Vascular Closure Devices Zoltan G Turi M D










- Slides: 31

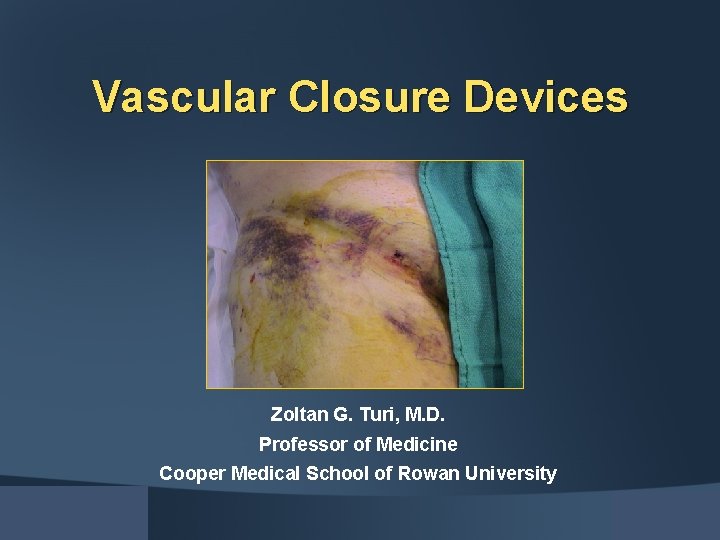
Vascular Closure Devices Zoltan G. Turi, M. D. Professor of Medicine Cooper Medical School of Rowan University

Zoltan G. Turi, MD § Contracted Research / Grant Support: § Abbott Vascular § Arstasis, Inc §Atritech §Cardiva §Marine Polymer Technologies, Inc. §St. Jude Medical, Inc. § Consulting Fees: § Boston Scientific Corporation § Cordis Corporation §St. Jude Medical

Categories of closure devices • • Anchored plugs Unanchored plugs Suture closure Clip/staple closure Topical patches “No footprint” devices Closure begins with access

Anchored Plugs Active Approximation Angio-Seal - Thrombosing agent High success rate, short learning curve, short deployment time Vascular occlusion, infection

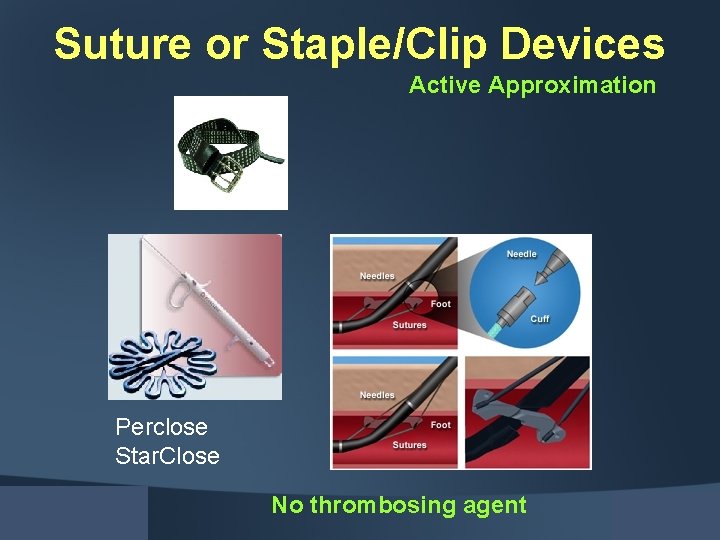
Suture or Staple/Clip Devices Active Approximation Perclose Star. Close No thrombosing agent

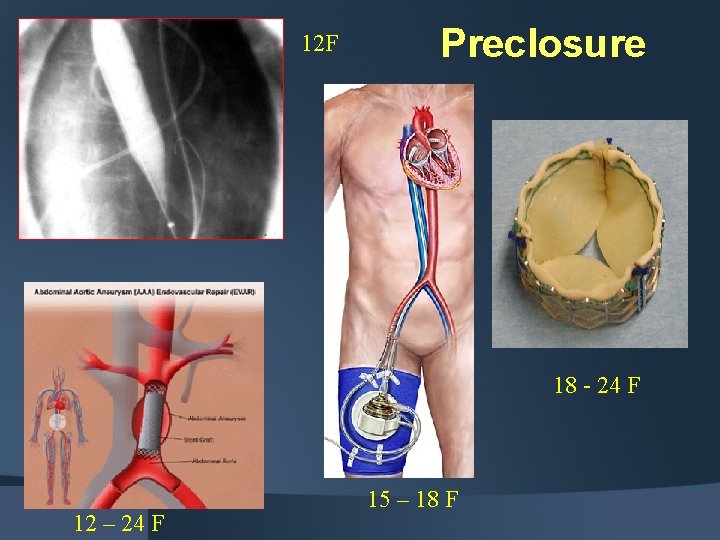
12 F Preclosure 18 - 24 F 12 – 24 F 15 – 18 F

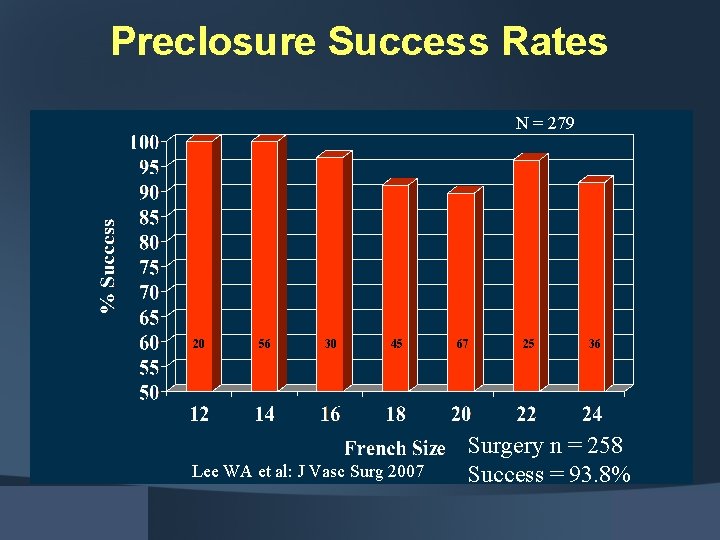
Preclosure Success Rates N = 279 20 56 30 45 Lee WA et al: J Vasc Surg 2007 67 25 36 Surgery n = 258 Success = 93. 8%

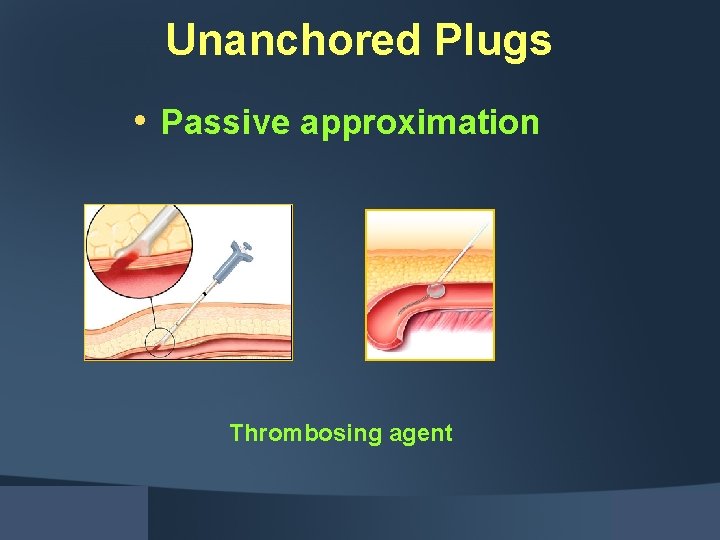
Unanchored Plugs • Passive approximation Thrombosing agent

Unanchored Plugs 1994 -2006 Vaso. Seal and Duett no longer marketed

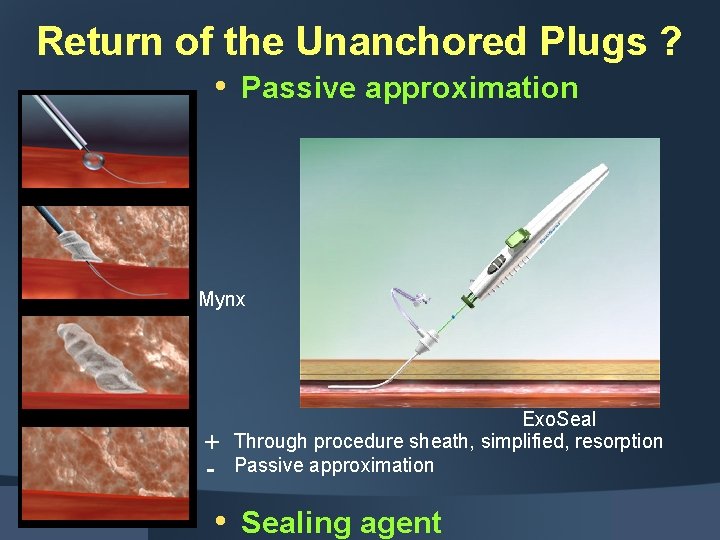
Return of the Unanchored Plugs ? • Passive approximation Mynx - Exo. Seal Through procedure sheath, simplified, resorption Passive approximation • Sealing agent

No footprint devices • Passive approximation Boomerang Closurewire/Catalyst • Mix of thrombosing materials no foreign body

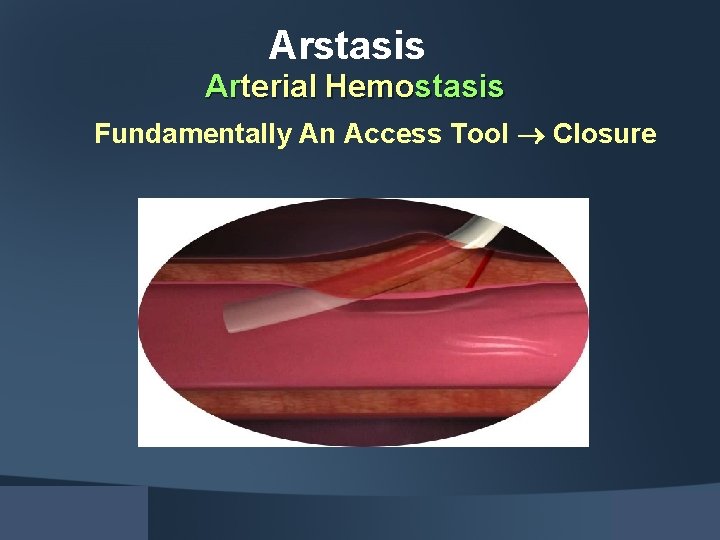
Start Closure with Access Arstasis

Arstasis Arterial Hemostasis Fundamentally An Access Tool Closure

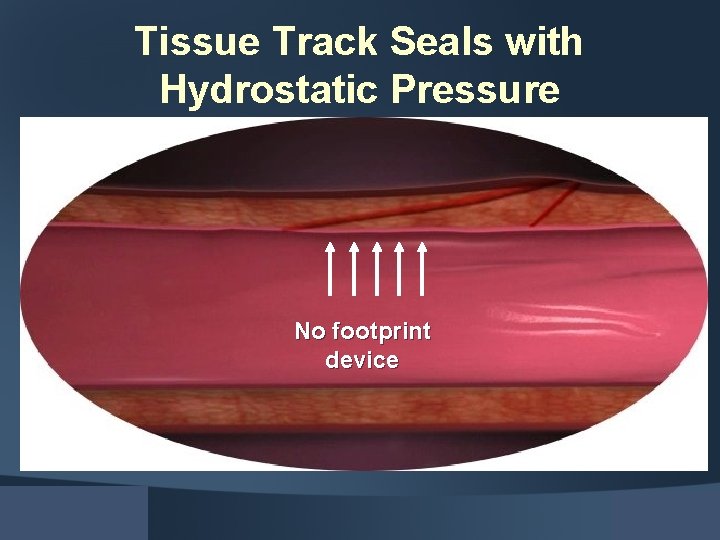
Tissue Track Seals with Hydrostatic Pressure No footprint device

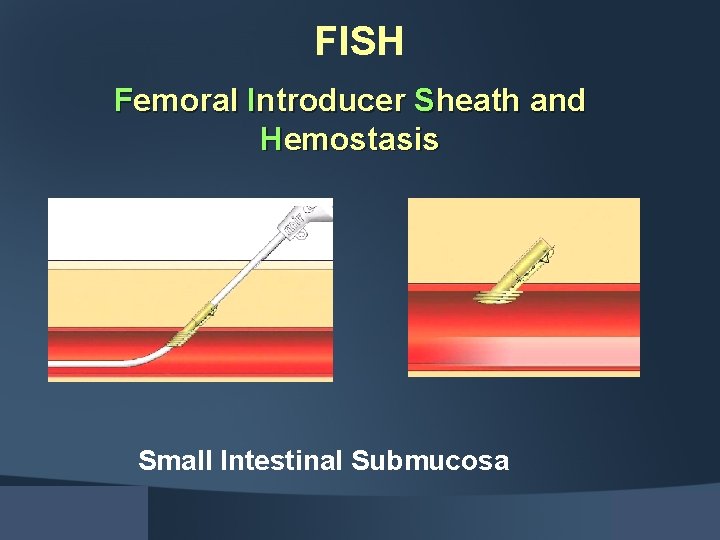
FISH Femoral Introducer Sheath and Hemostasis Small Intestinal Submucosa

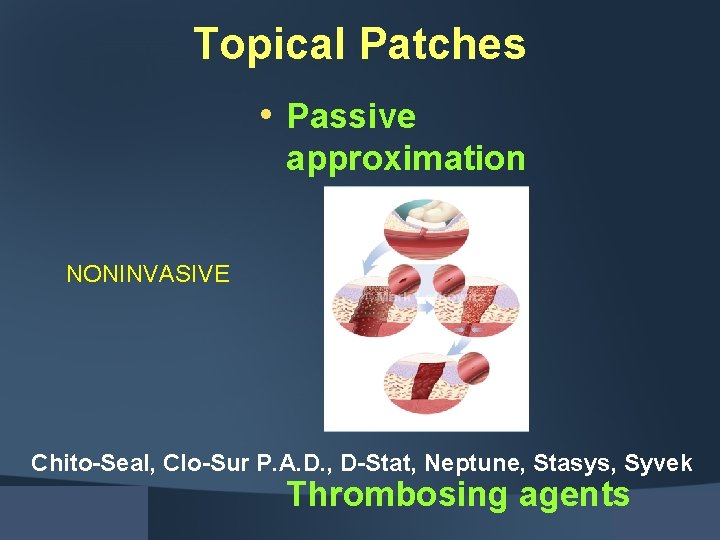
Topical Patches • Passive approximation NONINVASIVE Chito-Seal, Clo-Sur P. A. D. , D-Stat, Neptune, Stasys, Syvek Thrombosing agents

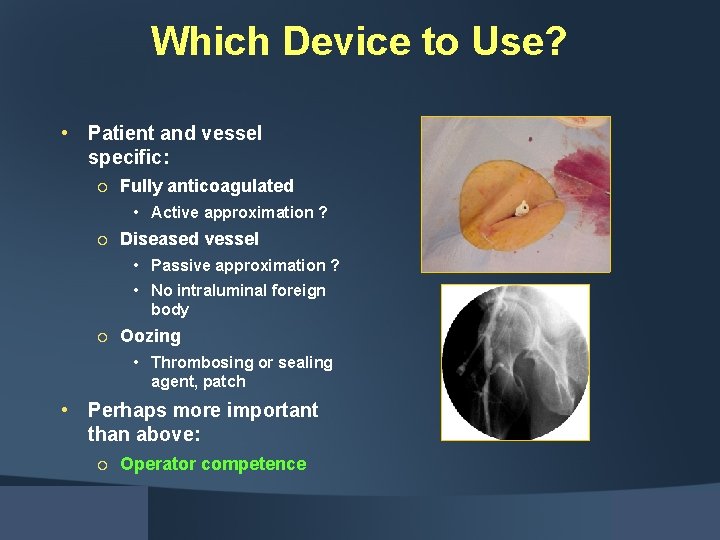
Which Device to Use? • Patient and vessel specific: ¡ Fully anticoagulated • Active approximation ? ¡ Diseased vessel • Passive approximation ? • No intraluminal foreign body ¡ Oozing • Thrombosing or sealing agent, patch • Perhaps more important than above: ¡ Operator competence

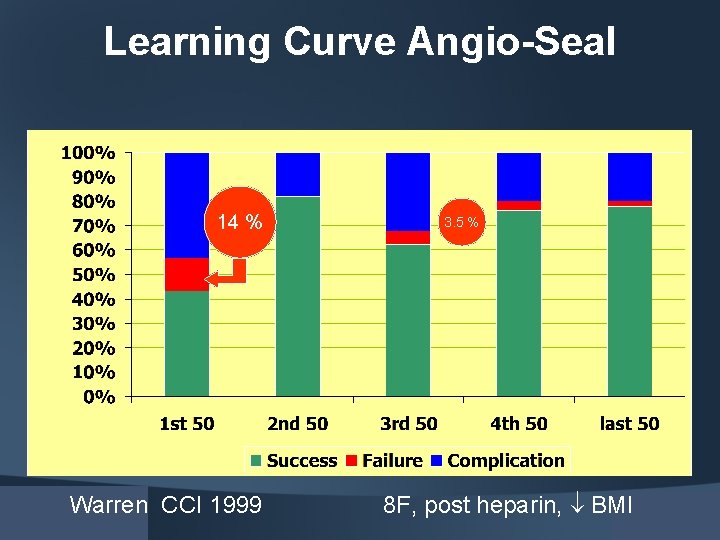
Learning Curve Angio-Seal 14 % Warren CCI 1999 3. 5 % 8 F, post heparin, BMI

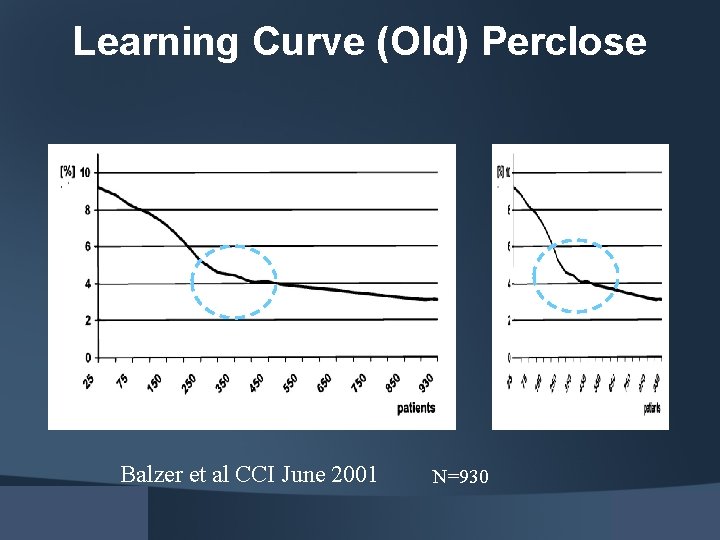
Learning Curve (Old) Perclose Balzer et al CCI June 2001 N=930

• Learning curve is steep • It does plateau • There is little valid evidence based comparison between devices

Moral of the Learning Curve • Learn one device and learn it well • Consider a second device for special situations • Keep in mind that manual compression is always an option

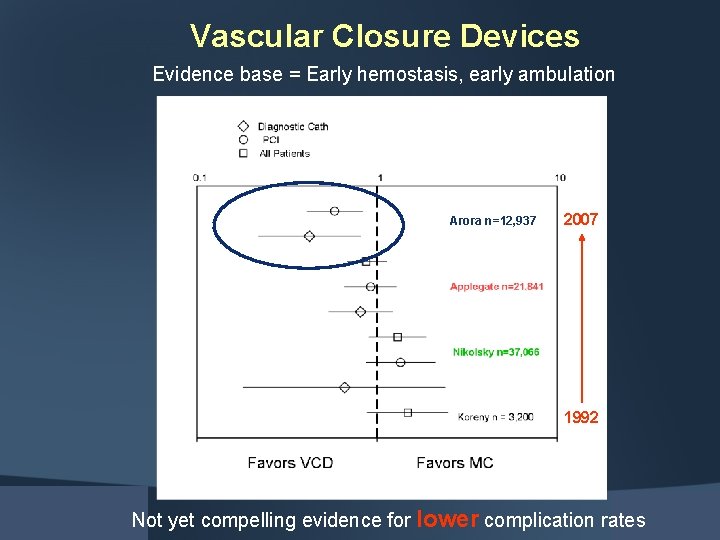
Vascular Closure Devices Evidence base = Early hemostasis, early ambulation Arora n=12, 937 2007 1992 Not yet compelling evidence for lower complication rates

Where I think twice • Diabetes, immune suppressed, poor hygiene • • Too thin, too fat < 5 F Low stick, high stick Significant vascular disease

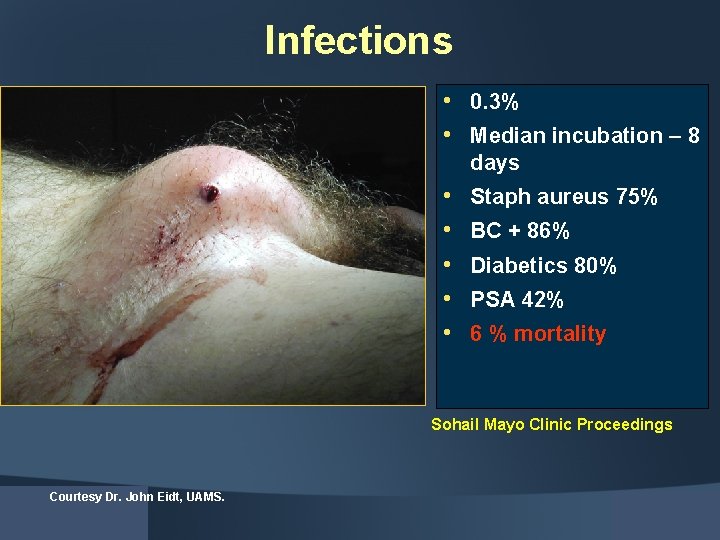
Infections • 0. 3% • Median incubation – 8 days • • • Staph aureus 75% BC + 86% Diabetics 80% PSA 42% 6 % mortality Sohail Mayo Clinic Proceedings Courtesy Dr. John Eidt, UAMS.

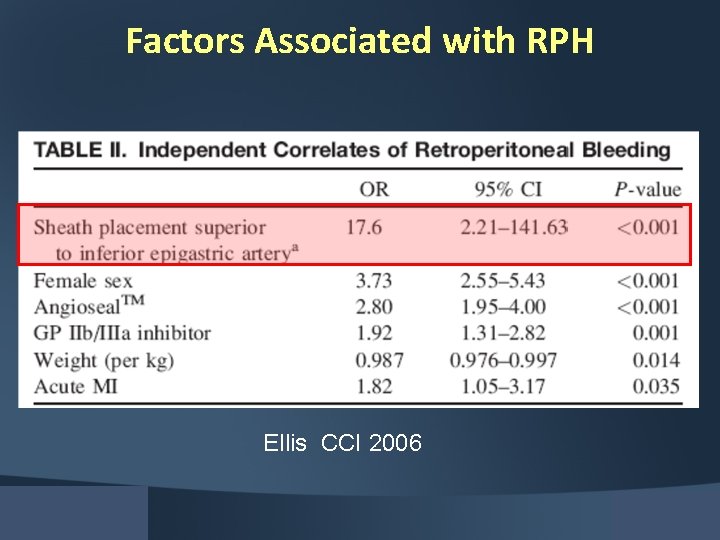
Factors Associated with RPH Ellis CCI 2006

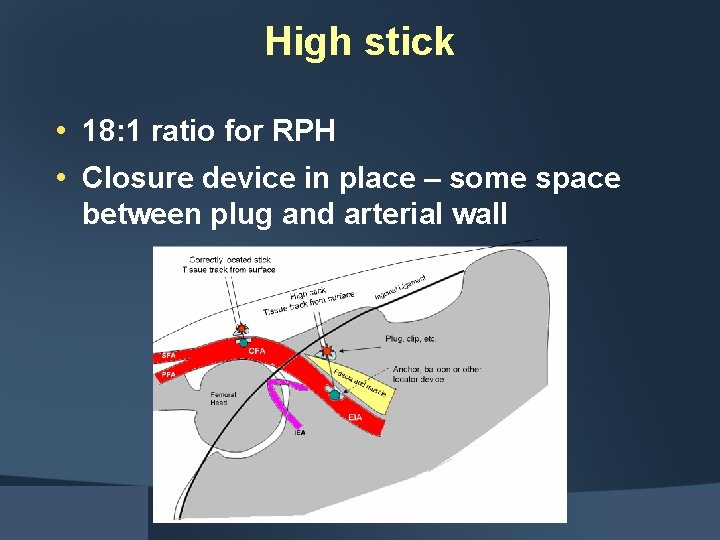
High stick • 18: 1 ratio for RPH • Closure device in place – some space between plug and arterial wall

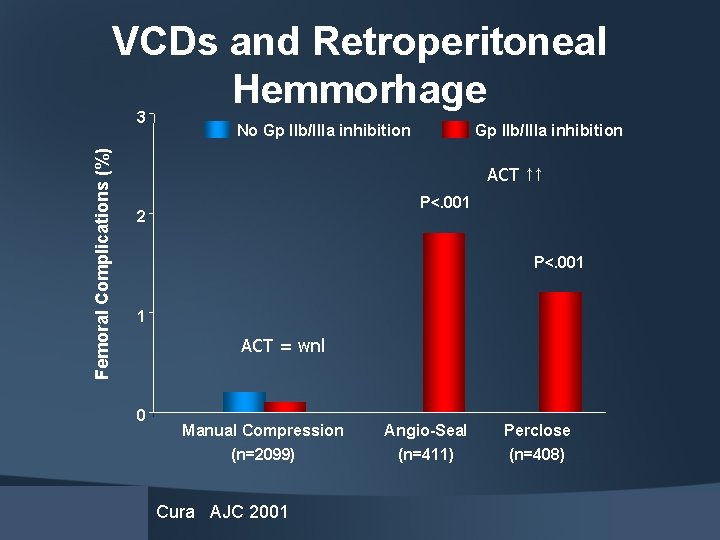
VCDs and Retroperitoneal Hemmorhage Femoral Complications (%) 3 No Gp IIb/IIIa inhibition ACT ↑↑ P<. 001 2 P<. 001 1 ACT = wnl 0 Manual Compression (n=2099) Cura AJC 2001 Angio-Seal (n=411) Perclose (n=408)

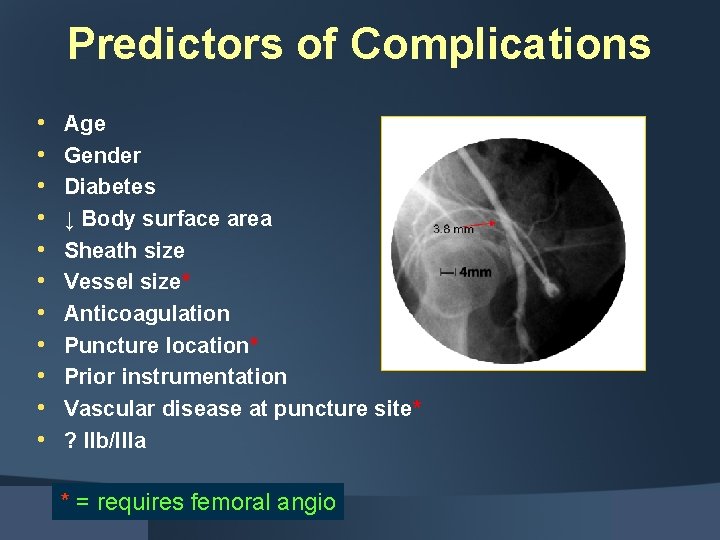
Predictors of Complications • • • Age Gender Diabetes ↓ Body surface area Sheath size Vessel size* Anticoagulation Puncture location* Prior instrumentation Vascular disease at puncture site* ? IIb/IIIa * = requires femoral angio


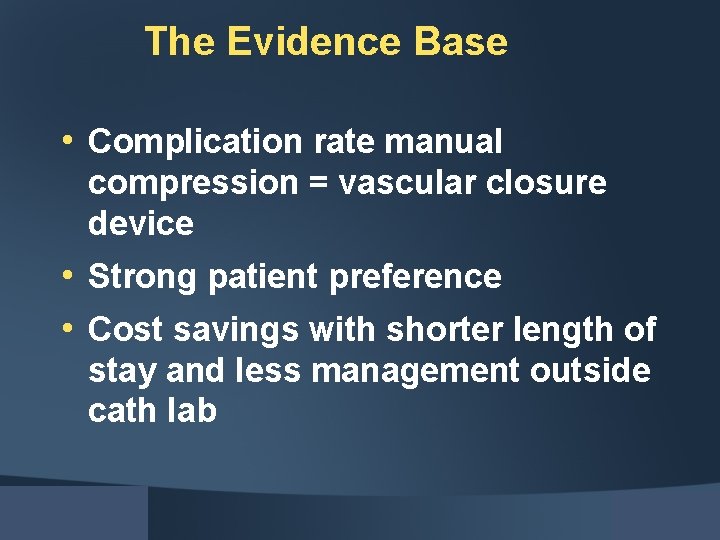
The Evidence Base • Complication rate manual compression = vascular closure device • Strong patient preference • Cost savings with shorter length of stay and less management outside cath lab

 Vascular and non vascular difference
Vascular and non vascular difference Vascular vs nonvascular plants
Vascular vs nonvascular plants Nonvascular plants
Nonvascular plants Kokia mažiausia dalelė turi nedalomą krūvį
Kokia mažiausia dalelė turi nedalomą krūvį Kokios energijos turi traukiamas automobilis
Kokios energijos turi traukiamas automobilis O'qituvchi faoliyatida pedagogik qobiliyat slayd
O'qituvchi faoliyatida pedagogik qobiliyat slayd Kasb tiplari
Kasb tiplari Turi honegger
Turi honegger Pedagogik qobiliyatning asosiy turlari
Pedagogik qobiliyatning asosiy turlari Kaip atrodo bukas kampas
Kaip atrodo bukas kampas Medžiagos tankis ppt
Medžiagos tankis ppt Komunikativ
Komunikativ Kiek tona turi centnerių
Kiek tona turi centnerių Talimni tashkil etishning yordamchi shakllari
Talimni tashkil etishning yordamchi shakllari Potencine energija formule
Potencine energija formule Oʻquvchi shaxsi tarbiyaning obyekti va subyekti sifatida
Oʻquvchi shaxsi tarbiyaning obyekti va subyekti sifatida Resta de donato giannini
Resta de donato giannini Didaktik qobiliyati nima
Didaktik qobiliyati nima Veiksmažodžio gramatiniai požymiai
Veiksmažodžio gramatiniai požymiai Yugurib kelib uzunlikka sakrash dars ishlanma
Yugurib kelib uzunlikka sakrash dars ishlanma Megametras
Megametras Ar visi medžiai turi lapus
Ar visi medžiai turi lapus Zoltan geler
Zoltan geler Dr. garamvölgyi zoltán
Dr. garamvölgyi zoltán Dr. aigner zoltán
Dr. aigner zoltán Dr lantos zoltán
Dr lantos zoltán Vaporline
Vaporline Dr lévai zoltán
Dr lévai zoltán Nagy zoltán sze
Nagy zoltán sze Polgár zoltán
Polgár zoltán Részei
Részei Zoltan geler
Zoltan geler