Welcome to Jeopardy Round 1 Category 2 Category





































































- Slides: 115

Welcome to Jeopardy

Round 1 Category 2 Category 3 Category 4 Category 5 100 100 100 200 200 200 300 300 300 400 400 400 500 500 500

Category 1 100 • Which field of science studies the composition of matter?

Category 1 200 • Which state of matter takes both the shape and volume of its container?

Category 1 300 • Are homogenous mixtures also known as solutions? Yes or No

Category 1 400 • What happens to matter during a chemical reaction?

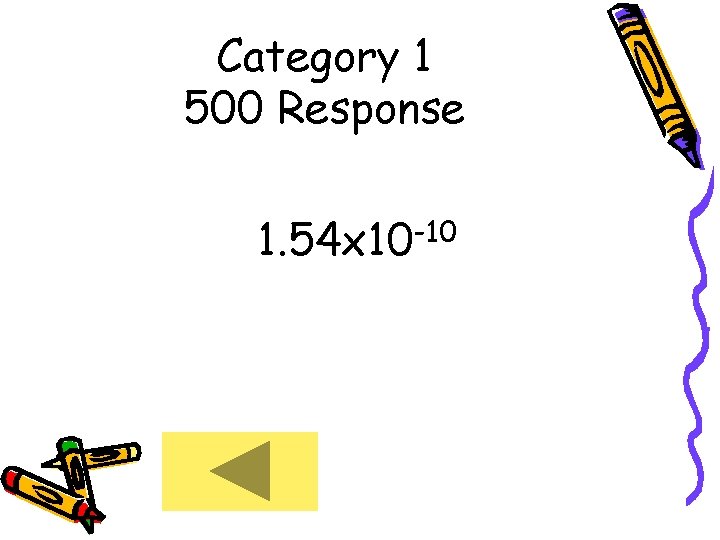
Category 1 500 • Express the following in scientific notation: 0. 00000154 m

Category 2 100 • How many significant figures are in the measurement 721. 30 grams?

Category 2 200 • What is the standard international unit for mass?

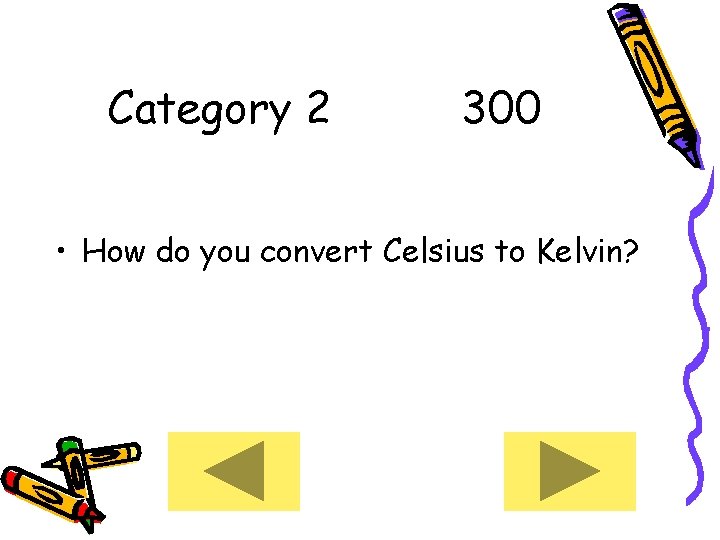
Category 2 300 • How do you convert Celsius to Kelvin?

Category 2 400 • What is the formula for density?

DAILY DOUBLE # 1!!! • Who was the philosopher that first suggested the concept of atoms?

Make a Wager

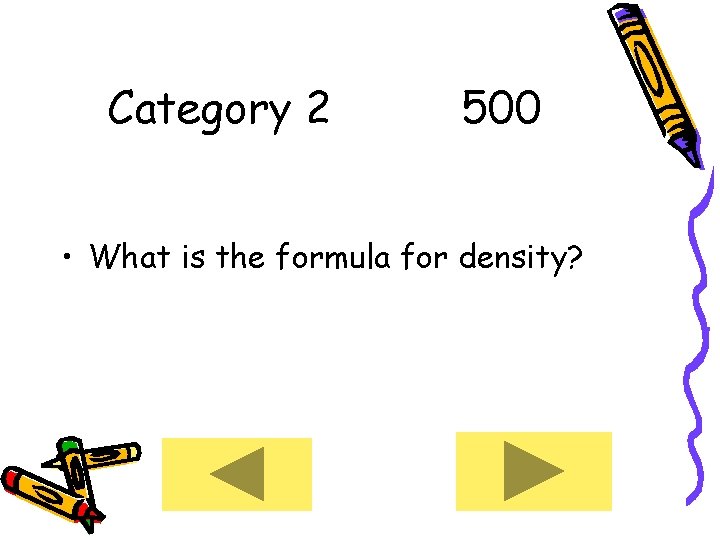
Category 2 500 • What is the formula for density?

Category 3 100 • What is the charge on an atom?

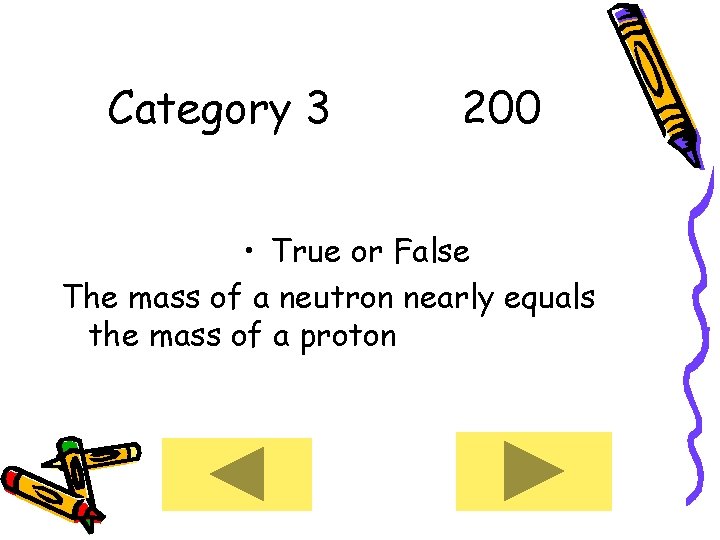
Category 3 200 • True or False The mass of a neutron nearly equals the mass of a proton

Category 3 300 • The Atomic number of an element represents which part of an atom

Category 3 400 • The atomic mass number equals the number of protons plus what?

Category 3 500 • What unit is used to measure atomic mass?

Category 4 100 How many electrons does He have?

Category 4 300 • How many electrons are needed to fill the d sublevel?

Category 4 400 • What is the maximum # of d orbitals in a principle energy level?

Category 4 500 • If one electron spins clockwise in an orbital, how does a second electron in that orbital spin?

Category 5 100 • What are quanta of light called?

Category 5 200 • Who developed the Quantum Mechanical Model of the atom?

Category 5 300 • What element has the electron configuration: 1 s 22 p 63 s 23 p 6

Category 5 400 • True or False An element is likely to be inactive if it’s highest energy level is full.

Category 5 500 • Will the metals in Groups 1, 2, & 3 gain or lose electrons to form ions?

Category 1 100 Response • Chemistry

Category 1 200 Response • Gas

Category 1 300 Response • YES

Category 1 400 Response • Matter is neither created or destroyed

Category 1 500 Response 1. 54 x 10 -10

Category 2 100 Response 5

Category 2 200 Response • kilogram

Category 2 300 Response • Celcius + 273 = Kelvin

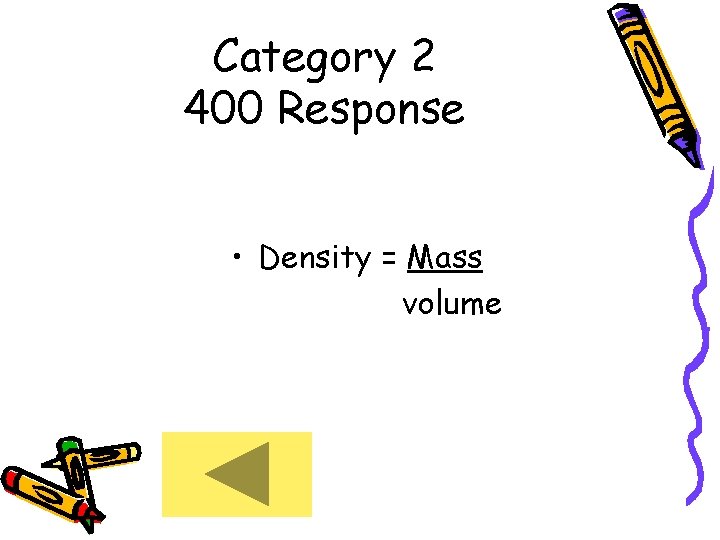
Category 2 400 Response • Density = Mass volume

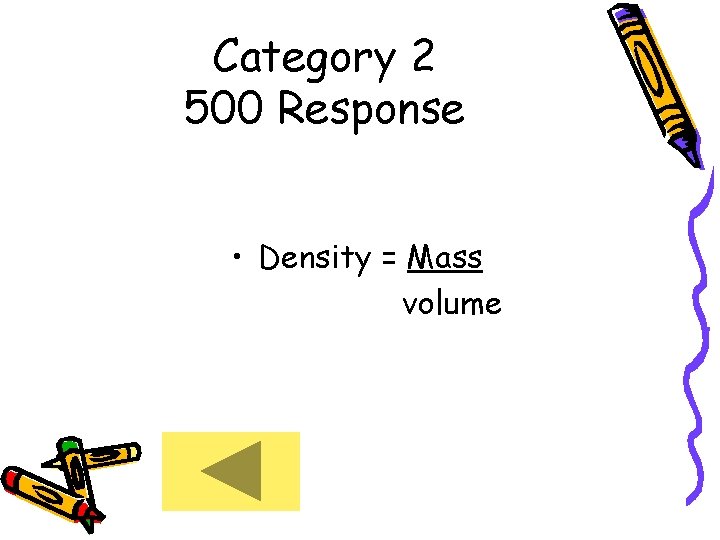
Category 2 500 Response • Density = Mass volume

DAILY DOUBLE # 1 Response • Democritus

Category 3 100 Response • Neutral

Category 3 200 Response • TRUE

Category 3 300 Response • The protons

Category 3 400 Response • The number of neutrons

Category 3 500 Response • amu

Category 4 100 Response 2

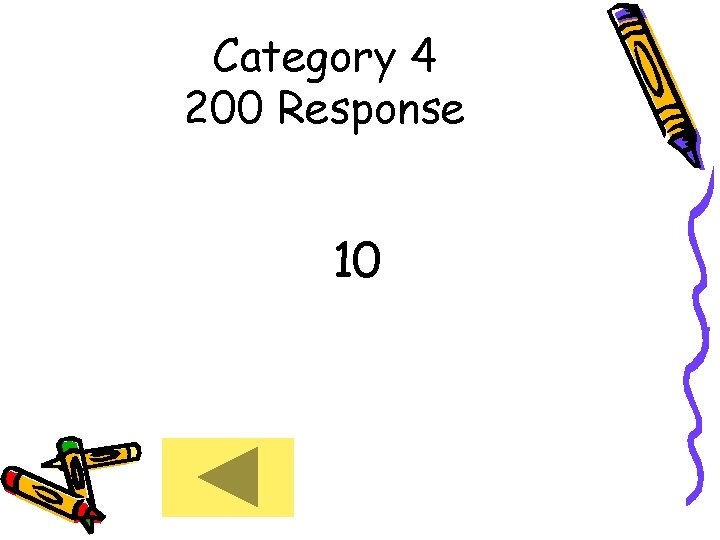
Category 4 200 Response 10

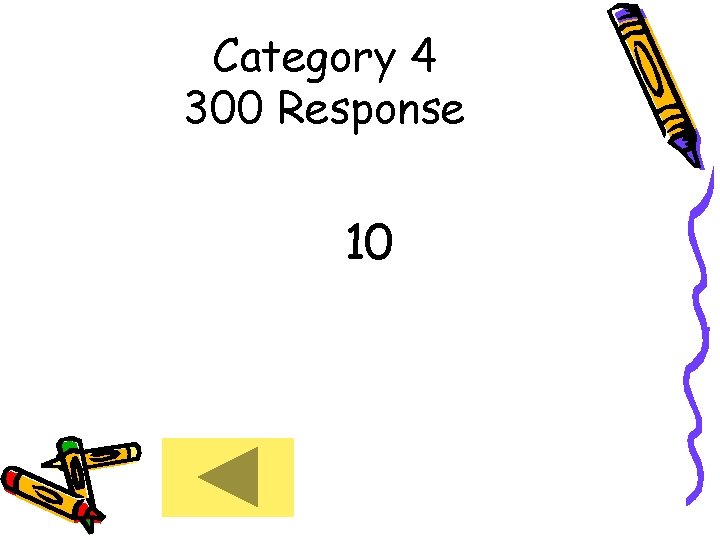
Category 4 300 Response 10

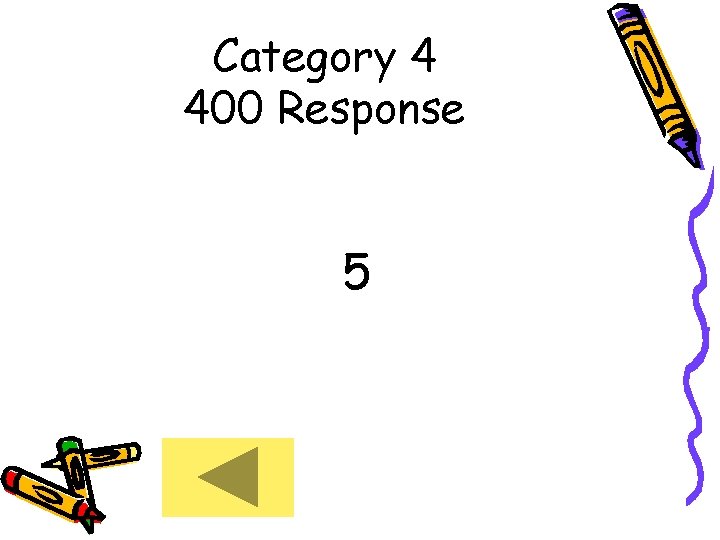
Category 4 400 Response 5

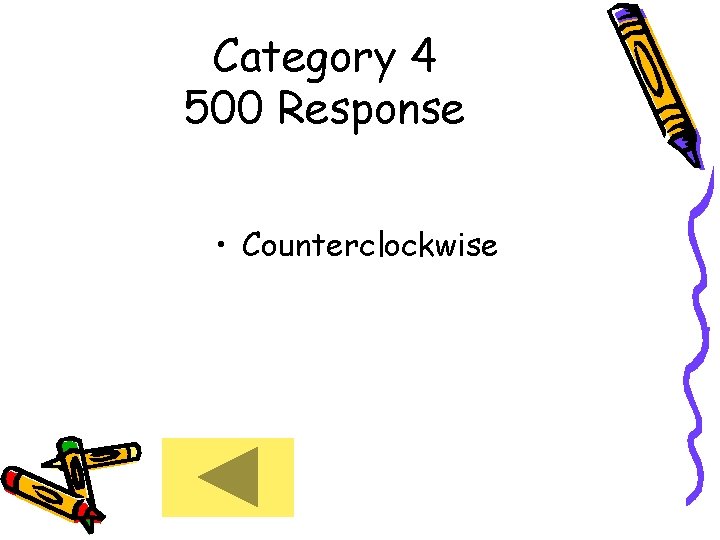
Category 4 500 Response • Counterclockwise

Category 5 100 Response • Photons

Category 5 200 Response • Schrodinger

Category 5 300 Response • Argon

Category 5 400 Response • TRUE

Category 5 500 Response • Lose electrons

Round 2 Category Category 6 7 8 9 10 200 200 200 400 400 400 600 600 600 800 800 800 1000 1000

Make a Wager

DAILY DOUBLE # 2!!! • Ionic compounds are normally in which physical state at room temperature?

DAILY DOUBLE # 2 Response solid

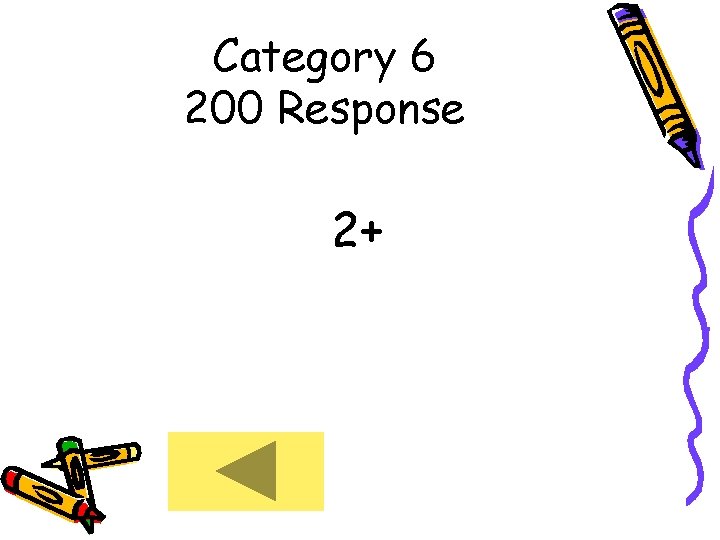
Category 6 200 • What is the charge on a Strontium ion?

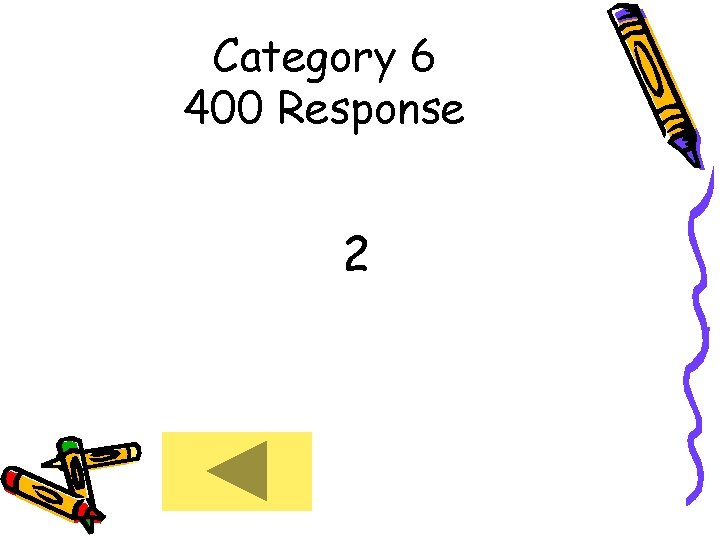
Category 6 400 • How many electrons does Barium give up to become more like a noble gas?

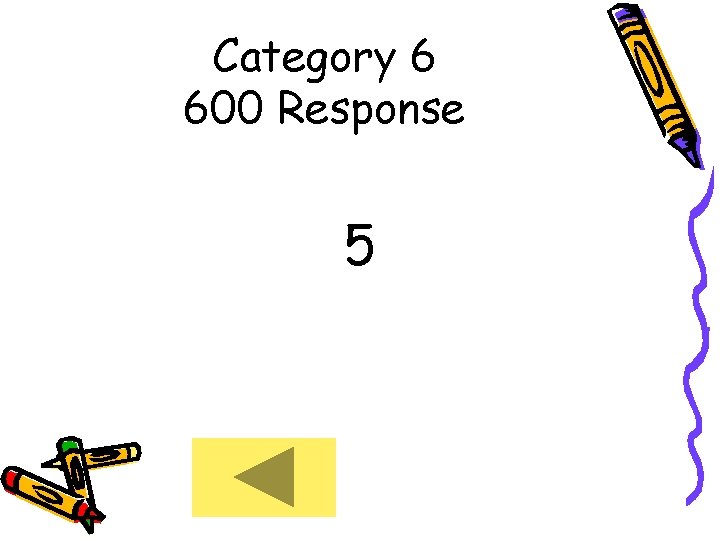
Category 6 600 • How many valance electrons does Phosphorus (P) have?

Category 6 800 • How many electrons does Nitrogen gain in order to be more like a noble gas?

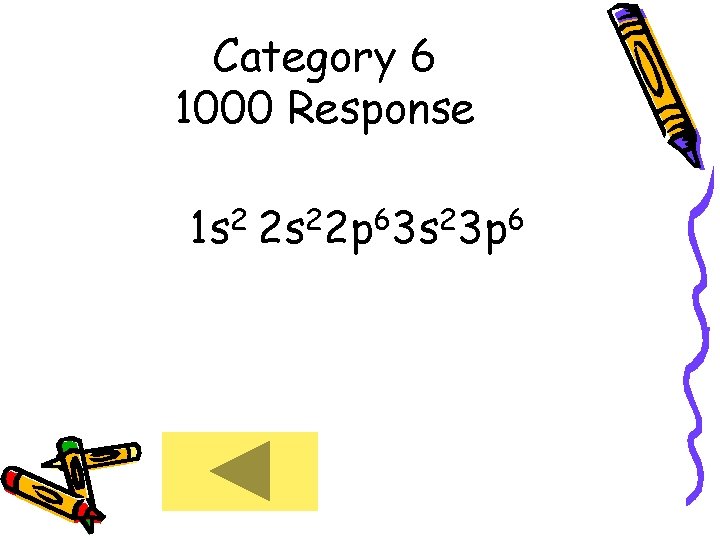
Category 6 1000 • What is the electron configuration of the calcium ion ?

Category 6 200 Response 2+

Category 6 400 Response 2

Category 6 600 Response 5

Category 6 800 Response 3

Category 6 1000 Response 1 s 2 2 s 22 p 63 s 23 p 6

Category 7 200 • Give an example of an alloy:

Category 7 400 • Ionic compounds are normally in which physical state at room temperature?

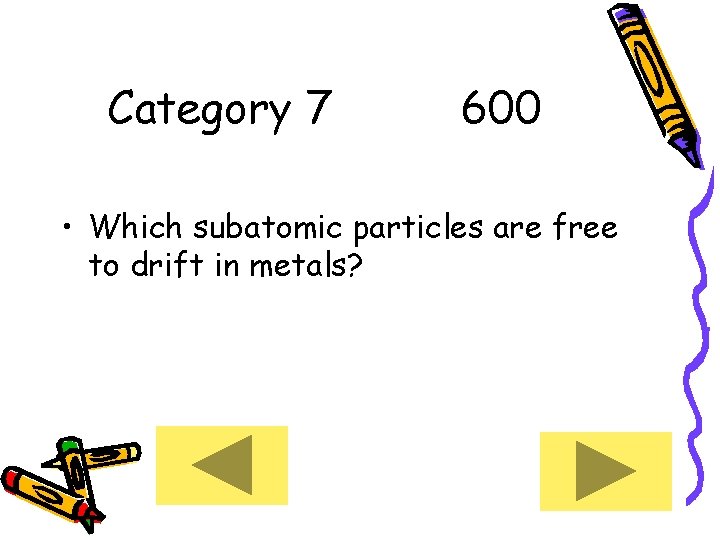
Category 7 600 • Which subatomic particles are free to drift in metals?

Category 7 800 • Why do atoms share electrons in covalent bonds?

Category 7 1000 • What type of ions have the ending ide ?

Category 7 200 Response • Brass, tin, bronze, etc.

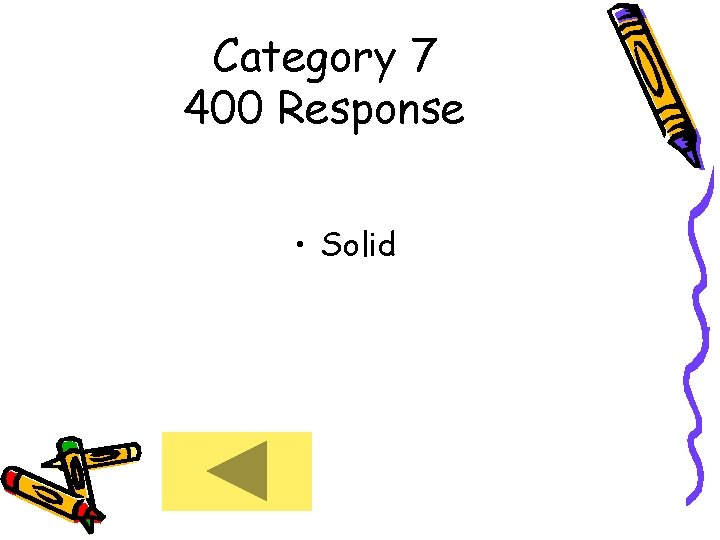
Category 7 400 Response • Solid

Category 7 600 Response • electrons

Category 7 800 Response • To attain a noble gas electron configuration.

Category 7 1000 Response • Anions

Category 8 200 • An –ate or –ite at the end of a compound name usually indicates the compound contains ?

Category 8 400 • In a combustion reaction between C 8 H 18 + O 2 what would the products be?

Category 8 600 • True or False: The kinetic theory states that particles of a gas move independently of each other, move rapidly and are relatively far apart.

Category 8 800 • What instrument is used to measure atmospheric pressure?

Category 8 1000 • What is the standard international unit for pressure and what is it at STP?

Category 8 200 Response • A polyatomic ion

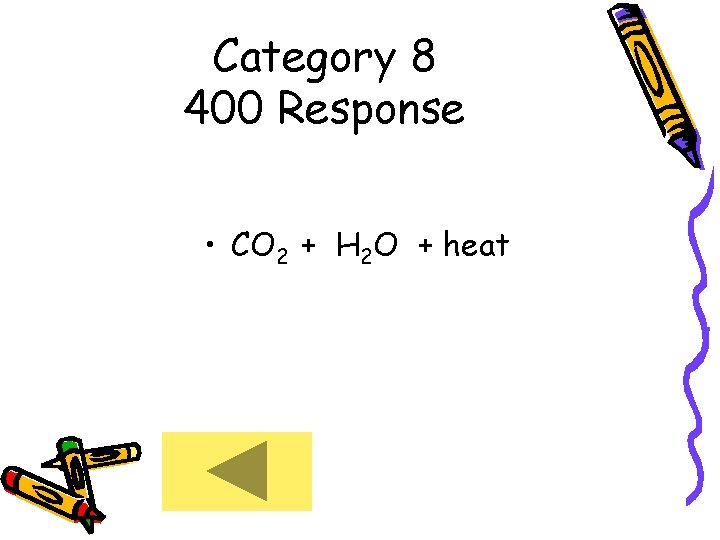
Category 8 400 Response • CO 2 + H 2 O + heat

Category 8 600 Response • True

Category 8 800 Response • A barometer

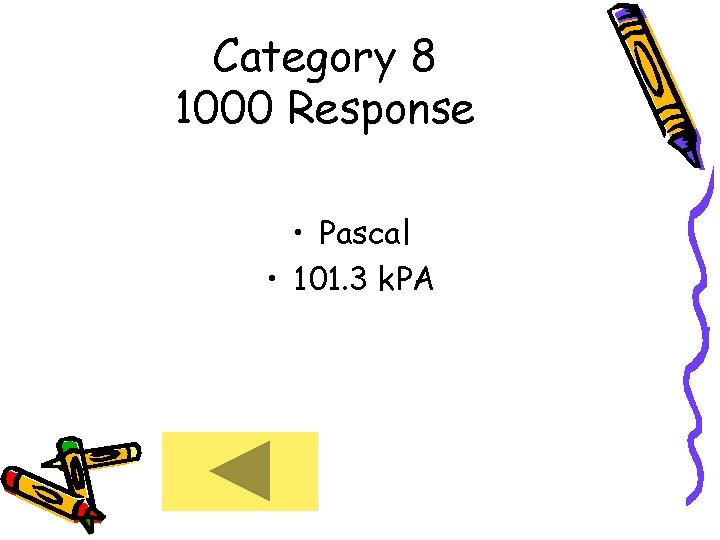
Category 8 1000 Response • Pascal • 101. 3 k. PA

Category 9 200 • True or False Most solids are not very dense and are easily compressed.

Category 9 400 • Why does air escape from a tire when the tire valve is opened?

Category 9 600 • How does the surface tension of water compare with the surface tension of other liquids?

Category 9 800 • The inward force which tends to minimize the surface area of a liquid is called?

Category 9 1000 • Will polar substances dissolve in nonpolar liquids?

Category 9 200 Response • False

Category 9 400 Response • The pressure outside the tire is lower than the pressure inside

Category 9 600 Response • It is higher

Category 9 800 Response • Surface Tension

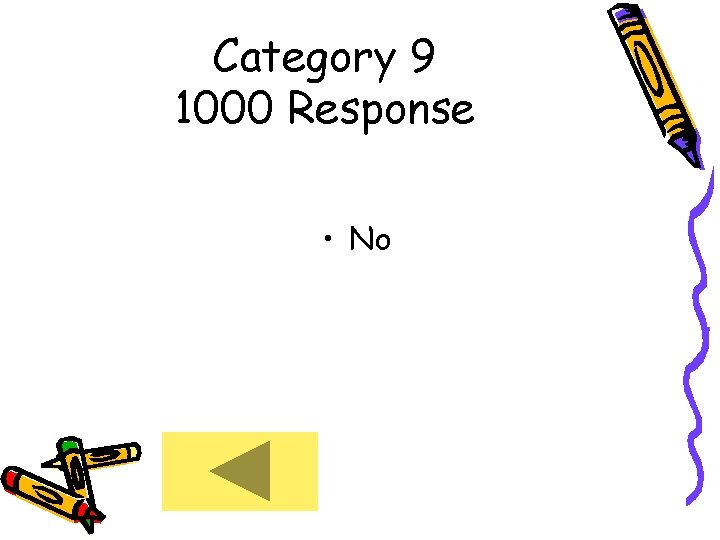
Category 9 1000 Response • No

Category 10 200 • What type of compound is always an electrolyte? , a ionic compound, a polar compound or a nonpolar compound?

Category 10 • An acid + a base yields ? 400

Category 10 600 • Name 2 properties of bases:

Category 10 800 • True or False: An Arrhenius acid is a substance that ionizes to yield protons (H+) in an aqueous solution

Category 10 1000 • In a neutral solution, the (H+) is equal to what other ion?

Category 10 200 Response • An ionic compound

Category 10 400 Response • A salt + H 2 O

Category 10 600 Response • Bitter • Slippery • p. H > 7

Category 10 800 Response • True

Make a Wager

DAILY DOUBLE # 3!!! • True or False: An Arrhenius acid is a substance that ionizes to yield protons (H+) in an aqueous solution

DAILY DOUBLE # 3 Response • TRUE

Category 10 1000 Response • The (OH-) ion

Final Jeopardy Category 11 ?

Final Jeopardy Question • Will you pass your CP Chemistry Final?

Final Jeopardy Response • Yes, if you paid attention to this game and you study your study guide!

The End!