Chapter 17 Classification of Matter Granite Section 1









- Slides: 14

Chapter 17 Classification of Matter Granite

Section 1 Composition of Matter • Substance: Either an element or a compound. They can be broken down into simpler components and still maintain the properties of the original substance • Exp. helium, aluminum, water, salt • Elements: All the atoms in a substance are alike • ONE KIND OF ATOM • Examples: graphite=all carbon atoms • outside of a copper=all copper atoms • inside of a penny = all zinc atoms • 90 elements are found in nature… 20 others have been manmade

• Compounds: a substance in which the atoms of two or more elements are combined in a fixed proportion. TWO OR MORE KINDS OF ATOMS Example: water = two hydrogen atoms and one oxygen salt= sodium and chlorine Mixtures: made up of two or more substances that can be easily separated by physical means Example: pizza and soft drinks

Mixtures • Heterogeneous: a mixture in which different materials can be distinguished easily by the eye or microscope. • UNEVENLY MIXED • Example: granite, concrete and dry soup mixes, shirts that are polyester and cotton

• Homogeneous mixtures: contain two or more gaseous, liquid, or solid substances blended evenly throughout. • EVENLY MIXED Solution • Example: soft drinks, vinegar • Solution: a homogeneous mixture of particles so small that they cannot be seen with a microscope and will never settle to the bottom of their container.

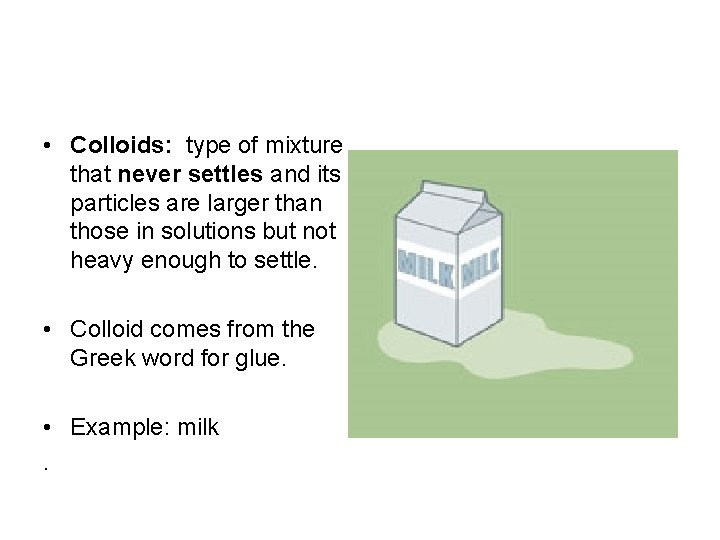
• Colloids: type of mixture that never settles and its particles are larger than those in solutions but not heavy enough to settle. • Colloid comes from the Greek word for glue. • Example: milk.

• Appearance distinguishes colloid from a solution • Tyndall Effect: the scattering of light by colloidal particles • This occurs because the particles in a colloid are large enough to scatter light, but those in the solution are not. • Colloids of liquids in liquids are emulsions • Colloids of solids in liquids are sols (when cooled=gel) • Colloids of liquids or solids in gases are called aerosols

• Suspensions: some mixtures are neither solutions nor colloids • Example: muddy pond water • If the pond water stands long enough, some mud particles will fall to the bottom, and the water clears. It is a heterogeneous mixture containing a liquid in which visible particles settle. • River delta’s are a large scale examples of how a suspension settles.

Section 2 Properties of Matter • Physical Properties: • 1. Appearance= shape, color, and state of matter, size • 2. Behavior=magnetic or high or low viscosity • Physical properties can be used to separate substances in a mixture. • Exp. - Magnetism separates iron from sand

Physical Change • A change in size, shape, or state of matter is called a physical change. • These changes may involve energy changes, but the kind of substance-the ID of the element or compounddoes not change. • Exp. Gum-tear in half…still tastes and chews the same. • Exp. Copper-heated glows red and then white but it is still copper • You can use physical changes to separate substances: sea water can be purified to fresh water by distillation. • Distillation=process for separating substances in a mixture by evaporating a liquid and re-condensing its vapor. • Two liquids with different boiling points can be separated by distillation. • Exp. - Natural oils like mint

Chemical Properties and Changes • Since burning produces new substances during a chemical change, flammability is an example of chemical change. • Chemical property=a characteristic of a substance that indicates whether it can undergo a certain chemical change. • Exp. – brown medicine bottles because the drug may react to light.

Detecting a Chemical Change • Chemical change- a change of one substance to another. • Exp. - chili cooking unattended= smell burnt odor • -smell of rotten eggs or the formation of rust are signs of a chemical change • -tarnished silver is also a sign of a chemical change • Using chemical change to separate: to remove tarnish from silver =place silver pot in a cooking pot of water with some aluminum foil in it and then heat the pot of water. A chemical reaction occurs and removes the tarnish. Tarnish is silver sulfide formed from sulfur compounds in the air! • This type of chemical separation is more common in factories.

Weathering- Chemical or Physical Change? • Physical= large rocks can split when water seeps into small cracks, freezes, and expands. • Chemical=limestone does not dissolve easily in water except when the water is acidic, then the calcium carbonate reacts and changes to a new substance calcium hydrogen carbonate, which does dissolve in water. • Exp. –White Cliffs of Dover lining the English Channel.

Conservation of Mass • • Burning wood is example of chemical change. Log does look different=ash Masses are different (log vs. ash) But if collected all oxygen and smoke and gases that escaped from the burning log and measure their masses too…you would find that the masses were equal and thus no mass was lost after all. • Law of conservation of mass: the mass of all substances that are present before a chemical change = the mass of all the substances that remain after the change.