1 Exploring Chemical Analysis Daniel C Harris Fourth


















- Slides: 65

1

Exploring Chemical Analysis Daniel C. Harris (Fourth Edition) 11 Polyprotic Acids and Bases 國防醫學院 生化學科 王明芳老師 2012 -3 -29 2

Erosion of carbonate stone 3

Outline l Amino acids are polyprotic l Finding the p. H in Diprotic systems l Which is the principal species? l Titration in polyprotic systems 4

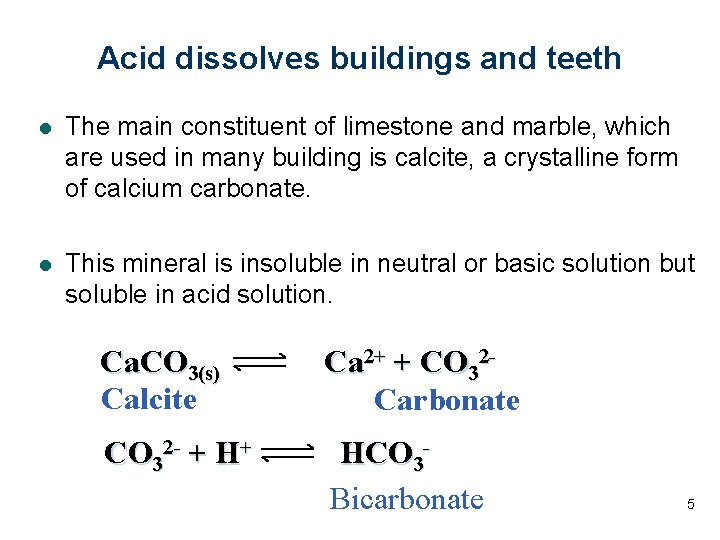
Acid dissolves buildings and teeth l The main constituent of limestone and marble, which are used in many building is calcite, a crystalline form of calcium carbonate. l This mineral is insoluble in neutral or basic solution but soluble in acid solution. Ca. CO 3(s) Calcite Ca 2+ + CO 32 Carbonate CO 32 - + H+ HCO 3 Bicarbonate 5

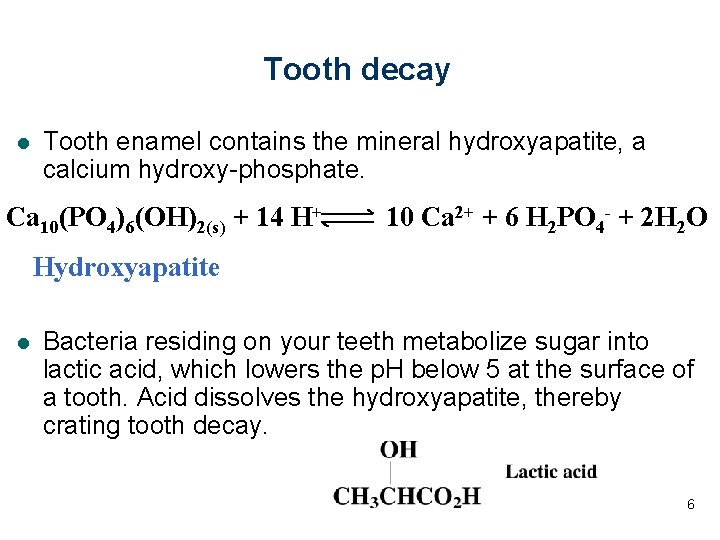
Tooth decay l Tooth enamel contains the mineral hydroxyapatite, a calcium hydroxy-phosphate. Ca 10(PO 4)6(OH)2(s) + 14 H+ 10 Ca 2+ + 6 H 2 PO 4 - + 2 H 2 O Hydroxyapatite l Bacteria residing on your teeth metabolize sugar into lactic acid, which lowers the p. H below 5 at the surface of a tooth. Acid dissolves the hydroxyapatite, thereby crating tooth decay. 6

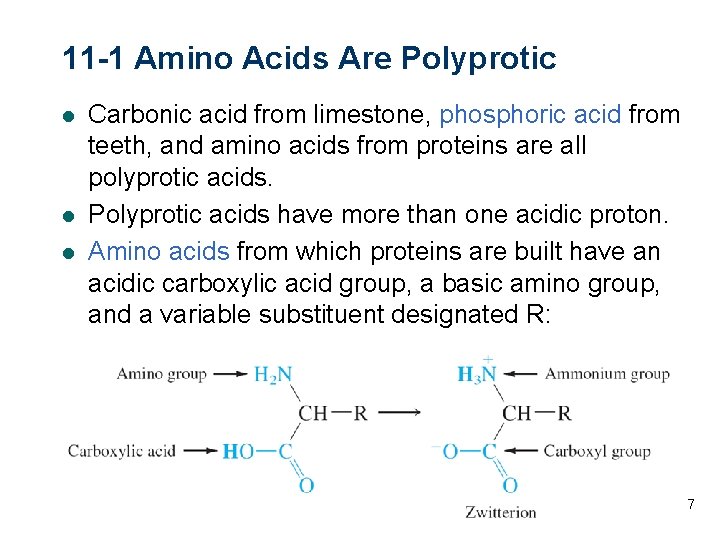
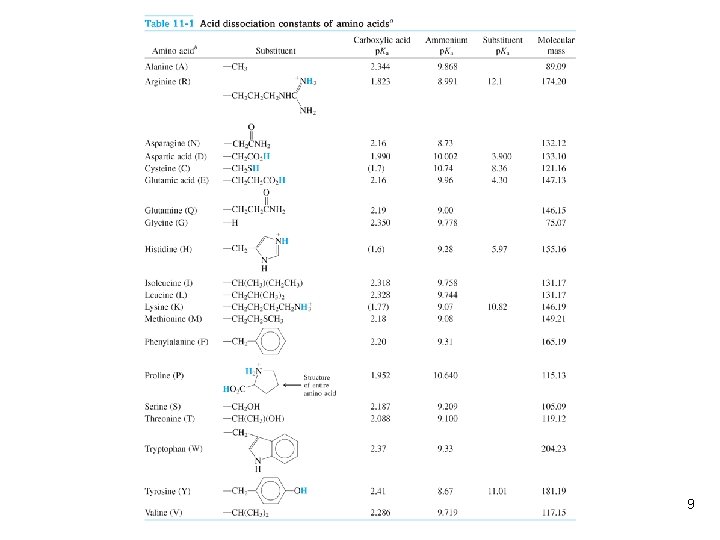
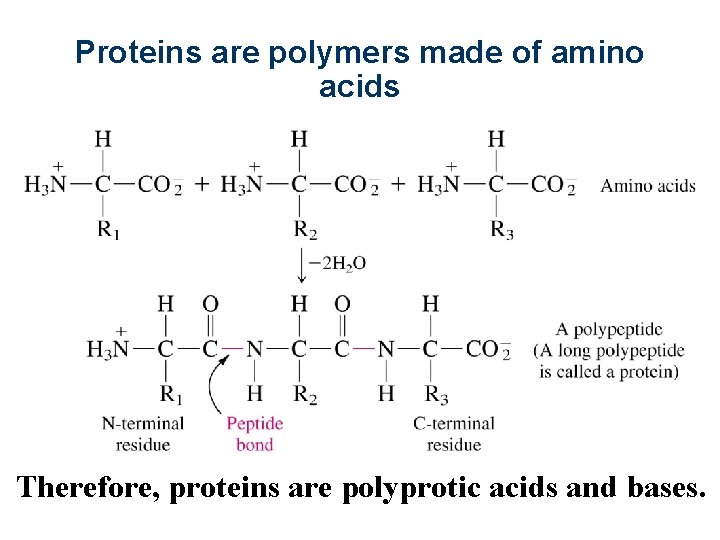
11 -1 Amino Acids Are Polyprotic l l l Carbonic acid from limestone, phosphoric acid from teeth, and amino acids from proteins are all polyprotic acids. Polyprotic acids have more than one acidic proton. Amino acids from which proteins are built have an acidic carboxylic acid group, a basic amino group, and a variable substituent designated R: 7

l Amino acid is a kind of inner salt. l The resulting structure, with positive and negative sites, is called a zwitterion. l A acid dissociation constants of the 20 common amino acids are given in Table 11 -1. 8

Table 11 -1 9

1. Nonpolar side chain: alanine, glycine, leucine, isoleucine, valine, proline, methionine, and phenylalanine. 2. Polar but uncharged side chain: serine, glutamine, threonine, cysteine, asparagine, tyrosine, and tryptophan. 3. Polar charged side chain: aspartic acid, glutamic acid, lysine, arginine, and histidine. 10

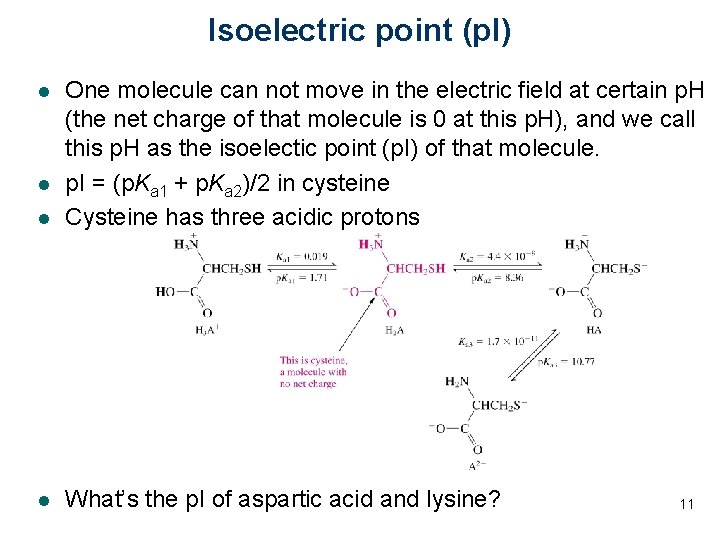
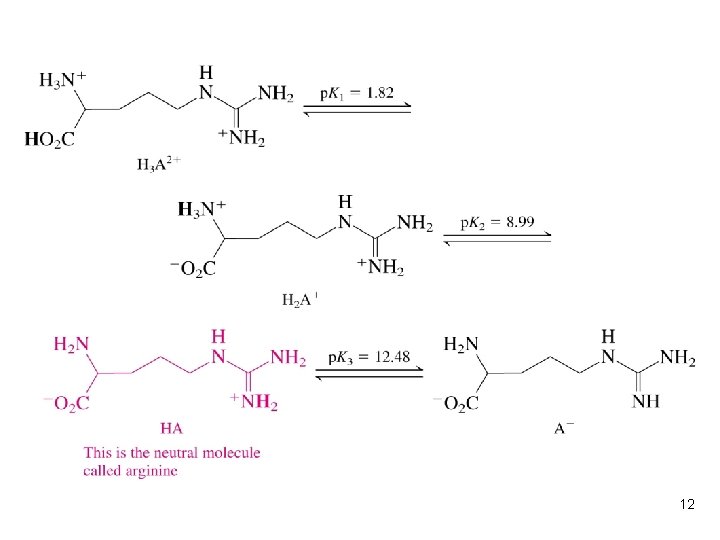
Isoelectric point (p. I) l One molecule can not move in the electric field at certain p. H (the net charge of that molecule is 0 at this p. H), and we call this p. H as the isoelectic point (p. I) of that molecule. p. I = (p. Ka 1 + p. Ka 2)/2 in cysteine Cysteine has three acidic protons l What’s the p. I of aspartic acid and lysine? l l 11

12

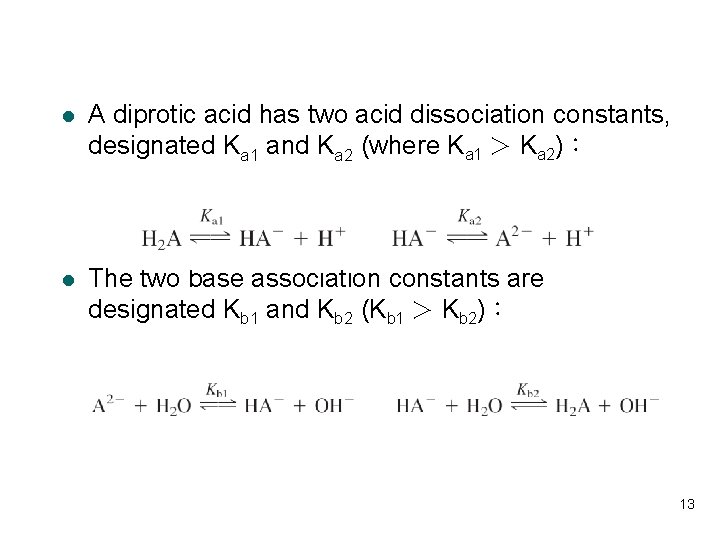
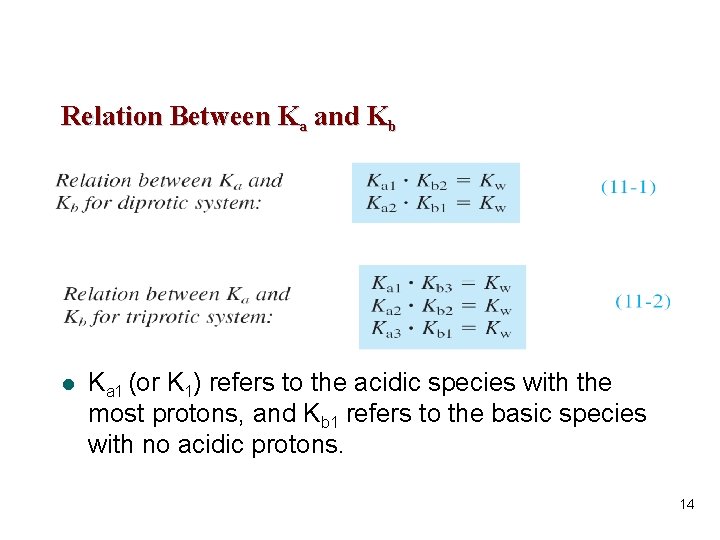
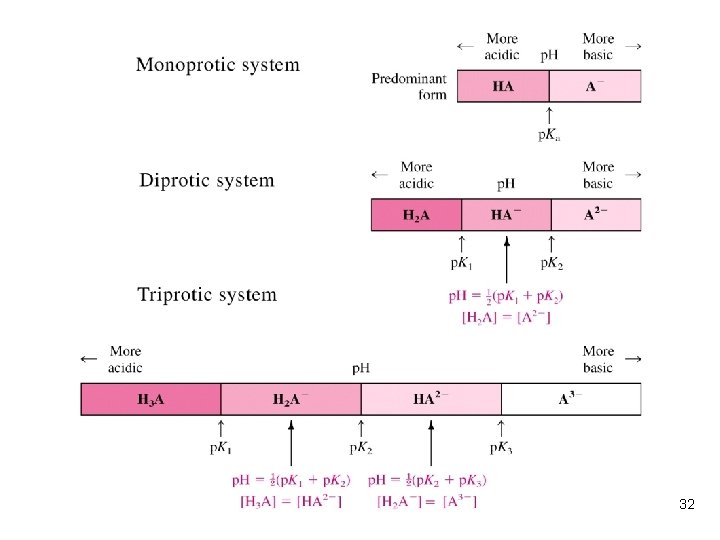
l A diprotic acid has two acid dissociation constants, designated Ka 1 and Ka 2 (where Ka 1 > Ka 2): l The two base association constants are designated Kb 1 and Kb 2 (Kb 1 > Kb 2): 13

Relation Between Ka and Kb l Ka 1 (or K 1) refers to the acidic species with the most protons, and Kb 1 refers to the basic species with no acidic protons. 14

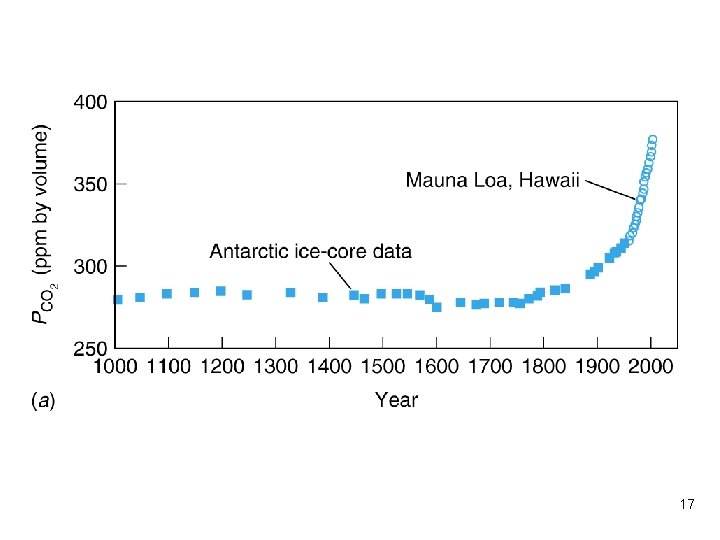
Box 11 -1 Carbon dioxide in the air and ocean l Carbon dioxide is the principal greenhouse gas in the atmosphere, with a significant role in regulating the temperature of earth’s surface. l Earth absorbs sunlight and then radiates energy away by discharging infrared radiation to space. l A greenhouse gas is so named because it absorbs infrared radiation emitted from the ground and reradiates back to the ground. 15

Carbon dioxide in the air and ocean (cont. ) l The balance between sunlight absorbed and radiation to space determines the surface temperature. l The ocean is a major reservoir for CO 2. When the concentration of dissolved CO 2 goes up, the p. H of the ocean goes down. l CO 2 could be trapped in ice cores in Antarctica and the measurement could track the CO 2 concentration in history. 16

17

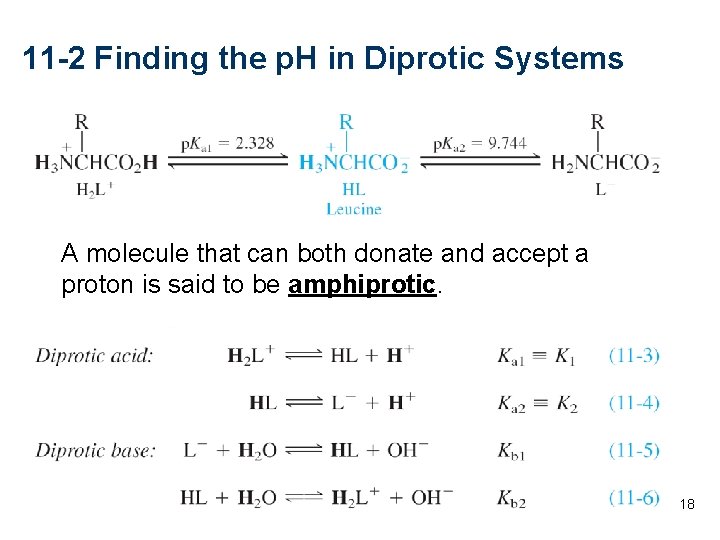
11 -2 Finding the p. H in Diprotic Systems A molecule that can both donate and accept a proton is said to be amphiprotic. . . 18

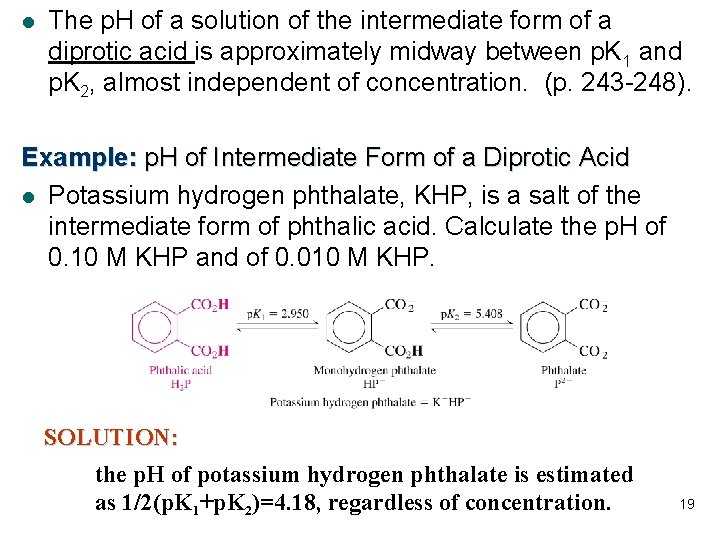
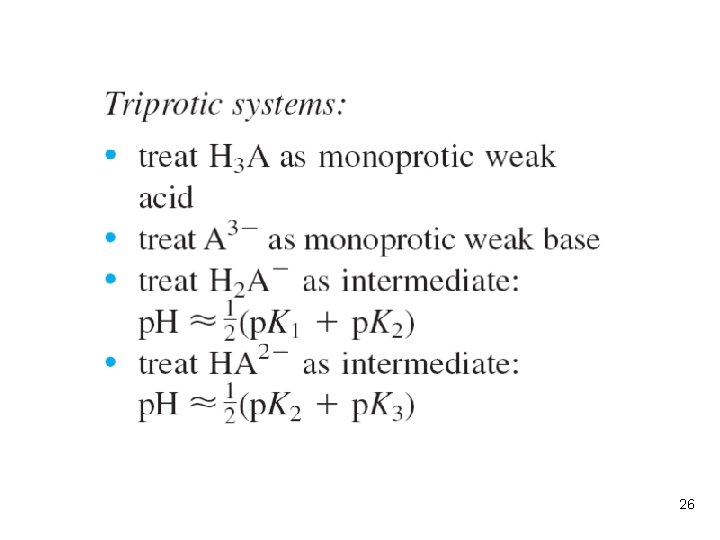
l The p. H of a solution of the intermediate form of a diprotic acid is approximately midway between p. K 1 and p. K 2, almost independent of concentration. (p. 243 -248). Example: p. H of Intermediate Form of a Diprotic Acid l Potassium hydrogen phthalate, KHP, is a salt of the intermediate form of phthalic acid. Calculate the p. H of 0. 10 M KHP and of 0. 010 M KHP. SOLUTION: the p. H of potassium hydrogen phthalate is estimated as 1/2(p. K 1+p. K 2)=4. 18, regardless of concentration. 19

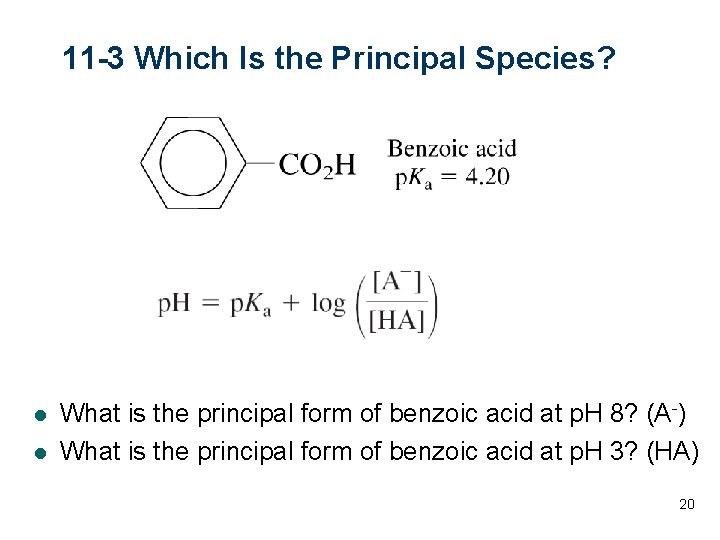
11 -3 Which Is the Principal Species? l l What is the principal form of benzoic acid at p. H 8? (A-) What is the principal form of benzoic acid at p. H 3? (HA) 20

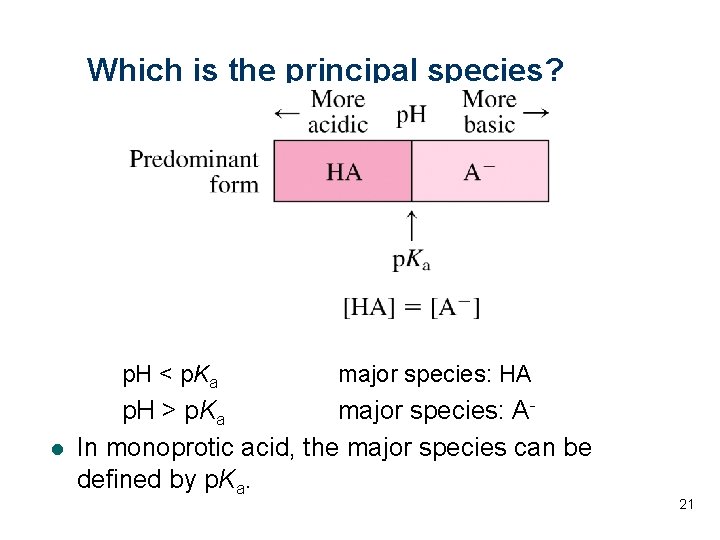
Which is the principal species? p. H < p. Ka l major species: HA p. H > p. Ka major species: AIn monoprotic acid, the major species can be defined by p. Ka. 21

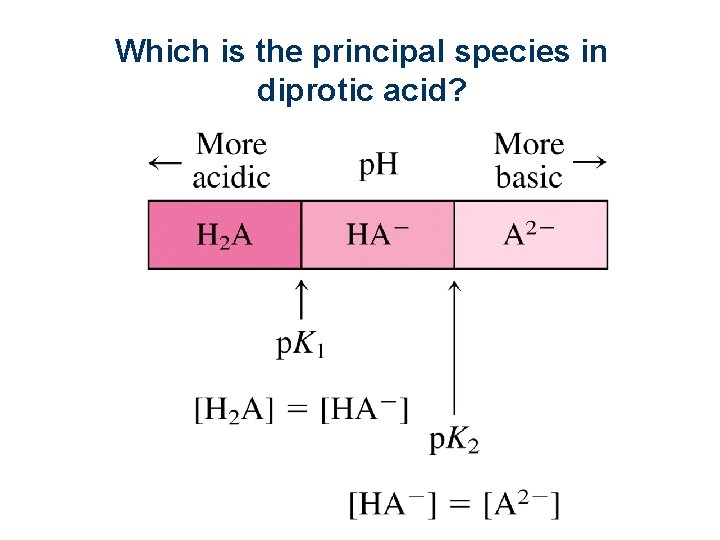
Which is the principal species in diprotic acid? Slide 22 of 119

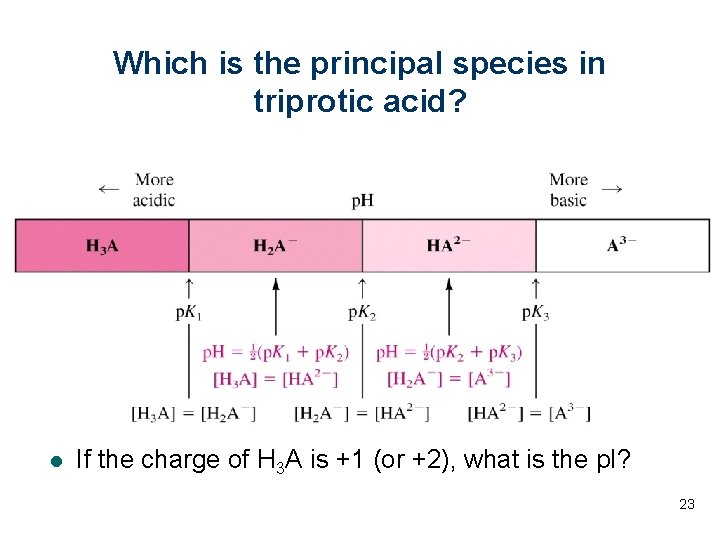
Which is the principal species in triprotic acid? l If the charge of H 3 A is +1 (or +2), what is the p. I? 23

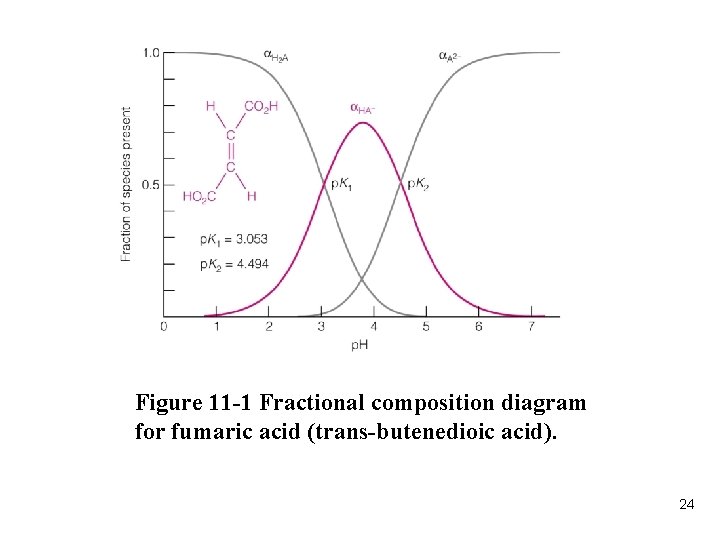
Figure 11 -1 Fractional composition diagram for fumaric acid (trans-butenedioic acid). 24

Example: Principal Species-Which One and How Much? l What is the predominated form of ammonia in a solution at p. H 7. 0? Approximately what fraction is in this form? SOLUTION: l At p. H=9. 24, [NH 4+] = [NH 3]. Below p. H 9. 24, NH 4+ will be the predominant form. Because p. H = 7. 0 is about 2 p. H units below p. Ka, the quotient [NH 3]/[NH 4+] will be about 1: 100. Approximately 99% is in the form NH 4+. 25

26

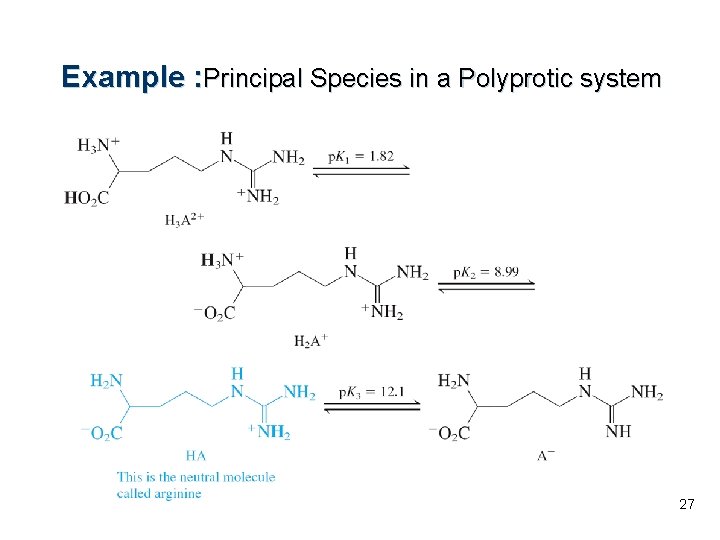
Example : Principal Species in a Polyprotic system 27

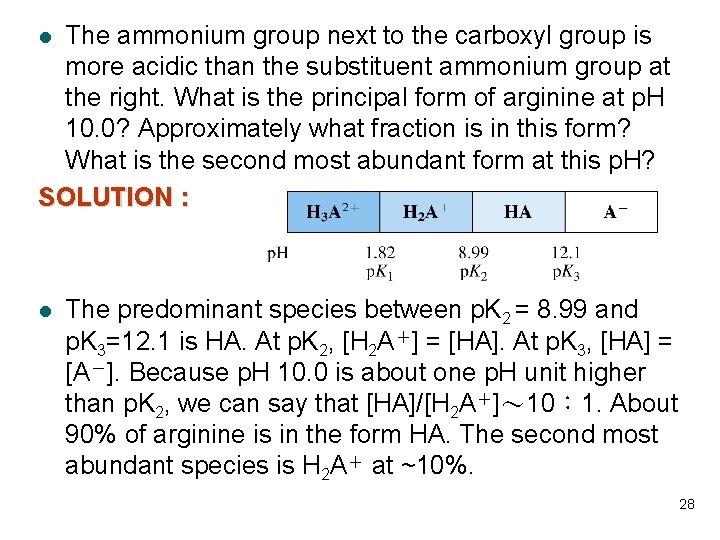
The ammonium group next to the carboxyl group is more acidic than the substituent ammonium group at the right. What is the principal form of arginine at p. H 10. 0? Approximately what fraction is in this form? What is the second most abundant form at this p. H? SOLUTION : l l The predominant species between p. K 2 = 8. 99 and p. K 3=12. 1 is HA. At p. K 2, [H 2 A+] = [HA]. At p. K 3, [HA] = [A-]. Because p. H 10. 0 is about one p. H unit higher than p. K 2, we can say that [HA]/[H 2 A+]~ 10: 1. About 90% of arginine is in the form HA. The second most abundant species is H 2 A+ at ~10%. 28

11 -4 Titrations in Polyprotic Systems l All Henderson-Hasselbalch equation are always true for a solution at equilibrium. l The p. K 1 and p. K 2 play important roles to show the character of this diprotic acid. 29

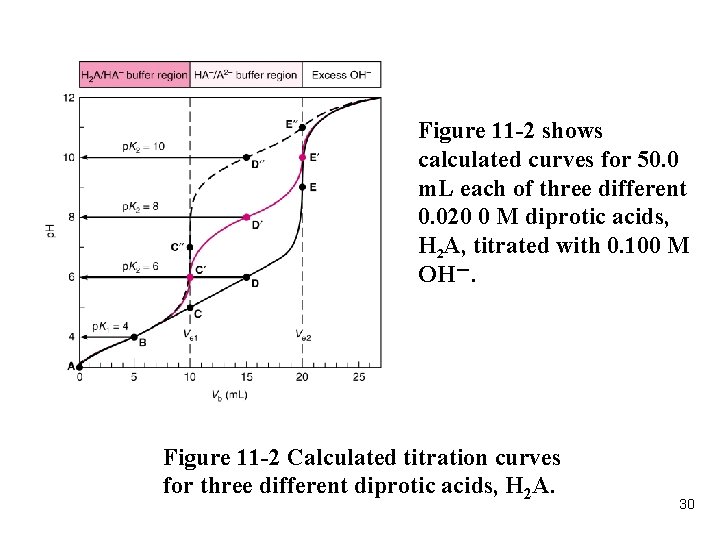
Figure 11 -2 shows calculated curves for 50. 0 m. L each of three different 0. 020 0 M diprotic acids, H 2 A, titrated with 0. 100 M OH-. Figure 11 -2 Calculated titration curves for three different diprotic acids, H 2 A. 30

Proteins are polymers made of amino acids Therefore, proteins are polyprotic acids and bases.

32

The End 33

Exploring Chemical Analysis Daniel C. Harris (Fourth Edition) 12 A Deeper Look at Chemical Equilibrium 國防醫學院 生化學科 王明芳老師 2012 -3 -29 34

Outline l The effect of ionic strength on solubility of salts l Activity coefficients 35

12 -1 Chemical equilibrium in the environment l Because of the acid drainage from abandoned coal mines, part of the north branch of the Potomac River is lifeless. l As the river passes a paper mill and wastewater treatment increase the p. H from 4. 5 to 7. 2. Ca. CO 3(s) + CO 2(aq) + H 2 O(l) Calcium carbonate Ca 2+(aq) + 2 HCO 3 -(aq) Dissolved calcium bicarbonate HCO 3 -(aq) + H+(aq) neutralization CO 2(g) + H 2 O(l) Acid from river 36

The effect of ionic strength on solubility of salts 37

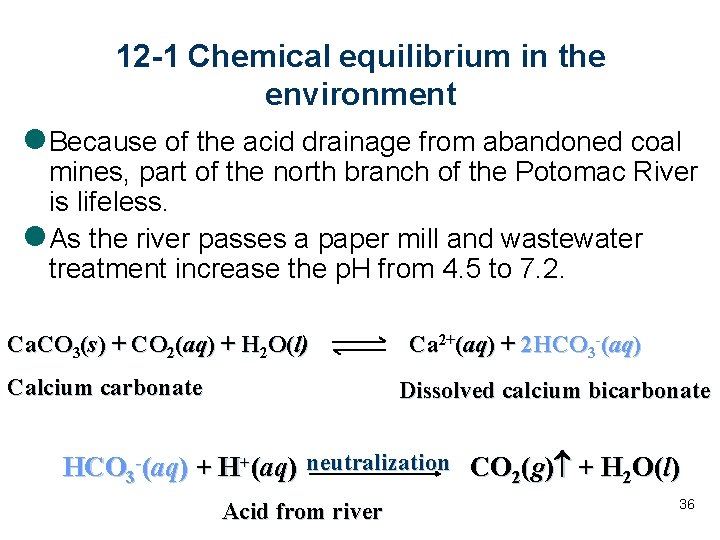
l When slightly soluble lead (II) iodide dissolves in pure water, many species are formed.

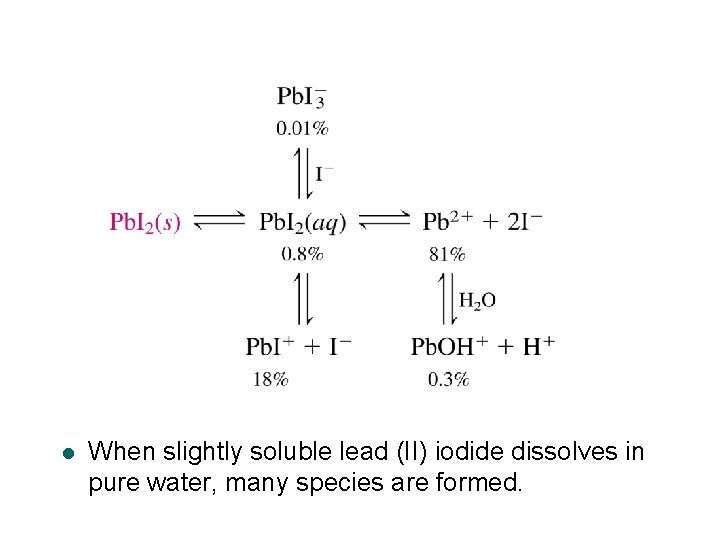
Effect of KNO 3 on the solubility of Pb. I 2 l When KNO 3 is added to the saturated Pb. I 2 solution, the total concentration of dissolved iodine increases. l The dissolved iodine includes free iodide and the iodide attached to lead. l Why does solubility increase when salts are added to the solution? 39


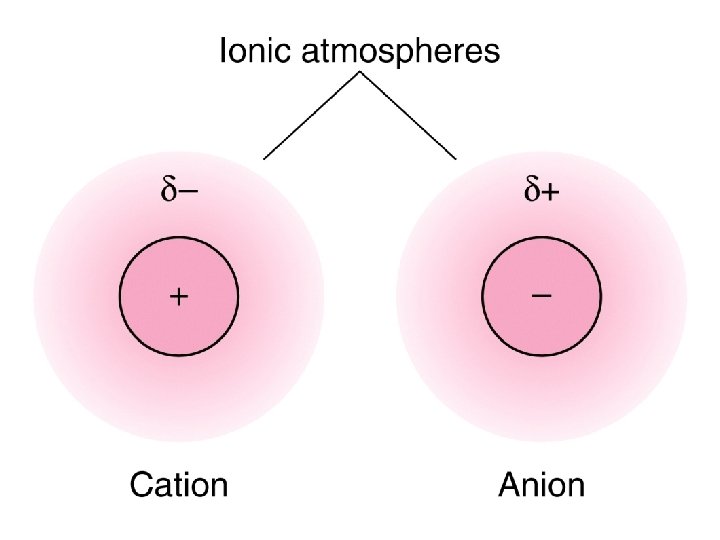
The effect of ionic strength on solubility of salts l The I- ion is surrounded by cations (K+, Pb 2+) and anions (NO 3 -, I-) in the solution. l The typical anion (I-) has more cations than anions near it. l We call this region the ionic atmosphere. l The net charge in the atmosphere is less than the charge of the anion at the center. l The ionic atmosphere attenuates (decrease) the attraction between ions in solutions. 41


The effect of ionic strength on solubility of salts (cont. ) l The higher the concentration of ions in a solution, the higher the charge in the ionic atmosphere. l Each ion-plus-atmosphere contains less net charge and there is less attraction between any particular cation and anion. l The greater the ionic strength of the solution, the greater the charge in each ionic atmosphere. 43

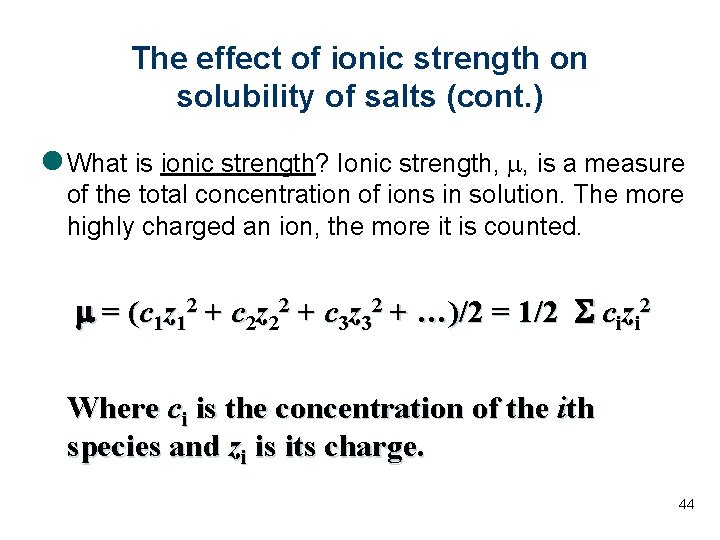
The effect of ionic strength on solubility of salts (cont. ) l What is ionic strength? Ionic strength, m, is a measure of the total concentration of ions in solution. The more highly charged an ion, the more it is counted. m = (c 1 z 12 + c 2 z 22 + c 3 z 32 + …)/2 = 1/2 S cizi 2 Where ci is the concentration of the ith species and zi is its charge. 44

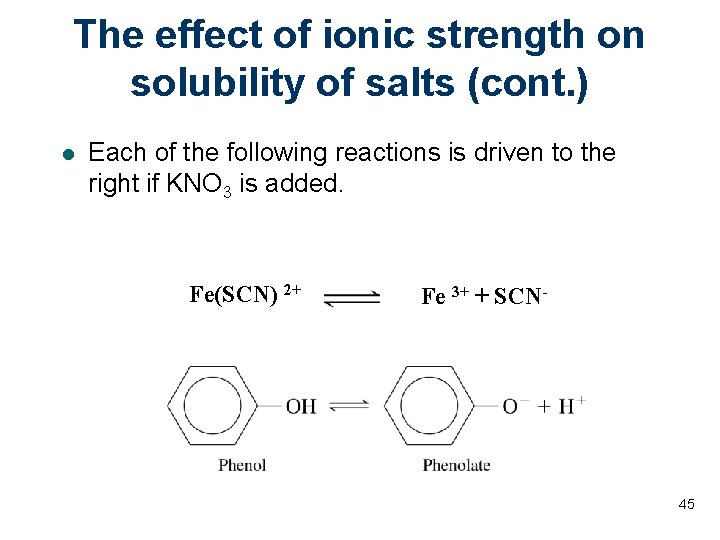
The effect of ionic strength on solubility of salts (cont. ) l Each of the following reactions is driven to the right if KNO 3 is added. Fe(SCN) 2+ Fe 3+ + SCN- 45

Example: Calculation of Ion Strength Find the ionic strength of (a) 0. 10 M Na. NO 3; (b) 0. 010 M Na 2 SO 4; and (c) 0. 020 M KBr plus 0. 010 M Na 2 SO 4. SOLUTION: 46
Activity coefficients of ions 47

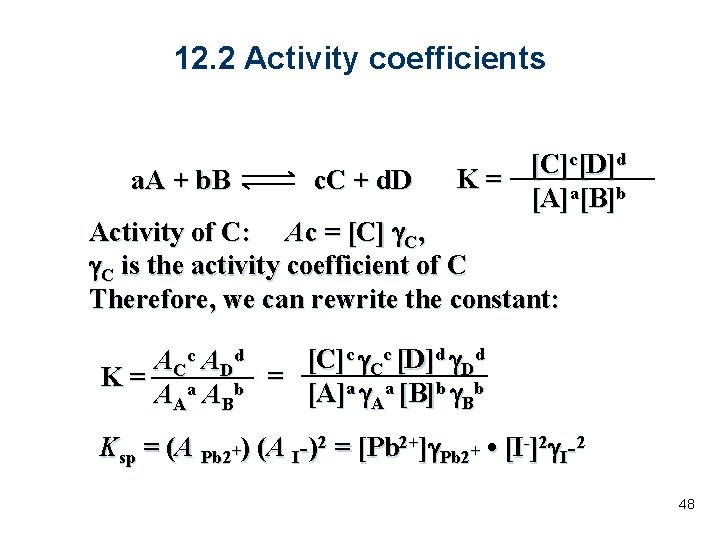
12. 2 Activity coefficients a. A + b. B c. C + d. D K= [C]c[D]d [A]a[B]b Activity of C: Ac = [C] g. C, g. C is the activity coefficient of C Therefore, we can rewrite the constant: [C]c g. Cc [D]d g. Dd A Cc A Dd K= a b = [A]a g. Aa [B]b g. Bb AA AB Ksp = (A Pb 2+) (A I-)2 = [Pb 2+]g. Pb 2+ • [I-]2 g. I-2 48

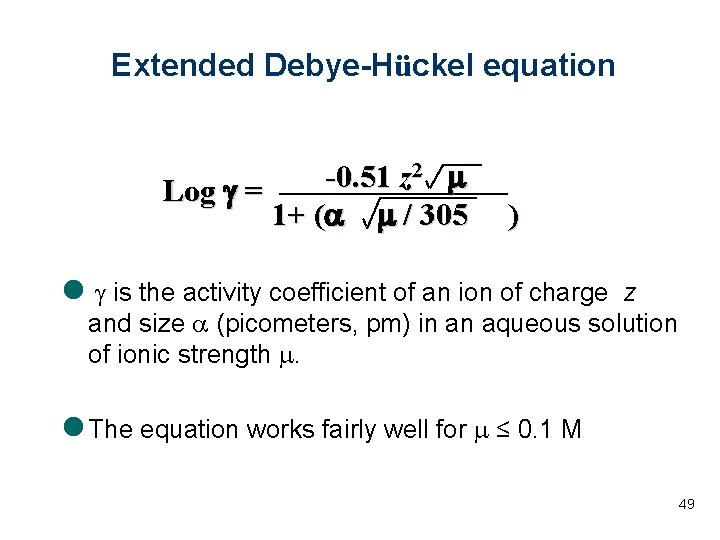
Extended Debye-Hückel equation 2 m -0. 51 z Log g = 1+ (a m / 305 ) l g is the activity coefficient of an ion of charge z and size a (picometers, pm) in an aqueous solution of ionic strength m. l The equation works fairly well for m ≤ 0. 1 M 49

Extended Debye-Hückel equation (cont. ) l The size a is the effective hydrated radius of the ion and its tightly bound sheath of water molecules. 50

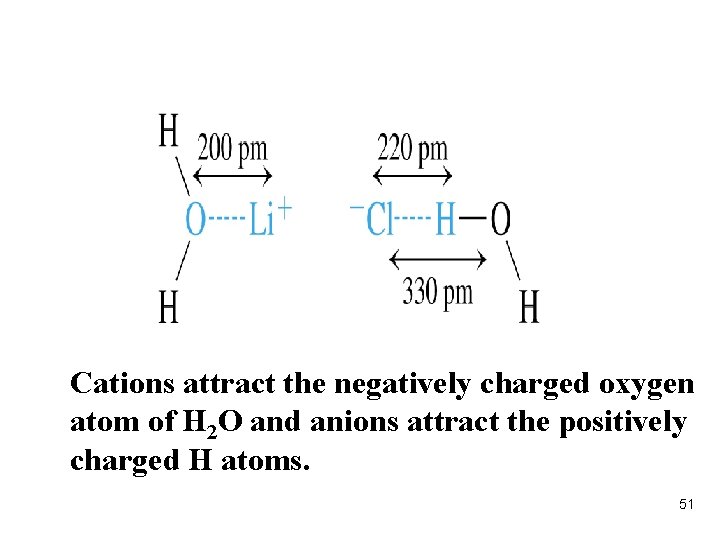
Cations attract the negatively charged oxygen atom of H 2 O and anions attract the positively charged H atoms. 51

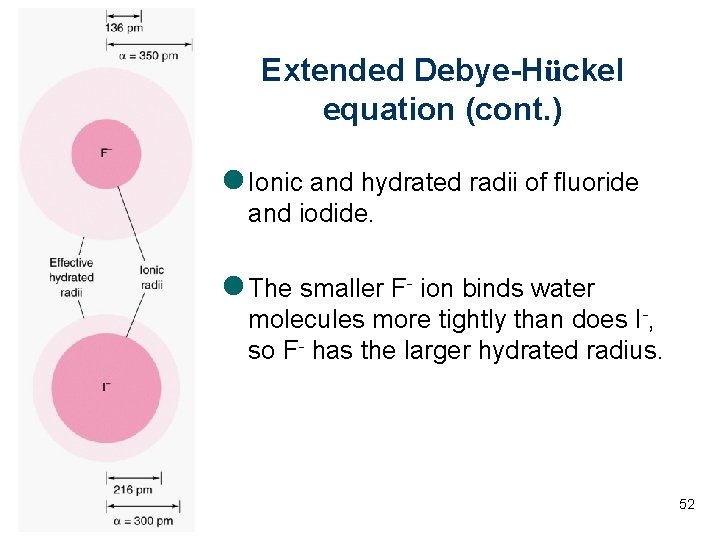
Extended Debye-Hückel equation (cont. ) l Ionic and hydrated radii of fluoride and iodide. l The smaller F- ion binds water molecules more tightly than does I-, so F- has the larger hydrated radius. 52

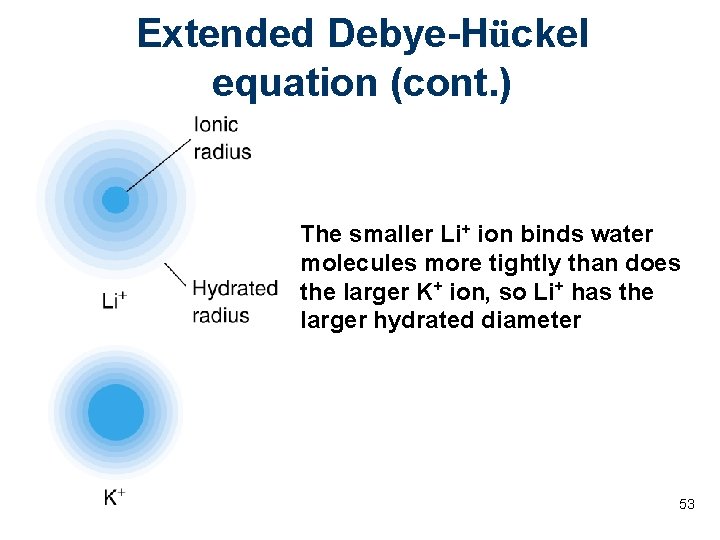
Extended Debye-Hückel equation (cont. ) The smaller Li+ ion binds water molecules more tightly than does the larger K+ ion, so Li+ has the larger hydrated diameter 53

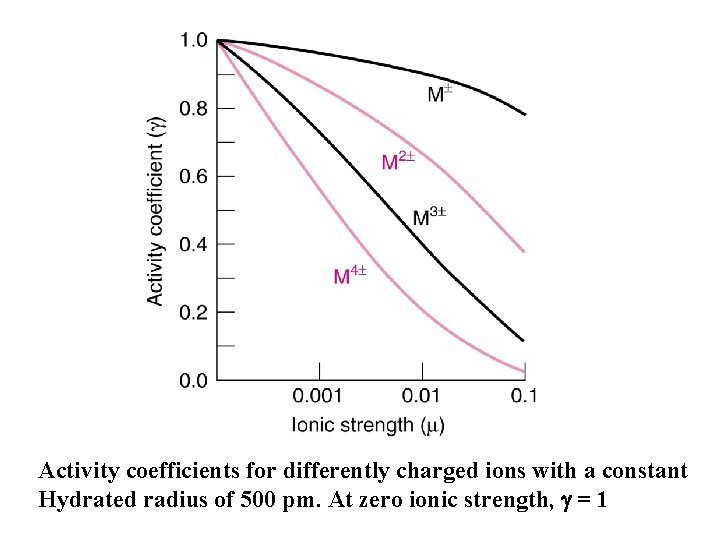
Effect of ionic strength, ion charge, and ion size on the activity coefficient l As ionic strength increases, the activity coefficient decreases. l As the charge of the ion increases, the departure of its activity from unity increases. l The smaller the hydrated radius of the ion, the more important activity effects become. 54

Activity coefficients for differently charged ions with a constant Hydrated radius of 500 pm. At zero ionic strength, g = 1

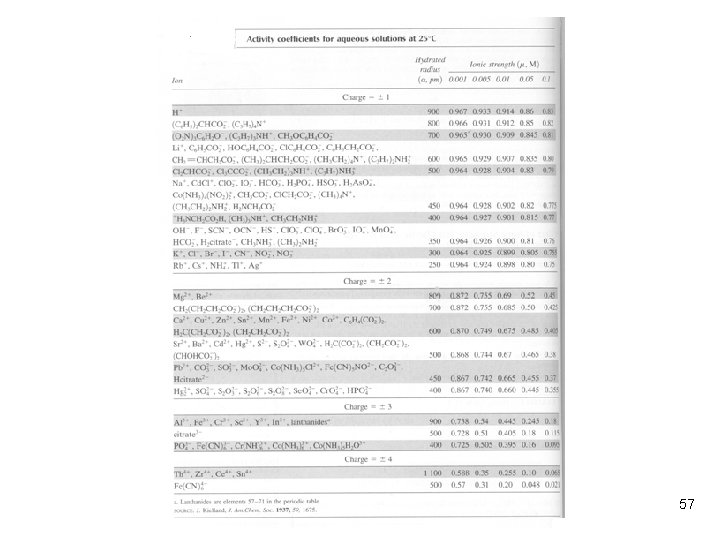
One easy way to obtain activity coefficients l We may obtain the activity coefficients by using the Extended Deby-Hückel equation. However, this method could be very complicated and time consuming. l We may utilize Table 12 -1 (p. 269) to obtain the activity coefficients. This method is very easy. l If the activity coefficient does not exist on the table, what would you do? 56

57

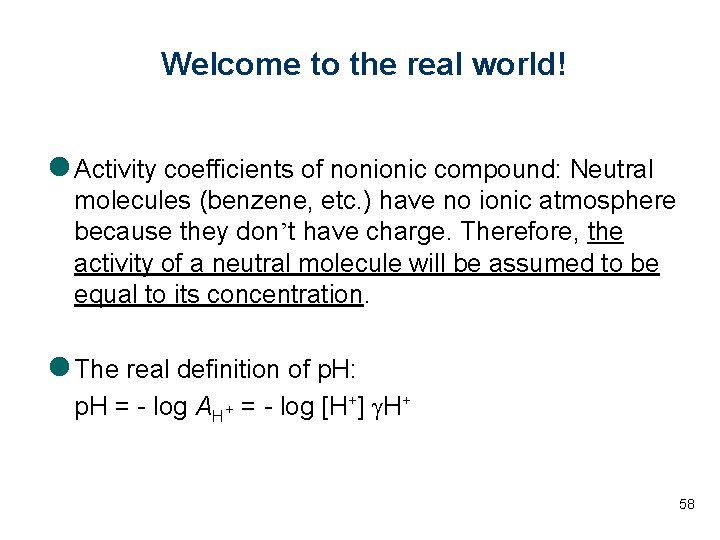
Welcome to the real world! l Activity coefficients of nonionic compound: Neutral molecules (benzene, etc. ) have no ionic atmosphere because they don’t have charge. Therefore, the activity of a neutral molecule will be assumed to be equal to its concentration. l The real definition of p. H: p. H = - log AH+ = - log [H+] g. H+ 58

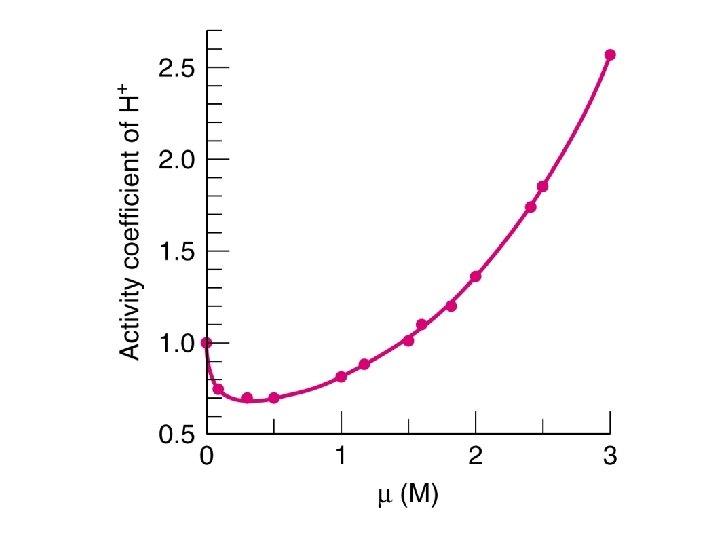
High ionic strength l In fact, above an ionic strength of approximately 1 M, activity coefficients of most ions increases. l In concentrated solutions, the ‘solvent’ is no longer just H 2 O. 59


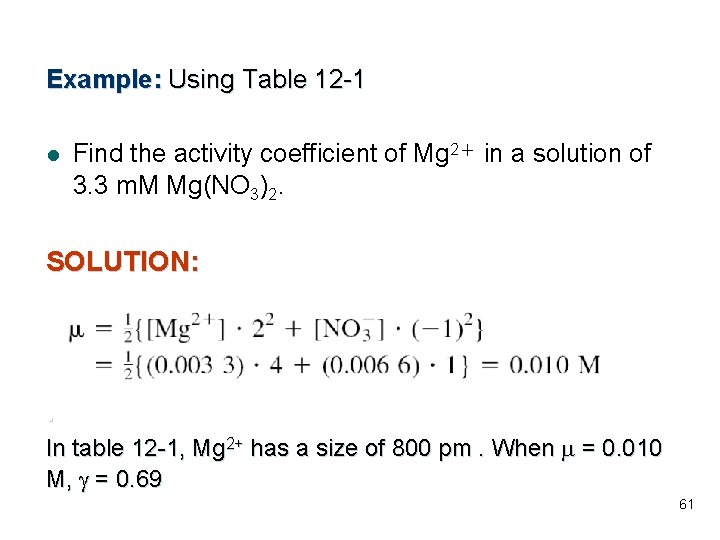
Example: Using Table 12 -1 l Find the activity coefficient of Mg 2+ in a solution of 3. 3 m. M Mg(NO 3)2. SOLUTION: . In table 12 -1, Mg 2+ has a size of 800 pm. When m = 0. 010 M, g = 0. 69 61

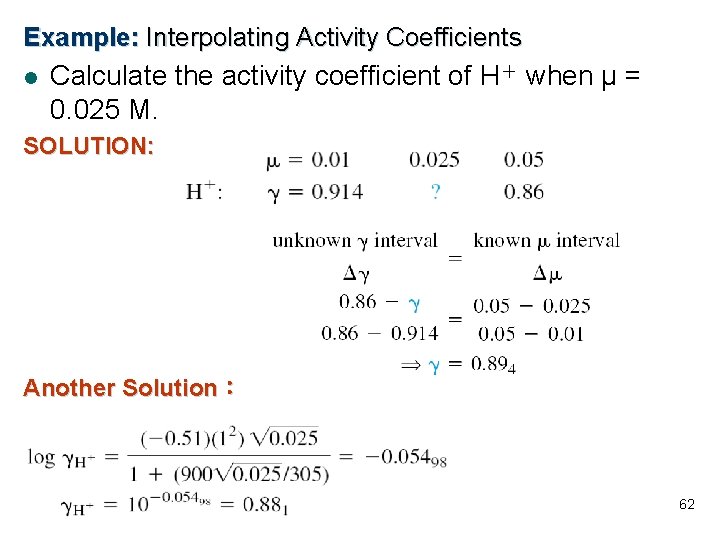
Example: Interpolating Activity Coefficients l Calculate the activity coefficient of H+ when μ = 0. 025 M. SOLUTION: Another Solution: 62

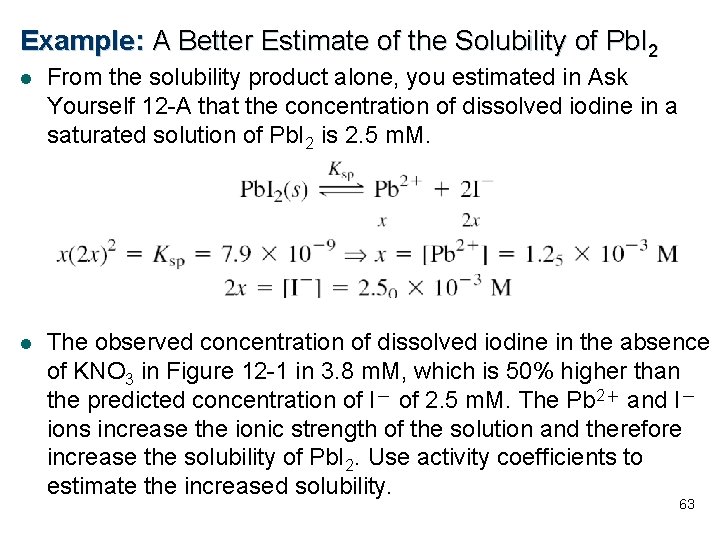
Example: A Better Estimate of the Solubility of Pb. I 2 l From the solubility product alone, you estimated in Ask Yourself 12 -A that the concentration of dissolved iodine in a saturated solution of Pb. I 2 is 2. 5 m. M. l The observed concentration of dissolved iodine in the absence of KNO 3 in Figure 12 -1 in 3. 8 m. M, which is 50% higher than the predicted concentration of I- of 2. 5 m. M. The Pb 2+ and I- ions increase the ionic strength of the solution and therefore increase the solubility of Pb. I 2. Use activity coefficients to estimate the increased solubility. 63

SOLUTION: l. 64

The End 65