Vascular Closure Devices Plugs Zoltan G Turi M






- Slides: 23

Vascular Closure Devices: Plugs Zoltan G. Turi, M. D. Professor of Medicine Cooper Medical School of Rowan University

Zoltan G. Turi, MD Consulting: Cordis Corporation Grant Support: Abbott Vascular Arstasis, Inc. St. Jude Medical, Inc. Marine Polymer Technologies, Inc.

Categories of closure devices • • Anchored plugs Unanchored plugs Suture closure Clip/staple closure Topical patches “No footprint” devices Closure begins with access

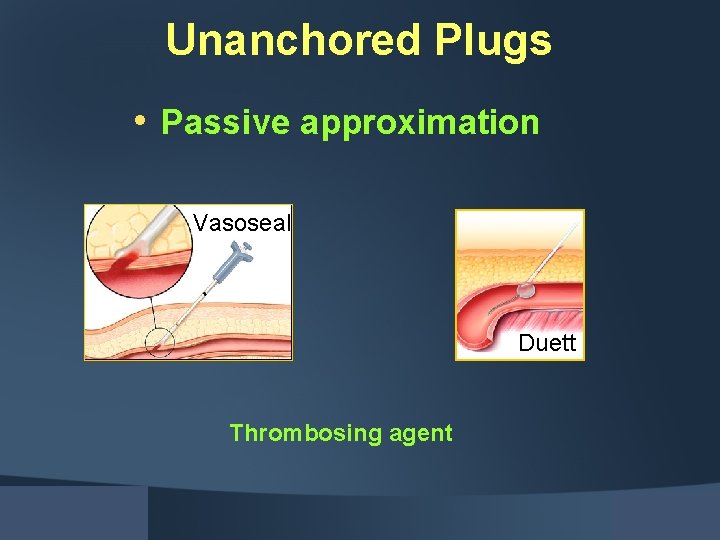
Unanchored Plugs • Passive approximation Vasoseal Duett Thrombosing agent

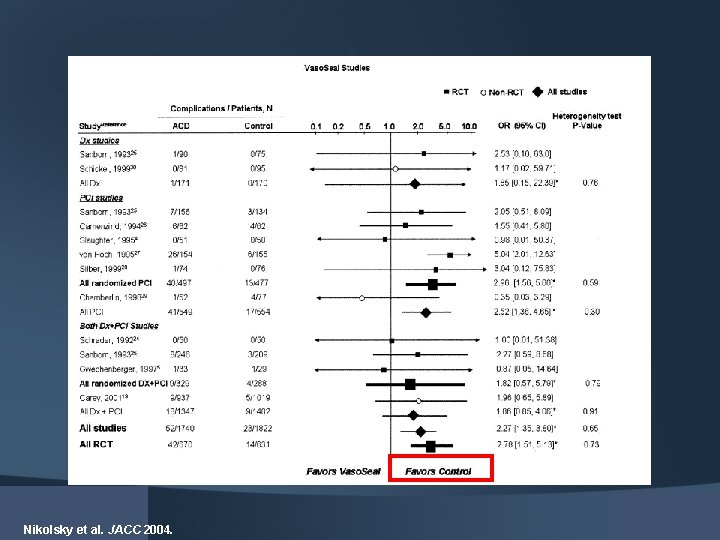
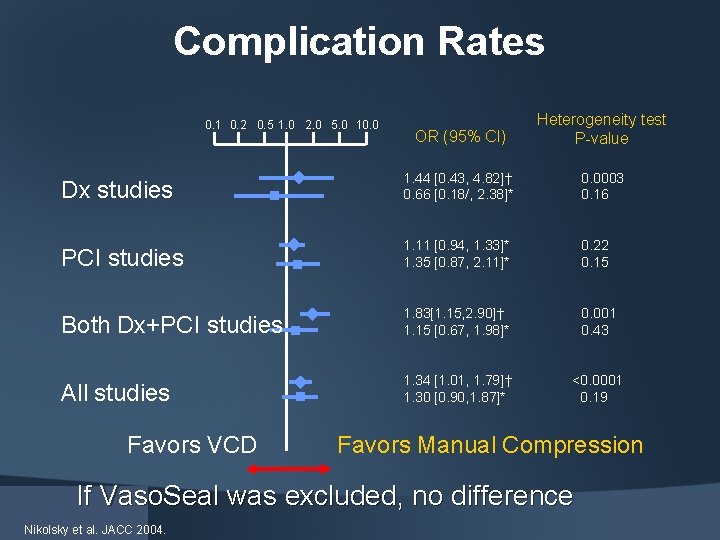
Nikolsky et al. JACC 2004.

Complication Rates OR (95% CI) Heterogeneity test P-value Dx studies 1. 44 [0. 43, 4. 82]† 0. 66 [0. 18/, 2. 38]* 0. 0003 0. 16 PCI studies 1. 11 [0. 94, 1. 33]* 1. 35 [0. 87, 2. 11]* 0. 22 0. 15 Both Dx+PCI studies 1. 83[1. 15, 2. 90]† 1. 15 [0. 67, 1. 98]* 0. 001 0. 43 All studies 1. 34 [1. 01, 1. 79]† 1. 30 [0. 90, 1. 87]* <0. 0001 0. 19 0. 1 0. 2 0. 5 1. 0 2. 0 5. 0 10. 0 Favors VCD Favors Manual Compression If Vaso. Seal was excluded, no difference Nikolsky et al. JACC 2004.

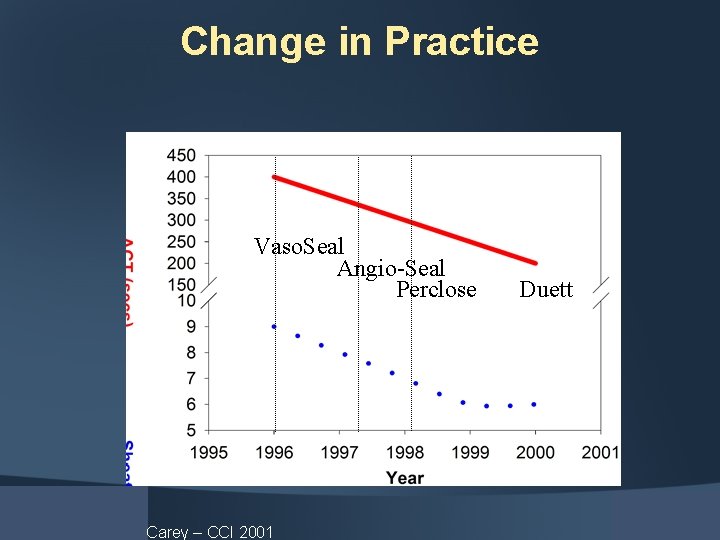
Change in Practice Vaso. Seal Angio-Seal Perclose Carey – CCI 2001 Duett

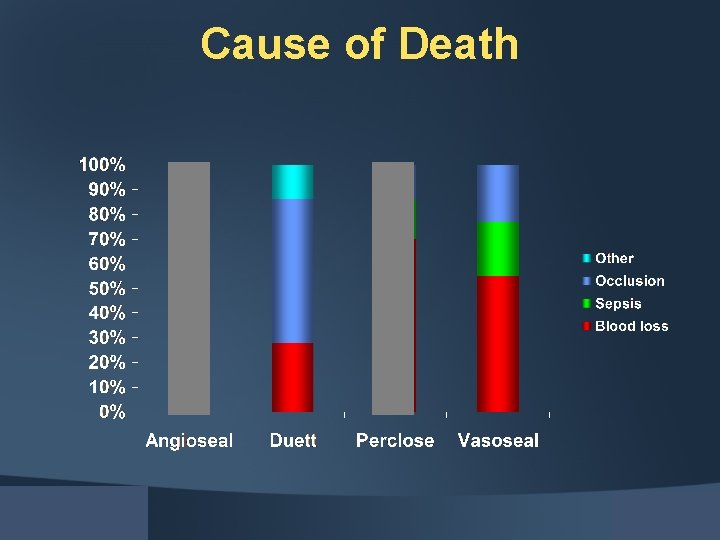
Cause of Death

Unanchored Plugs 1994 -2006 Vaso. Seal and Duett no longer marketed

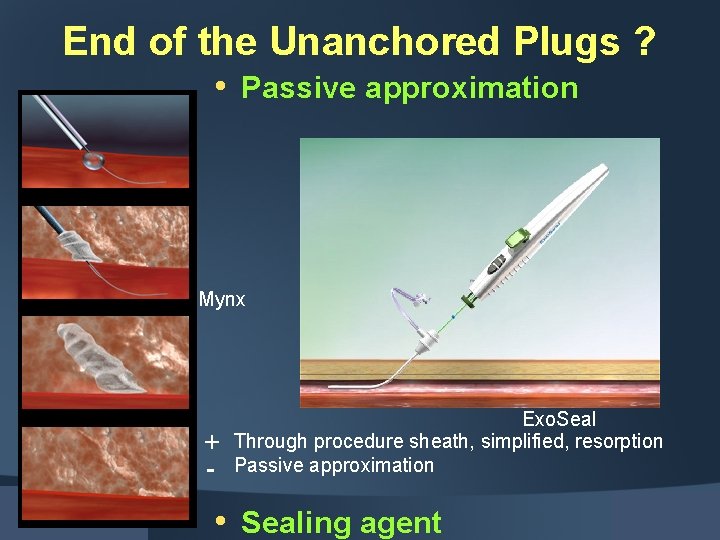
End of the Unanchored Plugs ? • Passive approximation Mynx - Exo. Seal Through procedure sheath, simplified, resorption Passive approximation • Sealing agent

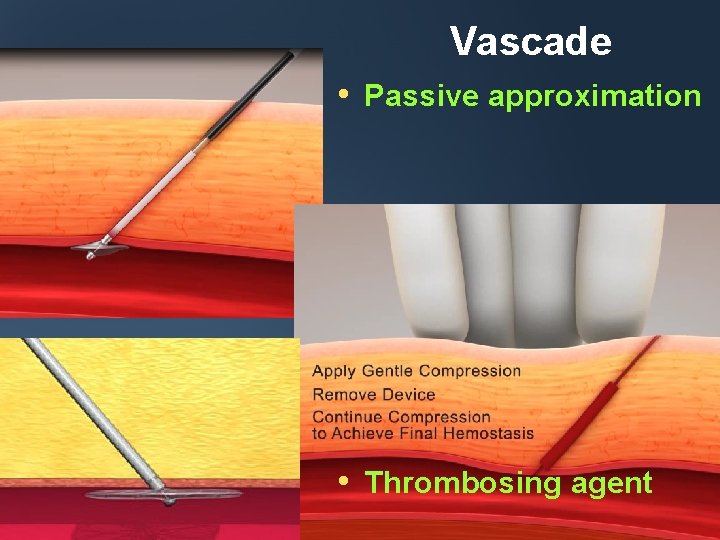
Vascade • Passive approximation • Thrombosing agent

Anchored Plugs Active Approximation Angio-Seal - Thrombosing agent High success rate, short learning curve, short deployment time Vascular occlusion, infection

Device Success VCD Device Closure Success - Interventions Most everything “works” in diagnostic cases Procedure success always looks good

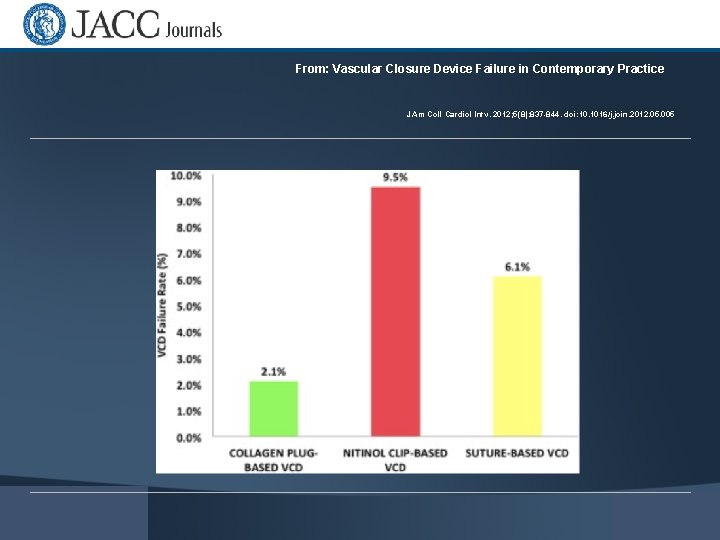
From: Vascular Closure Device Failure in Contemporary Practice J Am Coll Cardiol Intv. 2012; 5(8): 837 -844. doi: 10. 1016/j. jcin. 2012. 05. 005

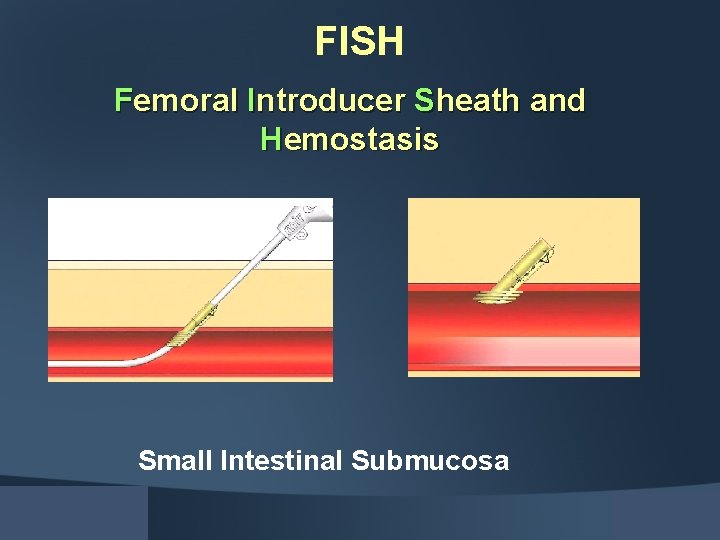
FISH Femoral Introducer Sheath and Hemostasis Small Intestinal Submucosa

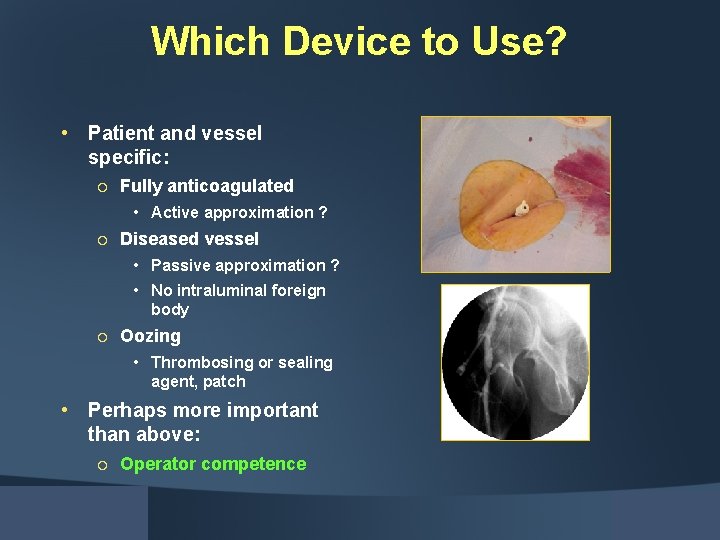
Which Device to Use? • Patient and vessel specific: ¡ Fully anticoagulated • Active approximation ? ¡ Diseased vessel • Passive approximation ? • No intraluminal foreign body ¡ Oozing • Thrombosing or sealing agent, patch • Perhaps more important than above: ¡ Operator competence

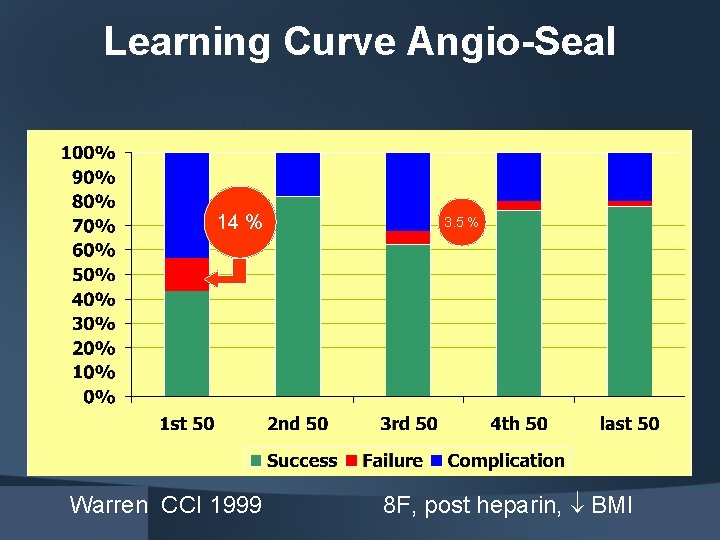
Learning Curve Angio-Seal 14 % Warren CCI 1999 3. 5 % 8 F, post heparin, BMI

Where I think twice • Diabetes, immune suppressed, poor hygiene • • Too thin, too fat < 5 F Low stick, high stick Significant vascular disease

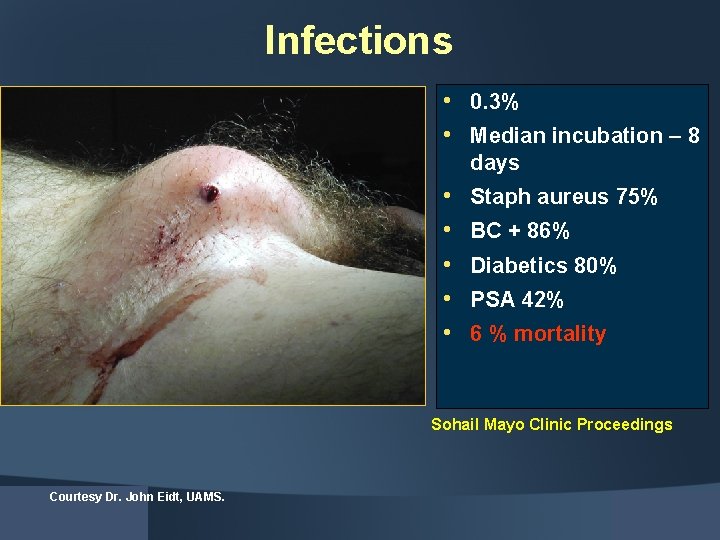
Infections • 0. 3% • Median incubation – 8 days • • • Staph aureus 75% BC + 86% Diabetics 80% PSA 42% 6 % mortality Sohail Mayo Clinic Proceedings Courtesy Dr. John Eidt, UAMS.

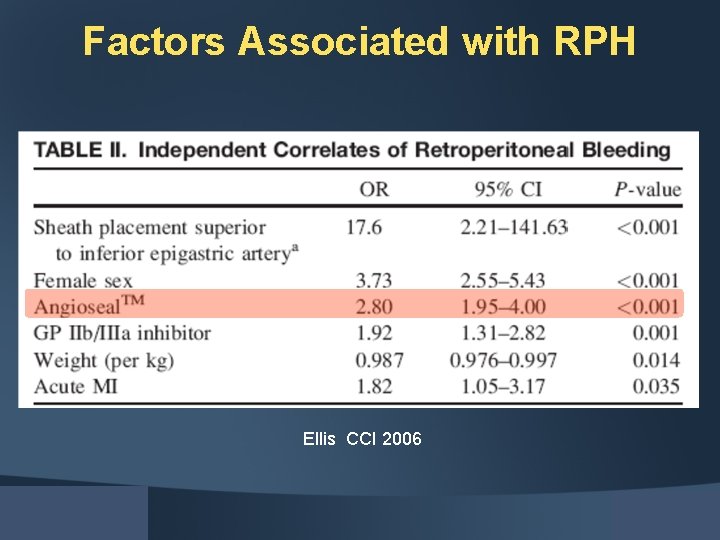
Factors Associated with RPH Ellis CCI 2006

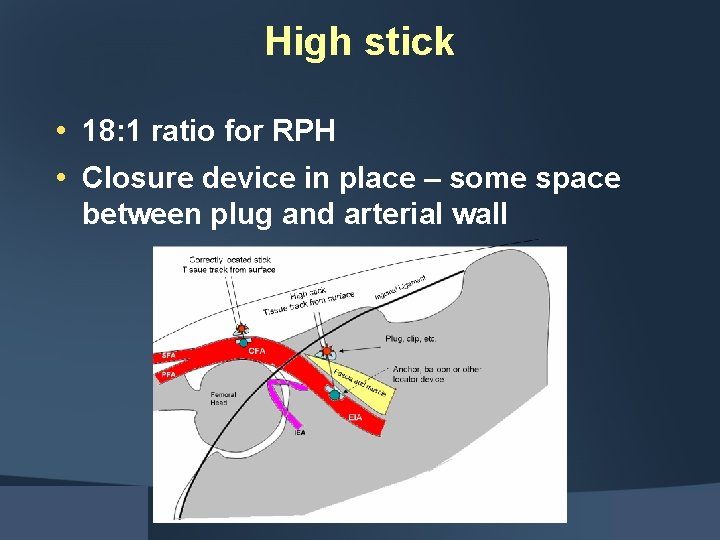
High stick • 18: 1 ratio for RPH • Closure device in place – some space between plug and arterial wall

The Evidence Base • Complication rate manual compression = vascular closure device • Strong patient preference • Cost savings with shorter length of stay and less management outside cath lab

 Vascular plants vs nonvascular plants
Vascular plants vs nonvascular plants Anthiridium
Anthiridium Vascular and non vascular difference
Vascular and non vascular difference Resta de donato giannini
Resta de donato giannini Pedagogik qobilyatni shakllantirish yo'llari
Pedagogik qobilyatni shakllantirish yo'llari Sakiniai su pusdalyviais
Sakiniai su pusdalyviais Yugurib kelib uzunlikka sakrash texnikasi
Yugurib kelib uzunlikka sakrash texnikasi Kas yra ilgis
Kas yra ilgis Ar visi medžiai turi lapus
Ar visi medžiai turi lapus Kokia mažiausia dalelė turi nedalomą krūvį
Kokia mažiausia dalelė turi nedalomą krūvį Kokios energijos turi traukiamas automobilis
Kokios energijos turi traukiamas automobilis Pedagogik mahorat fani haqida tushuncha
Pedagogik mahorat fani haqida tushuncha Ta'limni tashkil etish turlari va shakllari
Ta'limni tashkil etish turlari va shakllari Turi honegger
Turi honegger Konstruktiv qobiliyat nima
Konstruktiv qobiliyat nima Kiek laipsniu turi statusis kampas
Kiek laipsniu turi statusis kampas Medžiagos tankis ppt
Medžiagos tankis ppt Kommunikativlik haqida malumot
Kommunikativlik haqida malumot Kiek tona turi centnerių
Kiek tona turi centnerių Dars talimni tashkil etishning asosiy shakli
Dars talimni tashkil etishning asosiy shakli Mechanins
Mechanins O'quvchilar jamoasi
O'quvchilar jamoasi Dr egri zoltán
Dr egri zoltán Terenyi zoltán
Terenyi zoltán