gas is directly related to the number of moles (n) of gas § T and P are constant V 1 = V 2 n 1 n 2

Ideal Gas law The combination of those laws gives Usually written as: R is gas constant

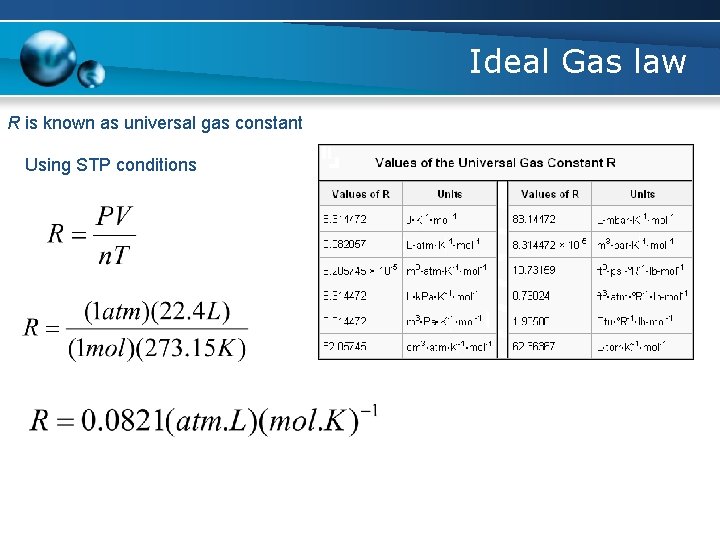
Ideal Gas law R is known as universal gas constant Using STP conditions

Ideal gas and Real gas Ideal gas The ideal gas law is used to describe the behavior of an ideal gas. Ideal gas: hypothetical gas that obeys kinetic molecular theory and the ideal gas law

Ideal gas and Real gas Ideal gas The ideal gas law was useful in determining the properties of a specific sample of gas at constant T, P, V, and n. We often need to know how a change in one (or more) properties impacts the other properties for a sample of a gas

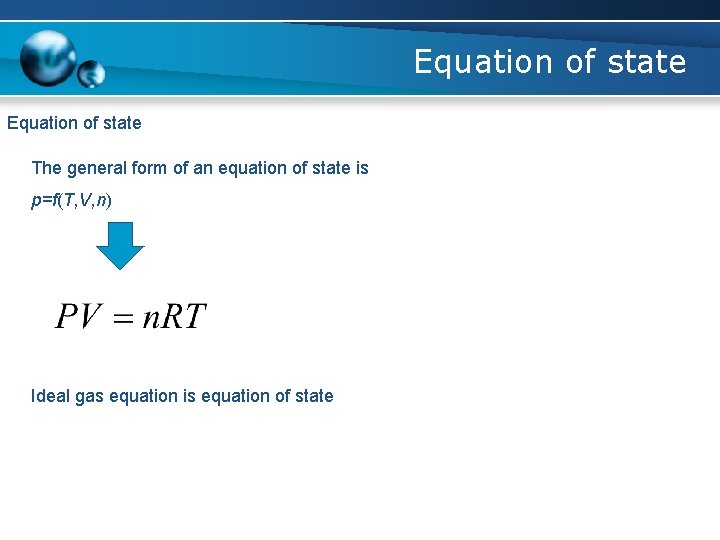
Equation of state The general form of an equation of state is p=f(T, V, n) Ideal gas equation is equation of state

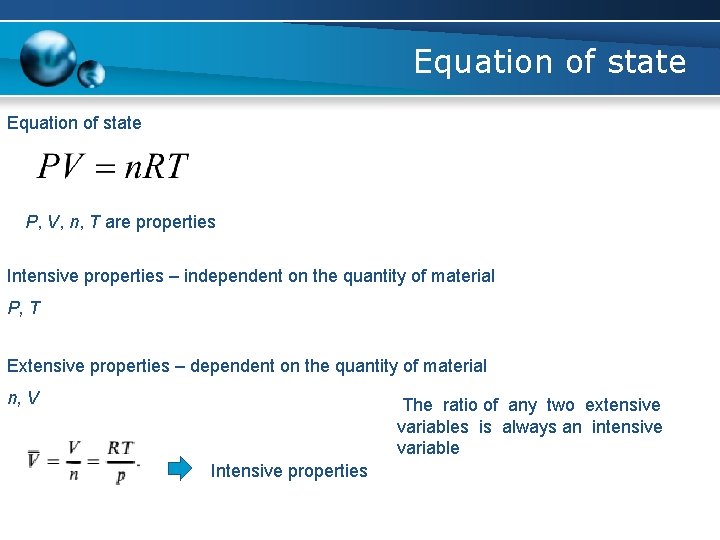
Equation of state P, V, n, T are properties Intensive properties – independent on the quantity of material P, T Extensive properties – dependent on the quantity of material n, V The ratio of any two extensive variables is always an intensive variable Intensive properties

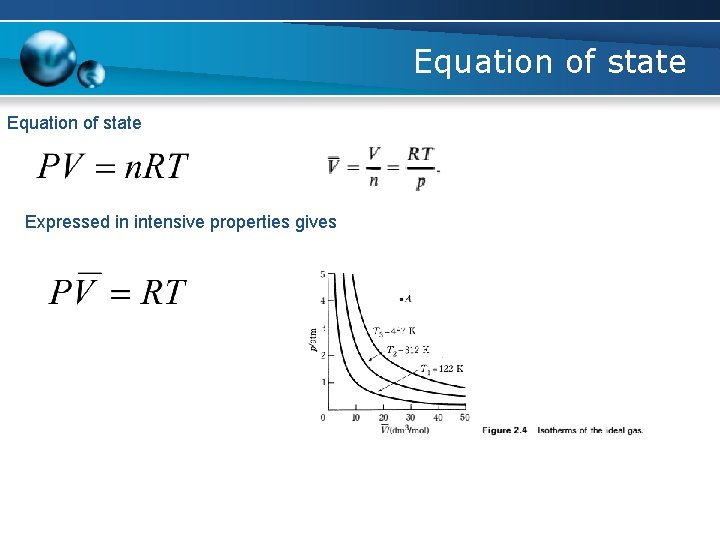
Equation of state Expressed in intensive properties gives

Ideal gas and Real gas deviations from the perfect gas law because molecules interact with one another Repulsive forces are significant only when molecules are almost in contact Attractive intermolecular forces have a relatively long range and are effective over several molecular diameters

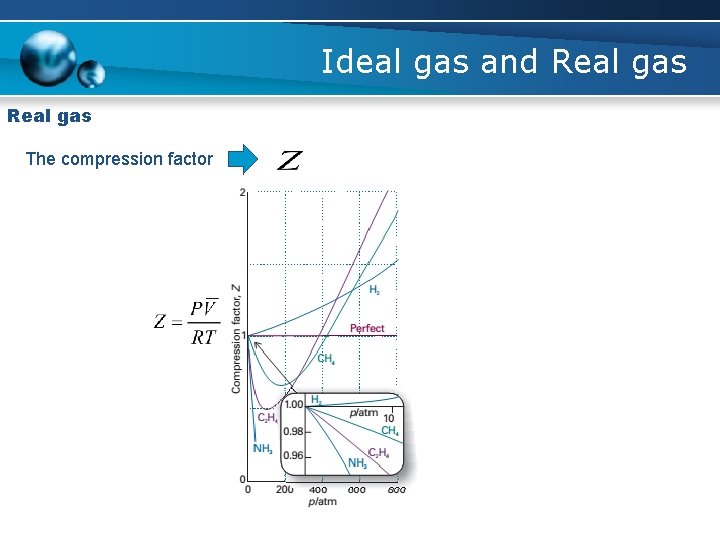
Ideal gas and Real gas The compression factor For ideal gas P moderate Z < 1

Ideal gas and Real gas The compression factor

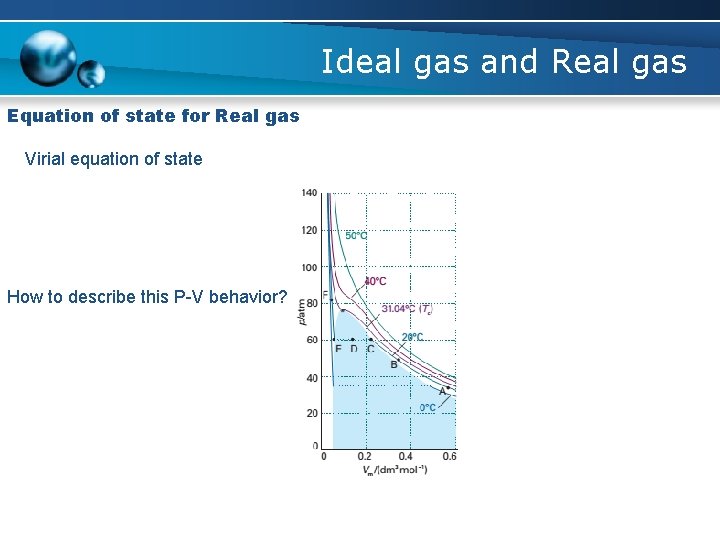
Ideal gas and Real gas Equation of state for Real gas Virial equation of state How to describe this P-V behavior?

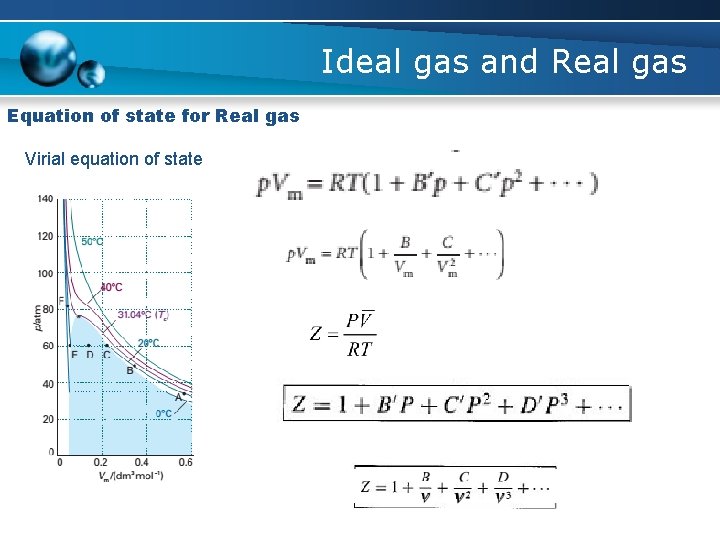
Ideal gas and Real gas Equation of state for Real gas Virial equation of state

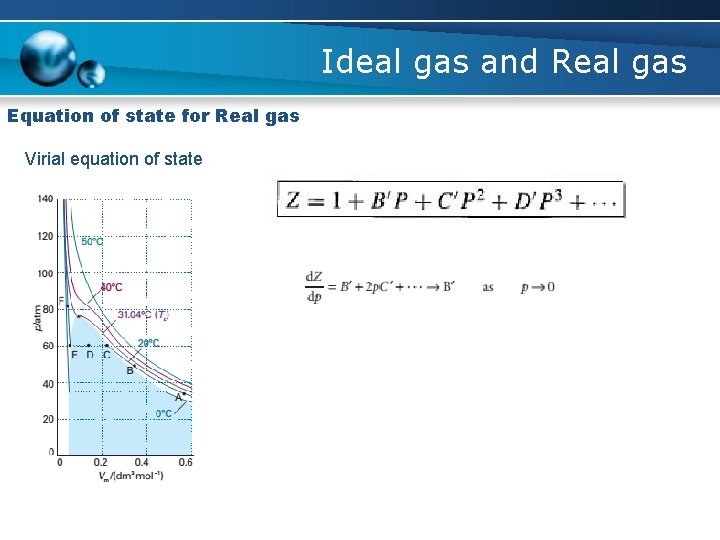
Ideal gas and Real gas Equation of state for Real gas Virial equation of state

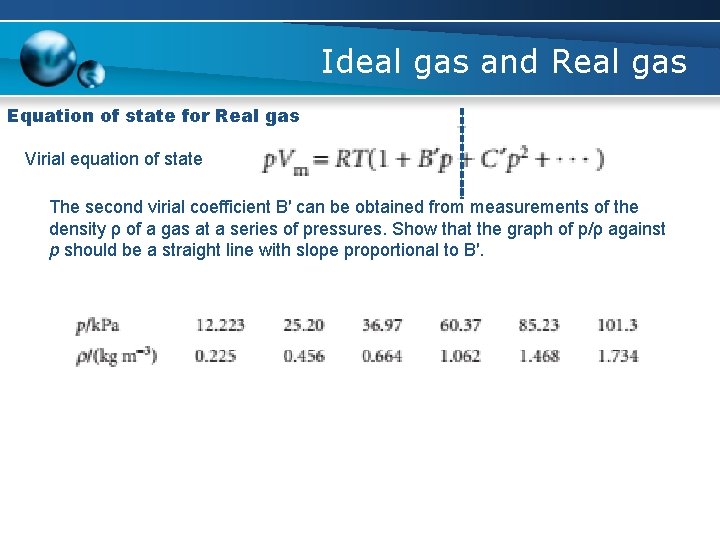
Ideal gas and Real gas Equation of state for Real gas Virial equation of state The second virial coefficient B′ can be obtained from measurements of the density ρ of a gas at a series of pressures. Show that the graph of p/ρ against p should be a straight line with slope proportional to B′.

 Ceritakan kitar hidup rama rama
Ceritakan kitar hidup rama rama Sri rama sri rama sri manoharama
Sri rama sri rama sri manoharama Chemistry semester 2 review unit 12 thermochemistry
Chemistry semester 2 review unit 12 thermochemistry Chemistry semester 1 exam study guide answers
Chemistry semester 1 exam study guide answers Chemistry fall semester exam review answers
Chemistry fall semester exam review answers If a laboratory fire erupts, immediately
If a laboratory fire erupts, immediately Chemistry semester exam review
Chemistry semester exam review Ib organic chemistry functional groups
Ib organic chemistry functional groups Organic vs inorganic chemistry
Organic vs inorganic chemistry Tipología de los participantes
Tipología de los participantes Basic instructor course tcole
Basic instructor course tcole Basic instructor course texas
Basic instructor course texas Basic instructor course texas
Basic instructor course texas Pepperball launcher nomenclature
Pepperball launcher nomenclature Not only the students but also the instructor
Not only the students but also the instructor Instructor vs teacher
Instructor vs teacher Cisco certified instructor
Cisco certified instructor Mptc firearms instructor manual
Mptc firearms instructor manual Basic instructor course #1014
Basic instructor course #1014 Basic instructor course #1014
Basic instructor course #1014 The virtual instructor
The virtual instructor Nfpa 1403
Nfpa 1403 Tp 12863
Tp 12863 Instructor operating station
Instructor operating station Catia instructor
Catia instructor Instructor
Instructor Instructor responsibilities and professionalism lesson plan
Instructor responsibilities and professionalism lesson plan Tcole 1014 basic instructor course
Tcole 1014 basic instructor course Usmc jrotc vacancies
Usmc jrotc vacancies Nrp instructor toolkit
Nrp instructor toolkit Cisco instructor certification
Cisco instructor certification Cbrf wisconsin registry
Cbrf wisconsin registry Nra certified instructor logo
Nra certified instructor logo Naismith was an instructor of
Naismith was an instructor of If your instructor were to ask if you cleaned up your room
If your instructor were to ask if you cleaned up your room Tcole advanced instructor course
Tcole advanced instructor course Tcole advanced instructor course
Tcole advanced instructor course Jrotc marksmanship instructor course online
Jrotc marksmanship instructor course online Which line is longer illusion
Which line is longer illusion Medical terminology instructor
Medical terminology instructor Basic instructor course #1014
Basic instructor course #1014 Basic instructor course tcole
Basic instructor course tcole Delmar cengage learning instructor resources
Delmar cengage learning instructor resources Instructor office hours
Instructor office hours Tmpi de rama secundaria
Tmpi de rama secundaria Rama ejecutiva orden municipal
Rama ejecutiva orden municipal Lant trofic om
Lant trofic om Cpe listado interinatos y suplencias 2021
Cpe listado interinatos y suplencias 2021 Despolarizacion
Despolarizacion