What happens when granite is weathered First unweathered


![Clays Sheet Silicates – aka Phyllosilicates [Si 2 O 5]2 Sheets of tetrahedra micas Clays Sheet Silicates – aka Phyllosilicates [Si 2 O 5]2 Sheets of tetrahedra micas](https://slidetodoc.com/presentation_image_h/9fe6827b0154a0343de1711335b010b3/image-4.jpg)
![Sheet Silicates – aka Phyllosilicates [Si 2 O 5]2 Sheets of tetrahedra micas talc Sheet Silicates – aka Phyllosilicates [Si 2 O 5]2 Sheets of tetrahedra micas talc](https://slidetodoc.com/presentation_image_h/9fe6827b0154a0343de1711335b010b3/image-5.jpg)


![Phyllosilicates Si. O 4 tetrahedra polymerized into 2 -D sheets: [Si 2 O 5] Phyllosilicates Si. O 4 tetrahedra polymerized into 2 -D sheets: [Si 2 O 5]](https://slidetodoc.com/presentation_image_h/9fe6827b0154a0343de1711335b010b3/image-8.jpg)

![Phyllosilicates Yellow = (OH) Kaolinite: Al 2 [Si 2 O 5] (OH)4 T-layers and Phyllosilicates Yellow = (OH) Kaolinite: Al 2 [Si 2 O 5] (OH)4 T-layers and](https://slidetodoc.com/presentation_image_h/9fe6827b0154a0343de1711335b010b3/image-13.jpg)
![Phyllosilicates Yellow = (OH) Serpentine: Mg 3 [Si 2 O 5] (OH)4 T-layers and Phyllosilicates Yellow = (OH) Serpentine: Mg 3 [Si 2 O 5] (OH)4 T-layers and](https://slidetodoc.com/presentation_image_h/9fe6827b0154a0343de1711335b010b3/image-15.jpg)
![Phyllosilicates Yellow = (OH) Pyrophyllite: Al 2 [Si 4 O 10] (OH)2 T-layer - Phyllosilicates Yellow = (OH) Pyrophyllite: Al 2 [Si 4 O 10] (OH)2 T-layer -](https://slidetodoc.com/presentation_image_h/9fe6827b0154a0343de1711335b010b3/image-17.jpg)
![Phyllosilicates Yellow = (OH) Talc: Mg 3 [Si 4 O 10] (OH)2 T-layer - Phyllosilicates Yellow = (OH) Talc: Mg 3 [Si 4 O 10] (OH)2 T-layer -](https://slidetodoc.com/presentation_image_h/9fe6827b0154a0343de1711335b010b3/image-18.jpg)
![Phyllosilicates Muscovite: K Al 2 [Si 3 Al. O 10] (OH)2 (coupled K - Phyllosilicates Muscovite: K Al 2 [Si 3 Al. O 10] (OH)2 (coupled K -](https://slidetodoc.com/presentation_image_h/9fe6827b0154a0343de1711335b010b3/image-19.jpg)
![Phyllosilicates Phlogopite: K Mg 3 [Si 3 Al. O 10] (OH)2 T-layer - triocathedral Phyllosilicates Phlogopite: K Mg 3 [Si 3 Al. O 10] (OH)2 T-layer - triocathedral](https://slidetodoc.com/presentation_image_h/9fe6827b0154a0343de1711335b010b3/image-20.jpg)
- Slides: 26

What happens when granite is weathered? ? • First, unweathered granite contains these minerals: – – Na Plagioclase feldspar K feldspar Quartz Lesser amounts of biotite, amphibole, or muscovite • What happens when granite is weathered? • The feldspars will undergo hydrolysis to form kaolinite (clay) and Na and K ions • The Na+ and K+ ions will be removed through leaching • The biotite and/or amphibole will undergo hydrolysis to form clay, and oxidation to form iron oxides.

Granite weathering, continued • The quartz (and muscovite, if present) will remain as residual minerals because they are very resistant to weathering. • Weathered rock is called saprolite. • What happens after this? – Quartz grains may be eroded, becoming sediment. The quartz in granite is sand- sized; it becomes quartz sand. The quartz sand will ultimately be transported to the sea (bed load), where it accumulates to form beaches. – Clays will ultimately be eroded and washed out to sea. Clay is finegrained and remains suspended in the water column (suspended load); it may be deposited in quiet water. – Dissolved ions will be transported by rivers to the sea (dissolved load), and will become part of the salts in the sea.

Sedimentary Minerals • We will focus on some minerals which form from precipitation of dissolved ions other minerals in sedimentary rocks are derived from the source rocks! • Clay, carbonate, and sulfate groups are key in sedimentary rocks – can ‘be’ the rock or cement fragments together! – Si. O 44 -, CO 32 -, SO 42 - anionic groups, respectively • Also consider halides (anion is Cl- or F-) and mineralization of silica
![Clays Sheet Silicates aka Phyllosilicates Si 2 O 52 Sheets of tetrahedra micas Clays Sheet Silicates – aka Phyllosilicates [Si 2 O 5]2 Sheets of tetrahedra micas](https://slidetodoc.com/presentation_image_h/9fe6827b0154a0343de1711335b010b3/image-4.jpg)
Clays Sheet Silicates – aka Phyllosilicates [Si 2 O 5]2 Sheets of tetrahedra micas talc clay minerals serpentine Phyllosilicates
![Sheet Silicates aka Phyllosilicates Si 2 O 52 Sheets of tetrahedra micas talc Sheet Silicates – aka Phyllosilicates [Si 2 O 5]2 Sheets of tetrahedra micas talc](https://slidetodoc.com/presentation_image_h/9fe6827b0154a0343de1711335b010b3/image-5.jpg)
Sheet Silicates – aka Phyllosilicates [Si 2 O 5]2 Sheets of tetrahedra micas talc clay minerals serpentine Phyllosilicates • Clays talc pyrophyllite micas • Display increasing order and lower variability of chemistry as T of formation increases

Clays • Term clay ALSO refers to a size (< 1 mm = <10 -6 m) • Sheet silicates, hydrous – some contain up to 20% H 2 O together with a layered structure and weak bonding between layers make them SLIPPERY WHEN WET • Very complex (even argued) chemistry reflective of specific solution compositions

Major Clay Minerals • Kaolinite – Al 2 Si 2 O 5(OH)4 • Illite – K 1 -1. 5 Al 4(Si, Al)8 O 20(OH)4 • Smectites: – Montmorillonite – (Ca, Na)0. 20. 4(Al, Mg, Fe)2(Si, Al)4 O 10(OH)2*n. H 2 O – Vermicullite - (Ca, Mg)0. 30. 4(Al, Mg, Fe)3(Si, Al)4 O 10(OH)2*n. H 2 O – Swelling clays – can take up extra water in their interlayers and are the major components of bentonite (NOT a mineral, but a mix of different clay minerals)
![Phyllosilicates Si O 4 tetrahedra polymerized into 2 D sheets Si 2 O 5 Phyllosilicates Si. O 4 tetrahedra polymerized into 2 -D sheets: [Si 2 O 5]](https://slidetodoc.com/presentation_image_h/9fe6827b0154a0343de1711335b010b3/image-8.jpg)
Phyllosilicates Si. O 4 tetrahedra polymerized into 2 -D sheets: [Si 2 O 5] Apical O’s are unpolymerized and are bonded to other constituents

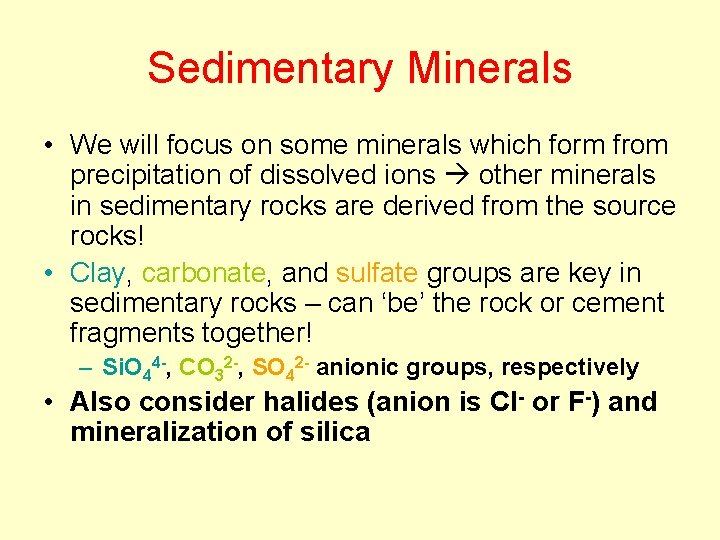
Phyllosilicates Tetrahedral layers are bonded to octahedral layers (OH) pairs are located in center of T rings where no apical O

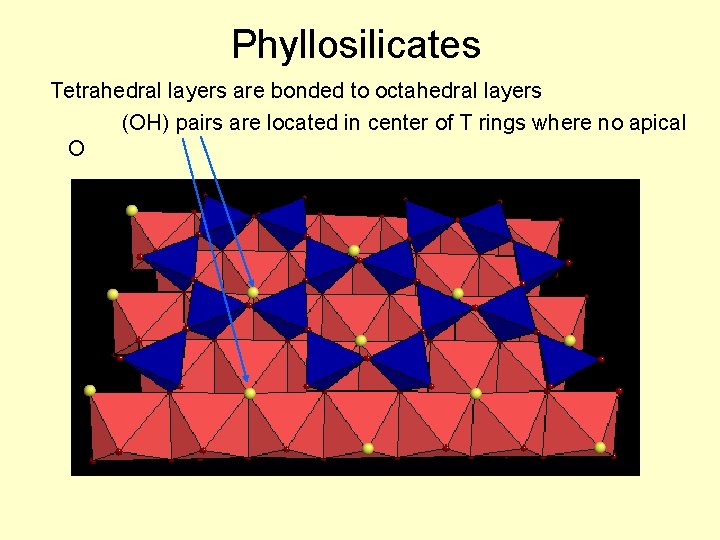
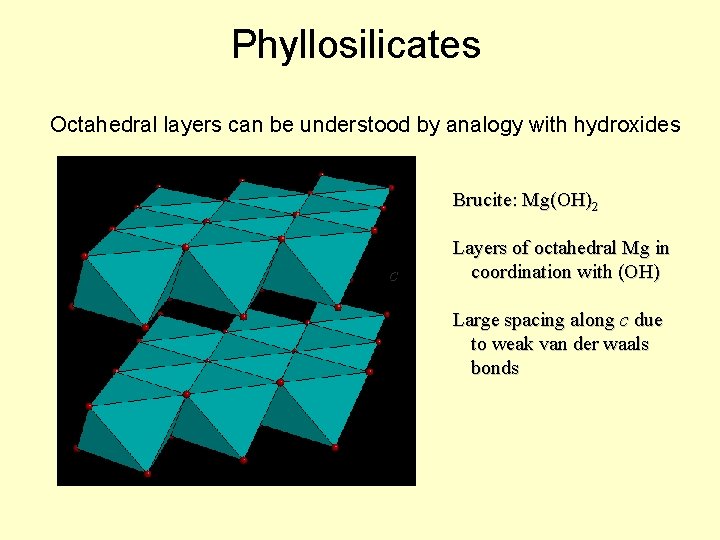
Phyllosilicates Octahedral layers can be understood by analogy with hydroxides Brucite: Mg(OH)2 c Layers of octahedral Mg in coordination with (OH) Large spacing along c due to weak van der waals bonds

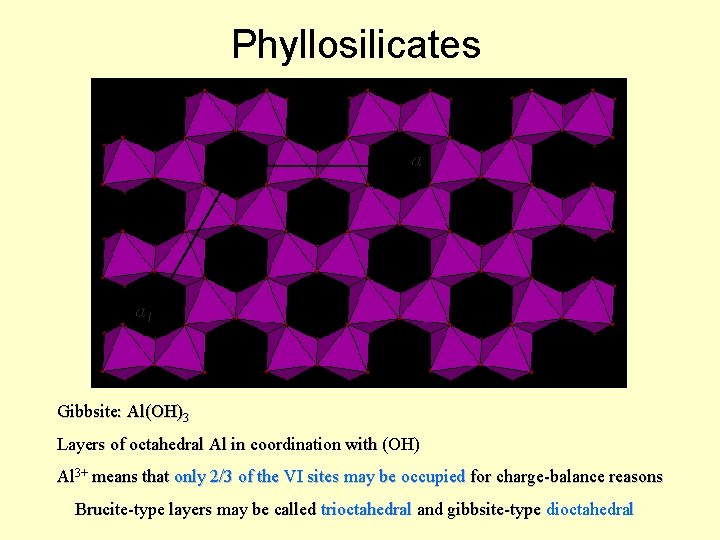
Phyllosilicates a 2 a 1 Gibbsite: Al(OH)3 Layers of octahedral Al in coordination with (OH) Al 3+ means that only 2/3 of the VI sites may be occupied for charge-balance reasons Brucite-type layers may be called trioctahedral and gibbsite-type dioctahedral

![Phyllosilicates Yellow OH Kaolinite Al 2 Si 2 O 5 OH4 Tlayers and Phyllosilicates Yellow = (OH) Kaolinite: Al 2 [Si 2 O 5] (OH)4 T-layers and](https://slidetodoc.com/presentation_image_h/9fe6827b0154a0343de1711335b010b3/image-13.jpg)
Phyllosilicates Yellow = (OH) Kaolinite: Al 2 [Si 2 O 5] (OH)4 T-layers and diocathedral (Al 3+) layers (OH) at center of T-rings and fill base of VI layer weak van der Waals bonds between T-O groups T O T O vdw

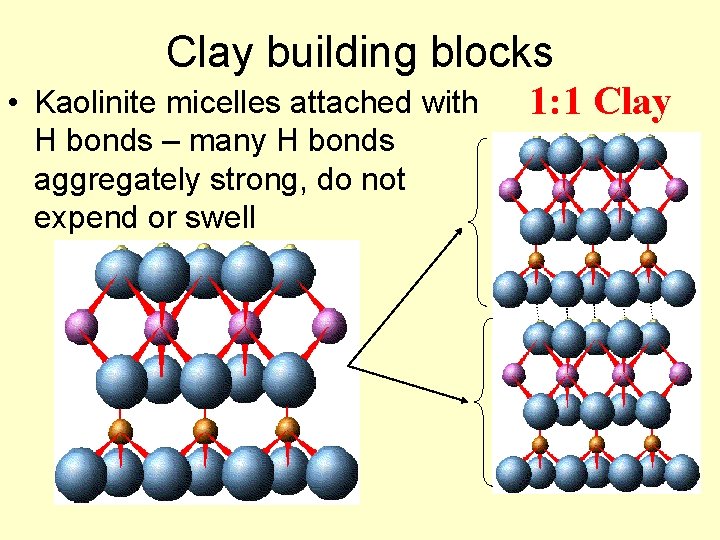
Clay building blocks • Kaolinite micelles attached with H bonds – many H bonds aggregately strong, do not expend or swell 1: 1 Clay
![Phyllosilicates Yellow OH Serpentine Mg 3 Si 2 O 5 OH4 Tlayers and Phyllosilicates Yellow = (OH) Serpentine: Mg 3 [Si 2 O 5] (OH)4 T-layers and](https://slidetodoc.com/presentation_image_h/9fe6827b0154a0343de1711335b010b3/image-15.jpg)
Phyllosilicates Yellow = (OH) Serpentine: Mg 3 [Si 2 O 5] (OH)4 T-layers and triocathedral (Mg 2+) layers (OH) at center of T-rings and fill base of VI layer weak van der Waals bonds between T-O groups T O T O vdw

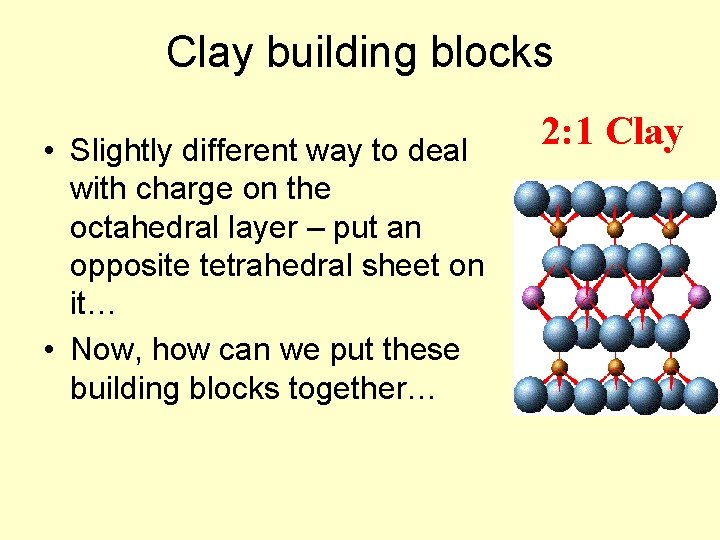
Clay building blocks • Slightly different way to deal with charge on the octahedral layer – put an opposite tetrahedral sheet on it… • Now, how can we put these building blocks together… 2: 1 Clay
![Phyllosilicates Yellow OH Pyrophyllite Al 2 Si 4 O 10 OH2 Tlayer Phyllosilicates Yellow = (OH) Pyrophyllite: Al 2 [Si 4 O 10] (OH)2 T-layer -](https://slidetodoc.com/presentation_image_h/9fe6827b0154a0343de1711335b010b3/image-17.jpg)
Phyllosilicates Yellow = (OH) Pyrophyllite: Al 2 [Si 4 O 10] (OH)2 T-layer - diocathedral (Al 3+) layer - T-layer weak van der Waals bonds between T - O - T groups T O T vdw
![Phyllosilicates Yellow OH Talc Mg 3 Si 4 O 10 OH2 Tlayer Phyllosilicates Yellow = (OH) Talc: Mg 3 [Si 4 O 10] (OH)2 T-layer -](https://slidetodoc.com/presentation_image_h/9fe6827b0154a0343de1711335b010b3/image-18.jpg)
Phyllosilicates Yellow = (OH) Talc: Mg 3 [Si 4 O 10] (OH)2 T-layer - triocathedral (Mg 2+) layer - T-layer weak van der Waals bonds between T - O - T groups T O T vdw
![Phyllosilicates Muscovite K Al 2 Si 3 Al O 10 OH2 coupled K Phyllosilicates Muscovite: K Al 2 [Si 3 Al. O 10] (OH)2 (coupled K -](https://slidetodoc.com/presentation_image_h/9fe6827b0154a0343de1711335b010b3/image-19.jpg)
Phyllosilicates Muscovite: K Al 2 [Si 3 Al. O 10] (OH)2 (coupled K - Al. IV) T-layer - diocathedral (Al 3+) layer - T-layer - K K between T - O - T groups is stronger than vdw T O T K T O T
![Phyllosilicates Phlogopite K Mg 3 Si 3 Al O 10 OH2 Tlayer triocathedral Phyllosilicates Phlogopite: K Mg 3 [Si 3 Al. O 10] (OH)2 T-layer - triocathedral](https://slidetodoc.com/presentation_image_h/9fe6827b0154a0343de1711335b010b3/image-20.jpg)
Phyllosilicates Phlogopite: K Mg 3 [Si 3 Al. O 10] (OH)2 T-layer - triocathedral (Mg 2+) layer - T-layer - K K between T - O - T groups is stronger than vdw T O T K T O T

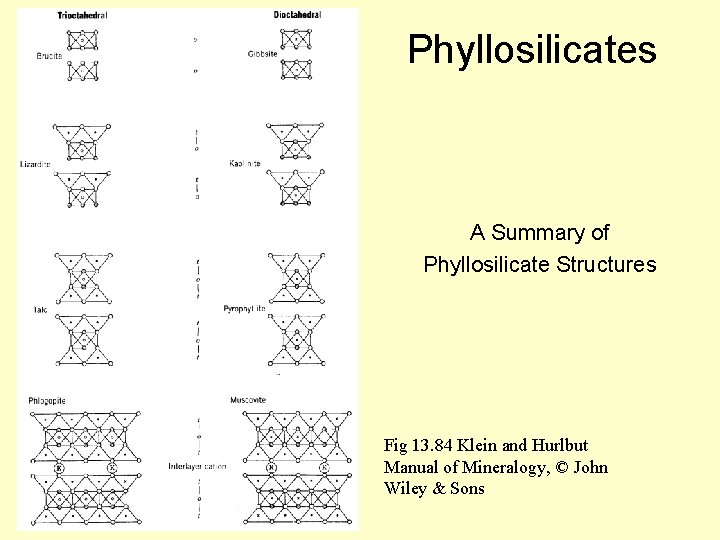
Phyllosilicates A Summary of Phyllosilicate Structures Fig 13. 84 Klein and Hurlbut Manual of Mineralogy, © John Wiley & Sons

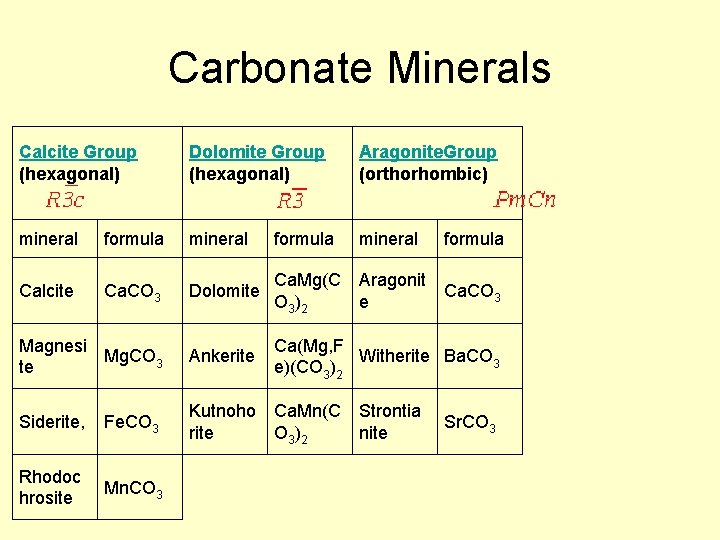
Carbonate Minerals Calcite Group (hexagonal) Dolomite Group (hexagonal) Aragonite. Group (orthorhombic) mineral formula Calcite Ca. CO 3 Dolomite Ca. Mg(C O 3 )2 Aragonit e Ca. CO 3 Magnesi Mg. CO 3 te Ankerite Ca(Mg, F Witherite Ba. CO 3 e)(CO 3)2 Siderite, Fe. CO 3 Kutnoho Ca. Mn(C rite O 3 )2 Rhodoc hrosite Mn. CO 3 Strontia nite Sr. CO 3

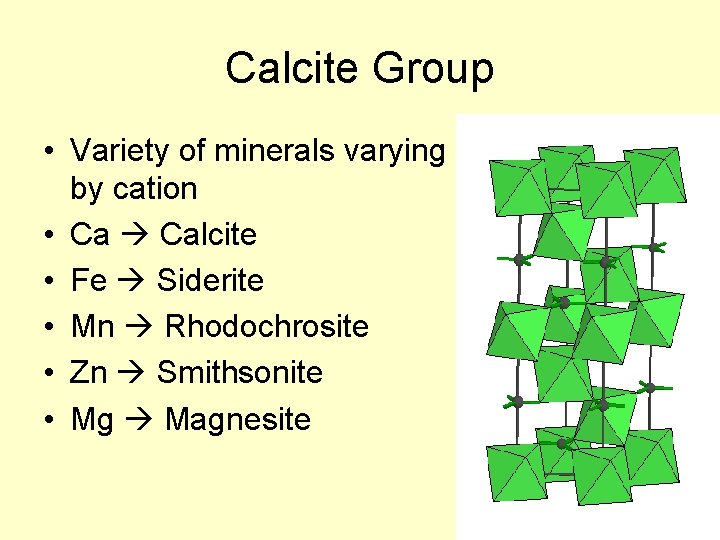
Calcite Group • Variety of minerals varying by cation • Ca Calcite • Fe Siderite • Mn Rhodochrosite • Zn Smithsonite • Mg Magnesite

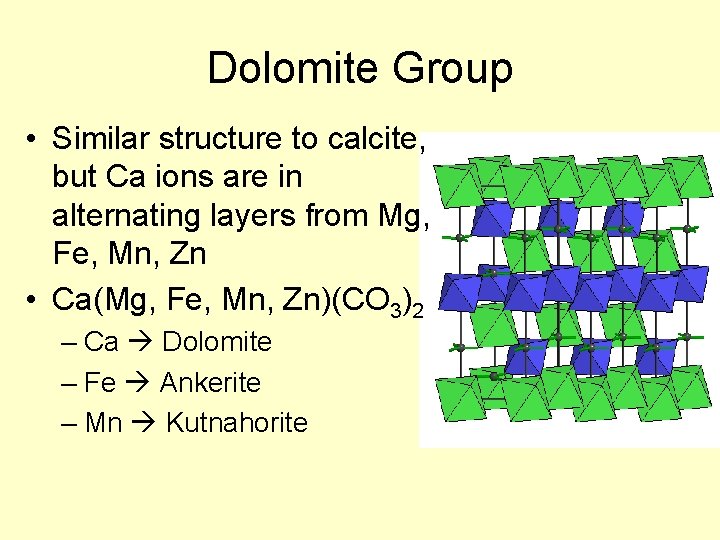
Dolomite Group • Similar structure to calcite, but Ca ions are in alternating layers from Mg, Fe, Mn, Zn • Ca(Mg, Fe, Mn, Zn)(CO 3)2 – Ca Dolomite – Fe Ankerite – Mn Kutnahorite

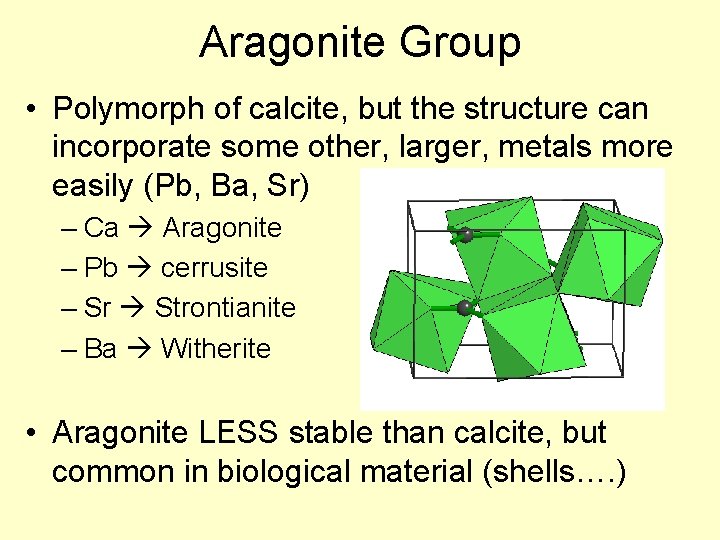
Aragonite Group • Polymorph of calcite, but the structure can incorporate some other, larger, metals more easily (Pb, Ba, Sr) – Ca Aragonite – Pb cerrusite – Sr Strontianite – Ba Witherite • Aragonite LESS stable than calcite, but common in biological material (shells…. )

Calcite vs. Dolomite • dolomite less reactive with HCl calcite has lower indices of refraction • calcite more commonly twinned • dolomite more commonly euhedral • calcite commonly colourless • dolomite may be cloudy or stained by iron oxide • Mg spectroscopic techniques! • Different symmetry cleavage same, but easily distinguished by XRD