Daniel C Harris Quantitative Chemical Analysis Seventh Edition



- Slides: 27

Daniel C. Harris Quantitative Chemical Analysis Seventh Edition Chapter 8 -12 Acid-Base Titrations Copyright © 2007 by W. H. Freeman and Company

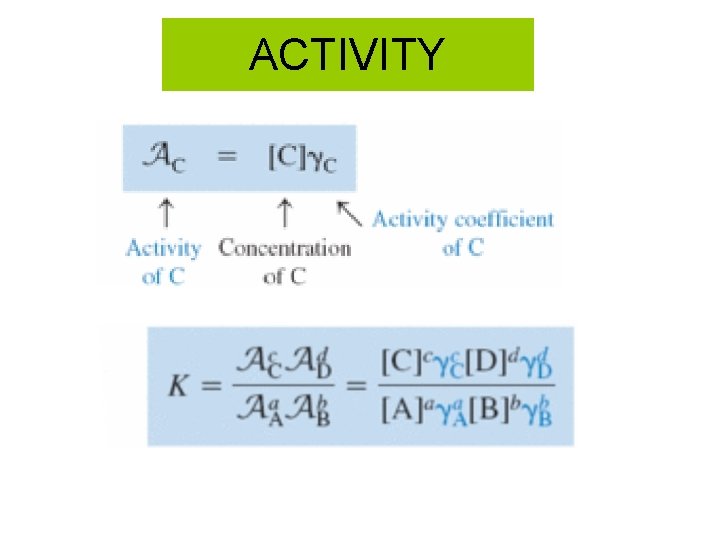
ACTIVITY

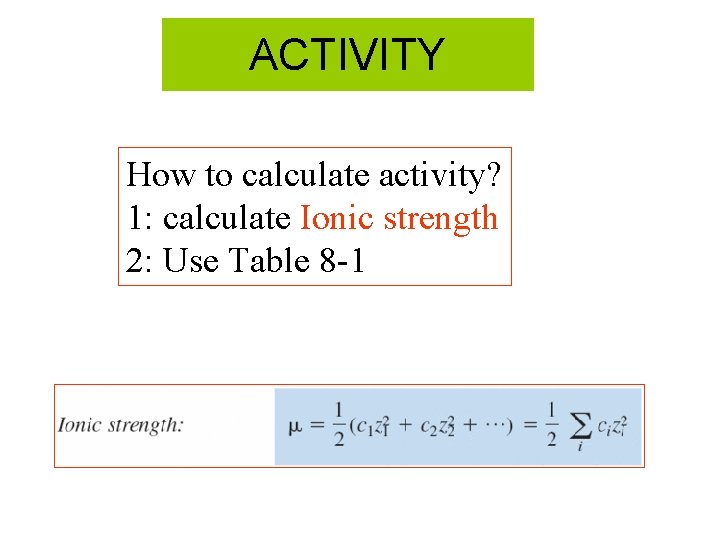
ACTIVITY How to calculate activity? 1: calculate Ionic strength 2: Use Table 8 -1

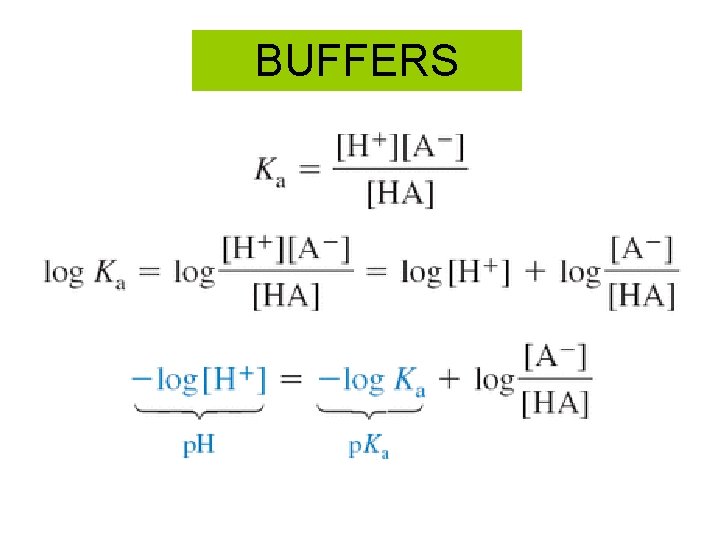
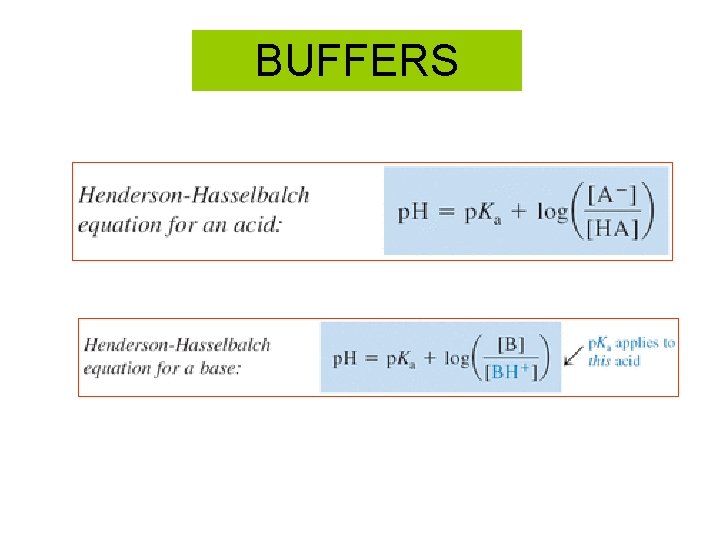
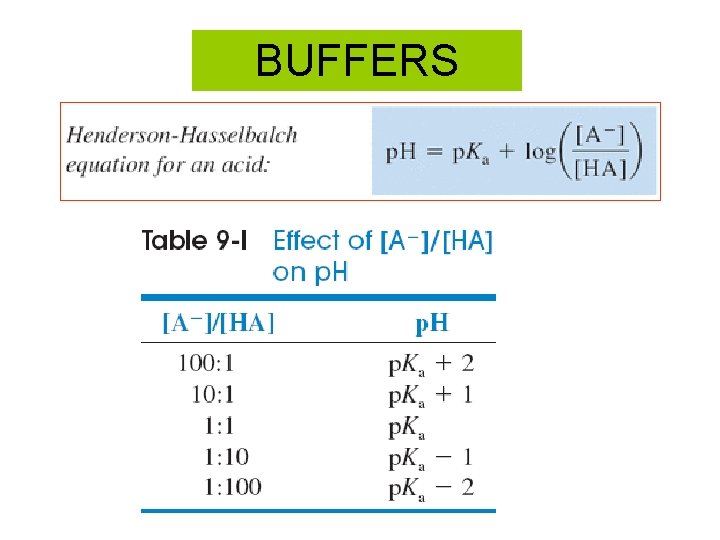
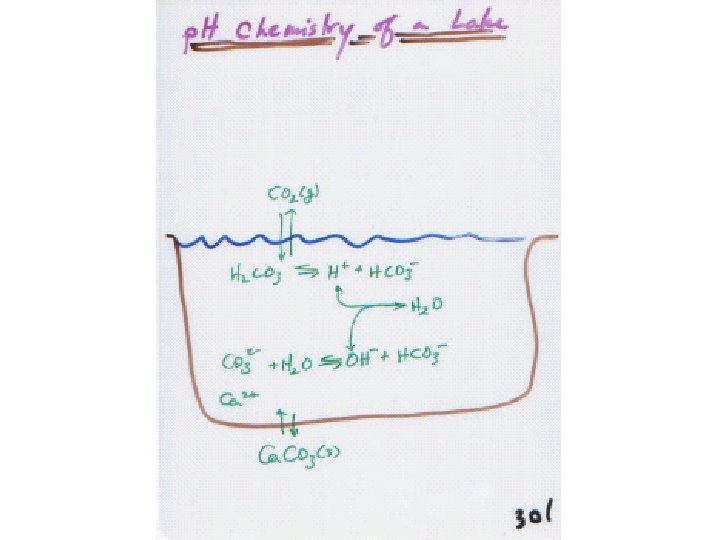
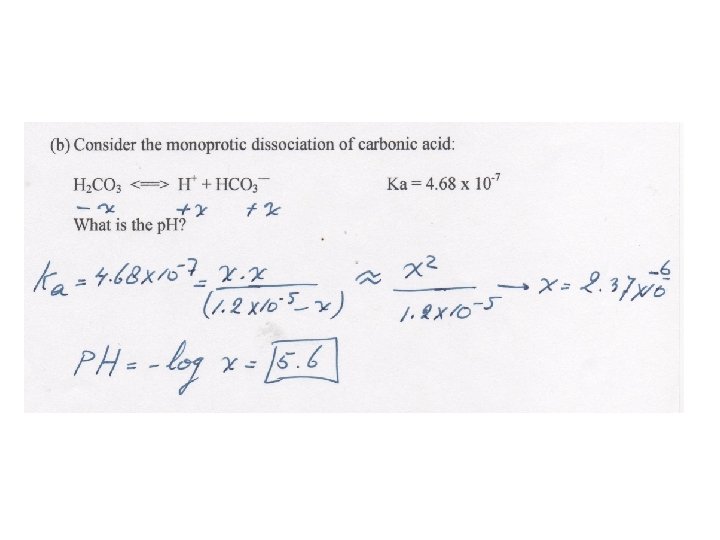
BUFFERS

BUFFERS

BUFFERS





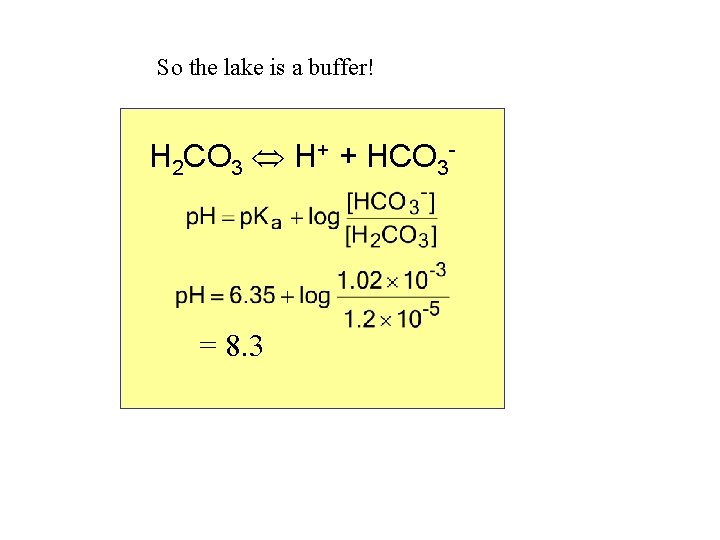
So the lake is a buffer! H 2 CO 3 H+ + HCO 3 - = 8. 3


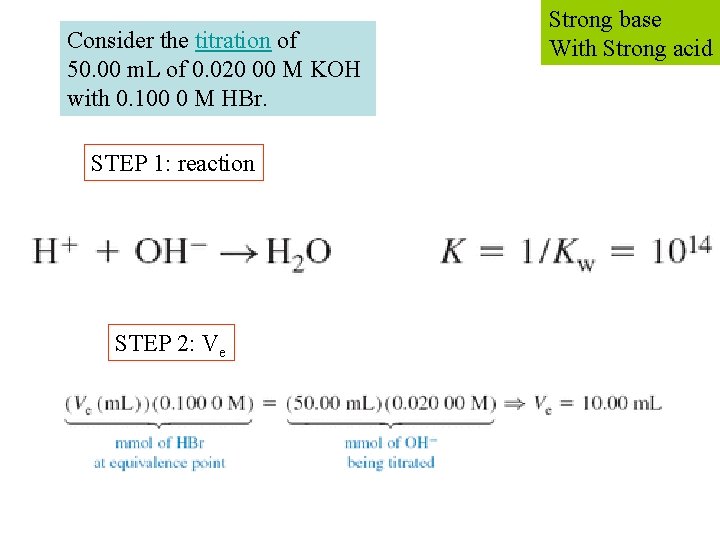
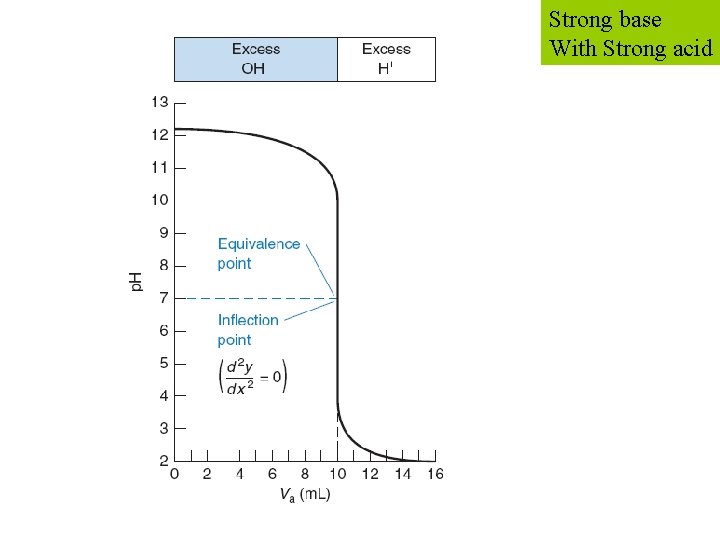
Consider the titration of 50. 00 m. L of 0. 020 00 M KOH with 0. 100 0 M HBr. STEP 1: reaction STEP 2: Ve Strong base With Strong acid

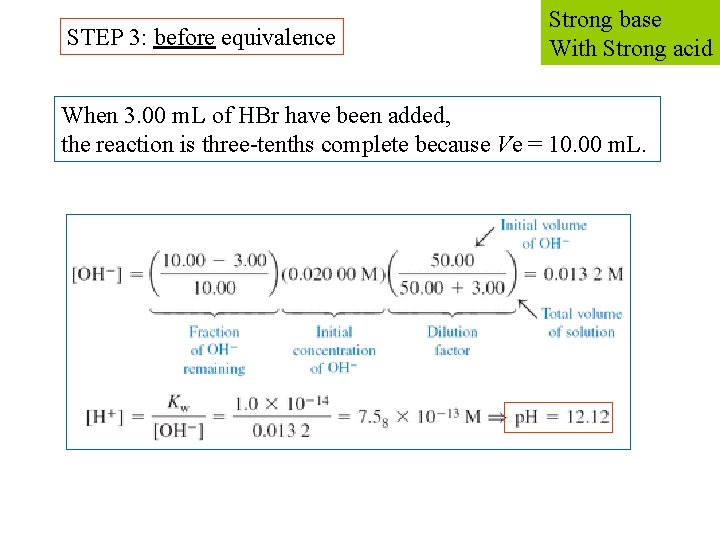
STEP 3: before equivalence Strong base With Strong acid When 3. 00 m. L of HBr have been added, the reaction is three-tenths complete because Ve = 10. 00 m. L.

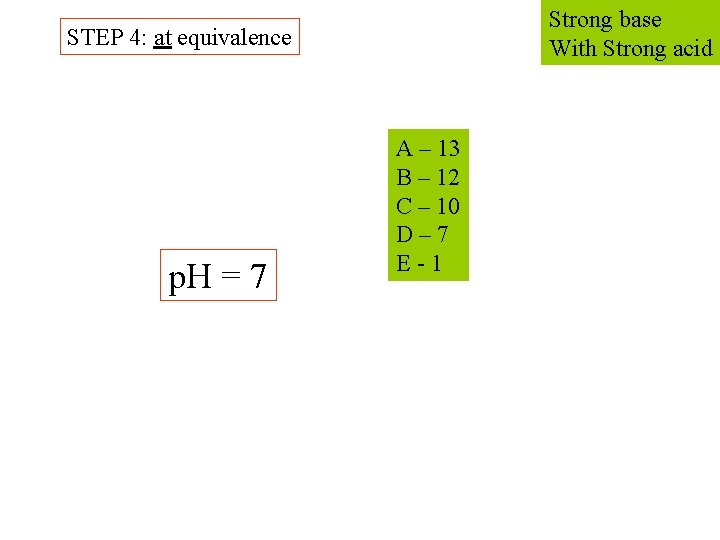
Strong base With Strong acid STEP 4: at equivalence p. H = 7 A – 13 B – 12 C – 10 D – 7 E - 1

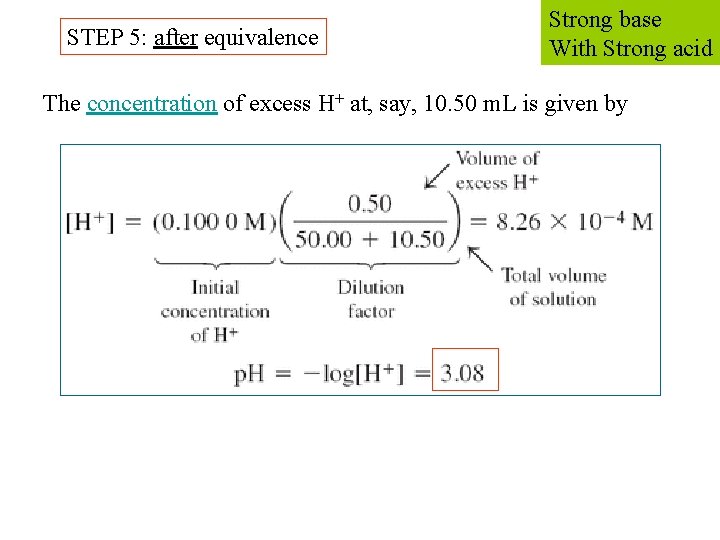
STEP 5: after equivalence Strong base With Strong acid The concentration of excess H+ at, say, 10. 50 m. L is given by

Strong base With Strong acid

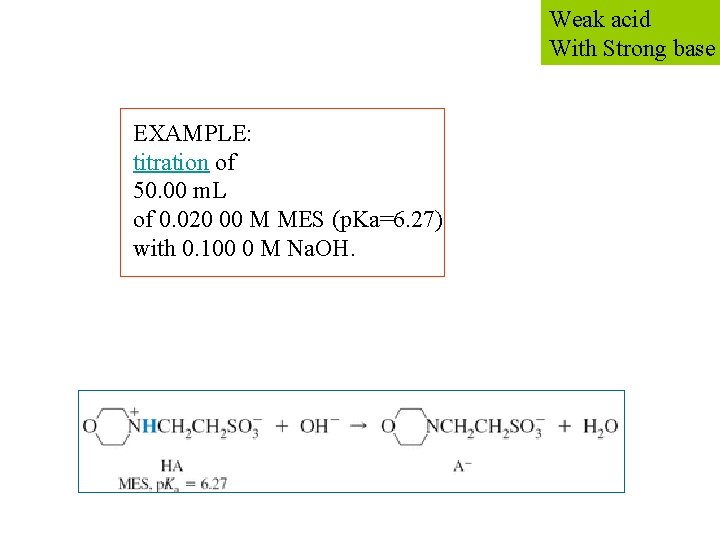
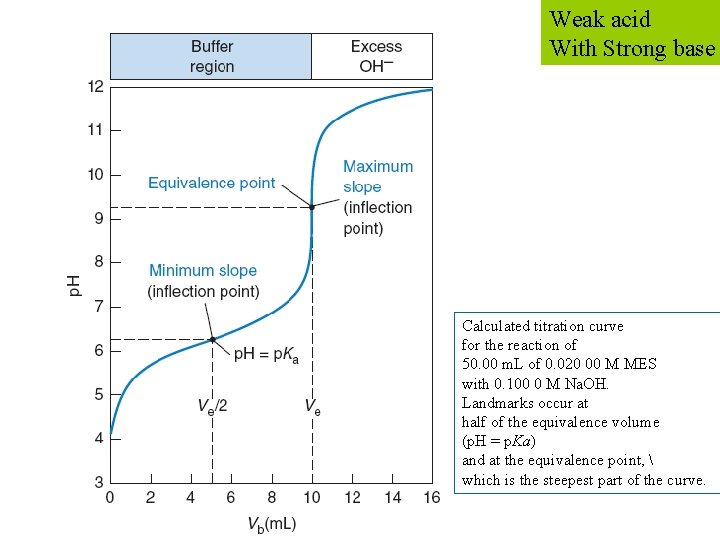
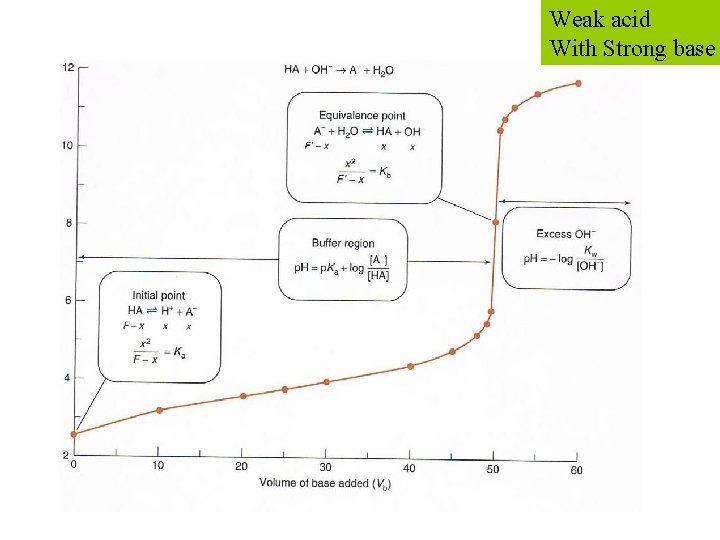
Weak acid With Strong base EXAMPLE: titration of 50. 00 m. L of 0. 020 00 M MES (p. Ka=6. 27) with 0. 100 0 M Na. OH.

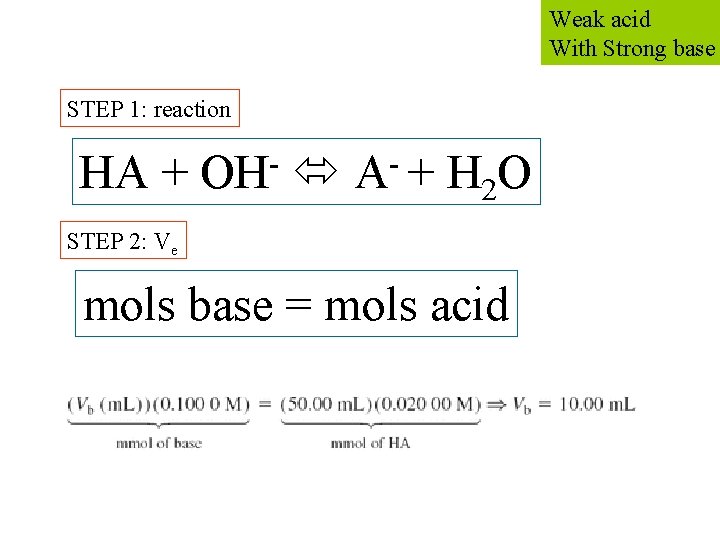
Weak acid With Strong base STEP 1: reaction - HA + OH A + H 2 O STEP 2: Ve mols base = mols acid

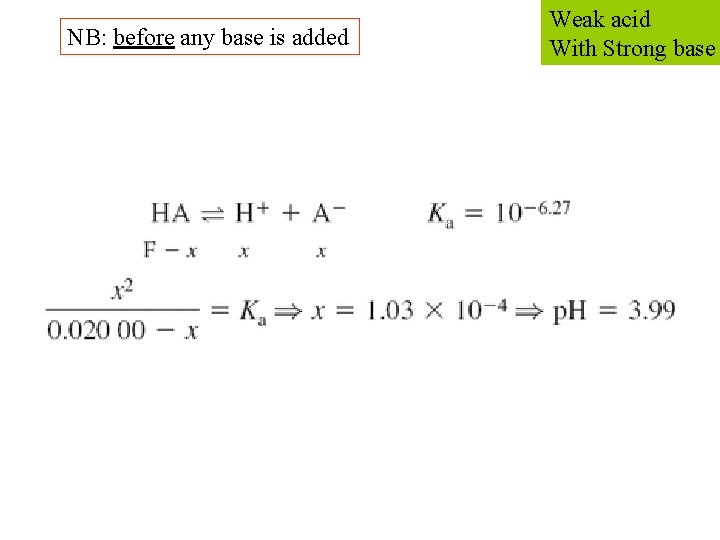
NB: before any base is added Weak acid With Strong base

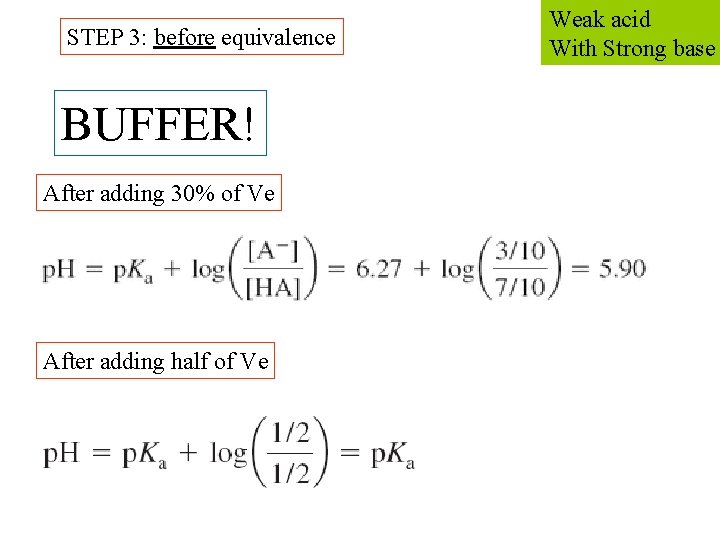
STEP 3: before equivalence BUFFER! After adding 30% of Ve After adding half of Ve Weak acid With Strong base

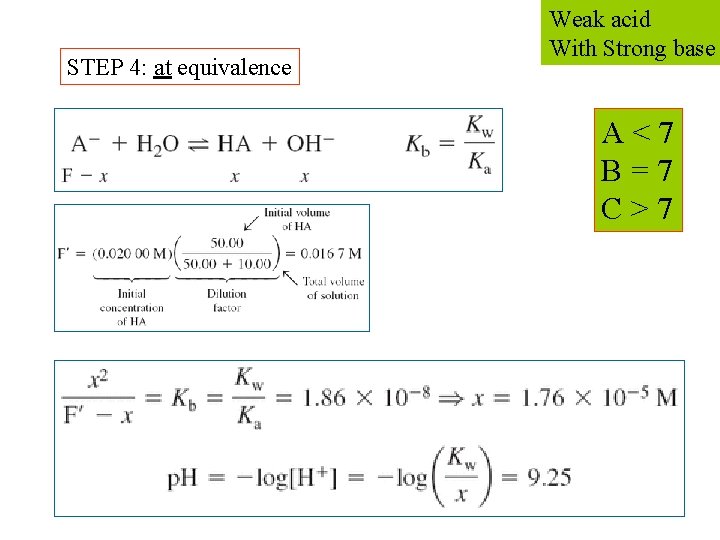
STEP 4: at equivalence Weak acid With Strong base A < 7 B = 7 C > 7

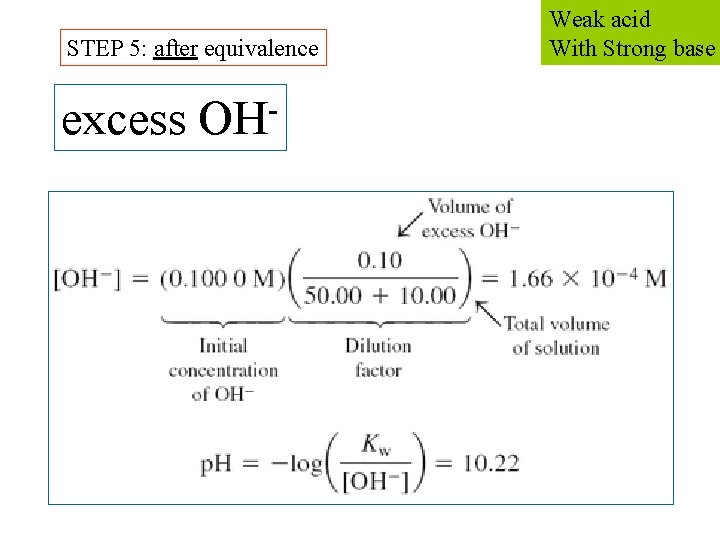
STEP 5: after equivalence excess OH Weak acid With Strong base

Weak acid With Strong base Calculated titration curve for the reaction of 50. 00 m. L of 0. 020 00 M MES with 0. 100 0 M Na. OH. Landmarks occur at half of the equivalence volume (p. H = p. Ka) and at the equivalence point, which is the steepest part of the curve.

Weak acid With Strong base

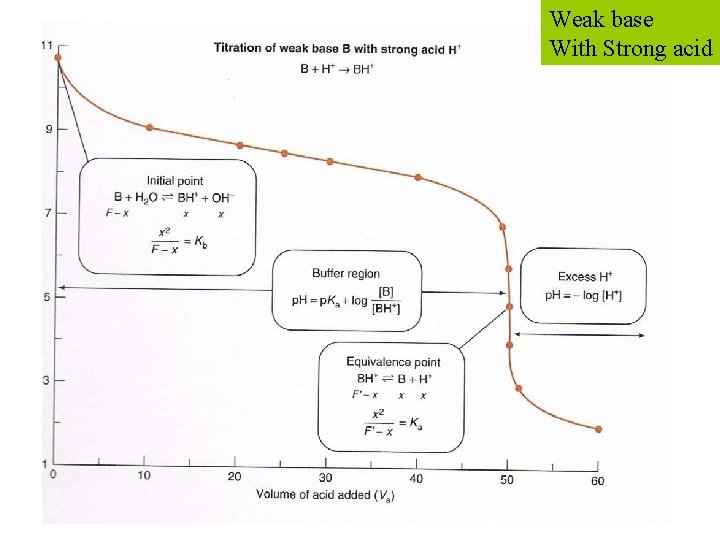
Weak base With Strong acid

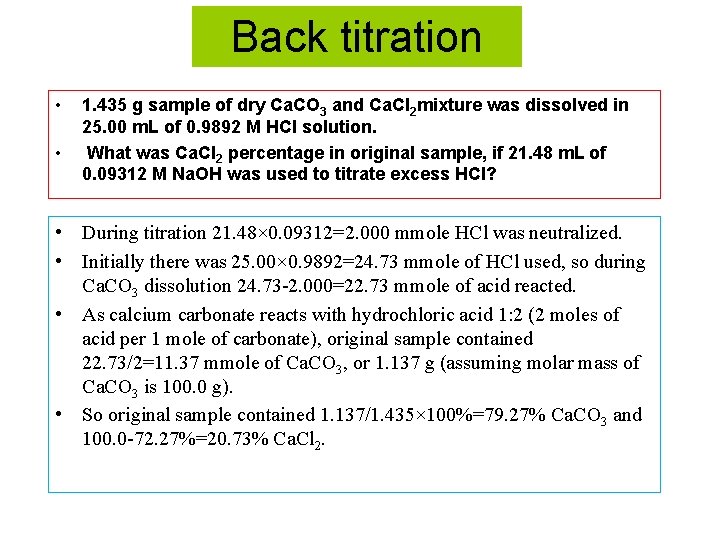
Back titration • • 1. 435 g sample of dry Ca. CO 3 and Ca. Cl 2 mixture was dissolved in 25. 00 m. L of 0. 9892 M HCl solution. What was Ca. Cl 2 percentage in original sample, if 21. 48 m. L of 0. 09312 M Na. OH was used to titrate excess HCl? • During titration 21. 48× 0. 09312=2. 000 mmole HCl was neutralized. • Initially there was 25. 00× 0. 9892=24. 73 mmole of HCl used, so during Ca. CO 3 dissolution 24. 73 -2. 000=22. 73 mmole of acid reacted. • As calcium carbonate reacts with hydrochloric acid 1: 2 (2 moles of acid per 1 mole of carbonate), original sample contained 22. 73/2=11. 37 mmole of Ca. CO 3, or 1. 137 g (assuming molar mass of Ca. CO 3 is 100. 0 g). • So original sample contained 1. 137/1. 435× 100%=79. 27% Ca. CO 3 and 100. 0 -72. 27%=20. 73% Ca. Cl 2.